Abstract
Objectives
In this study we aimed to evaluate the performance effects of chemiluminescence assay (CLIA) for Treponema pallidum specific antibodies detection, and to compare T. pallidum specific antibodies detection accuracy between CLIA and ELISA with TPPA (T. pallidum particle agglutination assay) as a confirmatory test.
Methods
A total of 865 samples from suspected syphilis patients and preoperative patients were included, in which T. pallidum specific antibodies were simultaneously detected by CLIA and ELISA. Among them, 457 samples were determined by TPPA.
Results
All coefficients of variation (CVs) of ELISA in high‐, median‐, and low‐level samples were more than 5% and the maximum CV was 54.39% in the low‐level sample. CVs of CLIA in different‐level samples were all below 5%. Among the three assays the Spearman correlation and Kappa coefficients were 0.771 (P ≤ 0.001) and 0.854 (P ≤ 0.001, CLIA vs. ELISA), 0.806 (P ≤ 0.001) and 0.897 (P ≤ 0.001, ELISA vs. TPPA), 0.937 (P ≤ 0.001) and 0.967 (P ≤ 0.001, CLIA vs. TPPA), respectively. The area under the receiver operating characteristic curve (AUC) of CLIA was higher than that of ELISA (0.994 vs. 0.989) with TPPA as the confirmatory test. In 18 discrepant samples the consistency rate between CLIA and TPPA was elevated compared with that between ELISA and TPPA (72.22% vs. 27.78%, P = 0.008). In gray zone, the consistency rate of CLIA with TPPA was higher than that of ELISA with TPPA (90.91% vs. 41.67%, P = 0.027).
Conclusions
Compared with ELISA, CLIA is more reliable, sensitive and accurate to detect serum T. pallidum specific antibodies. In the future it may be an alternative test with higher sensitivity to ELISA.
Keywords: chemiluminescence assay (CLIA), Treponema pallidum particle agglutination assay (TPPA), enzyme immunoassay (EIA), Treponema pallidum specific antibodies, syphilis
INTRODUCTION
Treponema pallidum is the pathogenic factor for syphilis, which is a sexually transmitted disease (STD) and has the similar clinical signs and symptoms to other infectious diseases. Since the diagnosis of syphilis is difficult due to its diverse clinical manifestations, the detection of etiology or serology is very important and helpful in syphilis diagnosis. Serological tests play vital roles in the accurate diagnosis of syphilis and are divided into nontreponemal and treponemal tests. Treponemal tests are directed against T. pallidum proteins with high specificity; these include fluorescent treponemal antibody‐absorption (FTA‐ABS), T. pallidum hemagglutination assay (TPHA), T. pallidum particle agglutination assay (TPPA), and enzyme immunoassay (EIA) 1, 2. Among them FTA‐ABS and TPPA are considered as confirmatory tests, but recently some investigation in Morbidity and Mortality Weekly Report (MMWR) 3 mentioned that CDC of the United States recommended the FTA‐ABS test not to be used to confirm discordant treponemal screening results because of some shortcomings such as lower specificity and probably lower sensitivity 4. The TPPA test is considered to be the most suitable confirmatory treponemal test according to previous published sensitivity and specificity data 5. However, the above‐mentioned traditional treponemal tests have some inevitable limitations, such as complicated operation procedures, subjective results, time‐consuming nature, and difficult automation. Therefore, development of a new analytic method with efficient testing, easy automation, high sensitivity and high specificity is critical for effective diagnosis of syphilis.
With the development of methodology, chemiluminescence assay (CLIA) has come into being. CLIA can automatically and quickly detect serum T. pallidum specific antibodies with high sensitivity and specificity for syphilis diagnosis 6, 7, 8. Previous studies mainly evaluated the diagnostic efficiency of EIA in T. pallidum specific antibodies detection 9, 10. Till now most studies on methodology comparison focused on EIA with TPPA 5, 11 or CLIA with TPPA 12, and the researches on the consistency of performance effects for T. pallidum detection by ELISA and CLIA were scarce. Therefore, we aimed to evaluate the performance effects of CLIA for T. pallidum specific antibodies detection and to compare the detection accuracy between CLIA and ELISA with TPPA as a confirmatory test.
MATERIALS AND METHODS
Subjects
A total of 865 samples of suspected syphilis patients and preoperative patients collected from September 2012 to February 2013 in West China Hospital of Sichuan University were included; among the patients, there were 377 females and 488 males and their mean age was 47.48 years. All the samples were detected for T. pallidum specific antibodies by CLIA and ELISA, and 457 samples among them were detected by TPPA as the confirmatory test. Our study using human blood samples was performed in accordance with the current revision of the Helsinki Declaration.
Serum T. pallidum Specific Antibodies Determination
Serum was separated within 3 h after drawing and stored for 1 week at −20°C. LUMIPULSE G1200 analyzer and Lumipulse® G TP‐N reagent kits (Fujirebio Diagnostics, Tokyo, Japan) as well as Tecan freedom EVOlyzer automated ELISA system (Tecan Group, Männedorf, Switzerland) and ELISA Diagnostic Kits for Antibody to T. pallidum (InTec Products, Xiamen, China) and SERODIA®‐TP•PA reagents (Fujirebio Diagnostics, Inc. Tokyo, Japan) were used. All assays were carried out according to the manufacturers’ standard procedures. Detailed performance characteristics of three different assays were described in Table 1. According to the manufacturers' suggestions and our clinical practical experiences, the value of S/C.O. (the optical density ratio of sample to cutoff) between 0.8 and 1.2 and C.O.I (cutoff index) value between 0.9 and 1.1 were considered gray zones of ELISA and CLIA, respectively.
Table 1.
Performance Characteristics of Three Different Assays
| LUMIPULSE G system | ELISA system | Agglutination assay | |
|---|---|---|---|
| Principles of assays | Chemiluminescent immunoassay, CLIA (two steps) | Enzyme‐linked immunosorbent assay, ELISA (two steps) | Gelatin particle agglutination assay (one step) |
| Analyzer | LUMIPULSE G1200 analyzer | Tecan freedom EVOlyzer automated ELISA system | No special analyzer |
| Reagents | Lumipulse® G TP‐N reagent kits | ELISA Diagnostic Kits for antibody to T. pallidum | SERODIA®‐TP•PA reagents |
| Test time | 25 min | 120 min | 120 min |
| Antibodies’ types | IgG and IgM | IgG and IgM | IgG and IgM |
| Coated antigen(s) | Recombination Tp15–17 and TpN47 | T. pallidum antigens from genetic engineering | T. pallidum (Nichols Strain) antigen |
| Enzymes and substrates | ALP and AMPPD | HRP and TMB | / |
ALP: alkaline phosphatase; AMPPD: 3‐[2‐spiroadamatane]‐4‐methoxy‐4‐[3‐phosphoryloxy]‐phenyl‐1,2‐dioxetane)Dioxetane ; HRP: horse radish peroxidase; TMB: tetramethylbenzidine
CLIA automatically detected serum T. pallidum specific antibodies on LUMIPULSE G1200 system. A total of 50 μl serum or TP‐N calibrator reacted with 250 μl microparticle coated with Tp15–17 and TpN47 antigens, then with 250 μl ALP‐conjugated Tp15–17 and TpN47 antigens formed antigen‐antibody‐antigen complex. With the substrate AMPPD and under 477 nm, the complex was detected by chemiluminescence method.
ELISA detected serum T. pallidum specific antibodies on Tecan freedom EVOlyzer automated ELISA system with InTec ELISA kits. A total of 100 μl sample, positive control, and negative control reacted with genetic engineering antigens coated microplate, and then reacted with HRP‐labeled genetic engineering antigens. With the substrate TMB and under 450 nm, the optical density was automatically detected.
TPPA was based on the agglutination of colored gelatin particle carriers sensitized with T. pallidum (Nichols Strain) antigen. Serum samples were serially diluted in sample diluent in microplate wells. Sensitized gelatin particles were added to respective wells and the contents of the plate were mixed by hand. The mixture was incubated for 2 h at room temperature. Serum containing specific antibodies formed a smooth mat of agglutinated particles in the microtitration tray. A compact button formed by the settling of the nonagglutinated particles characterized negative reactions.
Statistics
All data were analyzed by statistical software SPSS 13.0. Kappa test was conducted to evaluate the consistency of qualitative results. Spearman correlation was used to show the correlation of quantitative results. With TPPA as the confirmatory test for T. pallidum specific antibodies detection, receiver operating characteristic (ROC) curve analysis was made to evaluate the areas under the ROC curve (AUCs), sensitivity and specificity of ELISA and CLIA in T. pallidum specific antibodies detection. Chi‐square test or Fisher's exact test was performed to analyze the qualitative data or categorical variable comparison. P‐value <0.05 was considered statistically significant.
RESULTS
Imprecision Analysis of ELISA and CLIA for Detecting T. pallidum Specific Antibodies
High‐, median‐, and low‐concentration samples were selected to be continuously detected “20 times” by ELISA and CLIA. The detection showed that all coefficients of variation (CVs) of ELISA in high‐, median‐, and low‐level samples were more than 5% and the maximum CV was 54.39% in the low‐level sample. CVs of CLIA in different‐level samples were all below 5% and less than CVs of ELISA in all the samples (Table 2).
Table 2.
Imprecision Analysis of ELISA and CLIA for Detecting T. pallidum Specific Antibodies
| Low concentrationa | Median concentrationb | High concentrationc | ||
|---|---|---|---|---|
| CV (%) | ELISA | 54.39 | 10.63 | 6.79 |
| CLIA | 4.42 | 1.43 | 2.09 |
Mean low concentration: CLIA—C.O.I = 0.1, ELISA—S/C.O. = 0.004.
Mean median concentration: CLIA—C.O.I = 11.7, ELISA—S/C.O. = 7.44.
Mean high concentration: CLIA—C.O.I = 73.76, ELISA—S/C.O. = 17.01.
Consistency Analysis of CLIA and ELISA in Quantitative and Qualitative Results
In quantitative data or numerical variable, consistency analysis showed that Spearman correlation coefficient between CLIA and ELISA was 0.771 (P ≤ 0.001, Fig. 1a) and when data were described as qualitative or categorical variable Kappa coefficient was 0.854 (P ≤ 0.001, Table 3).
Figure 1.
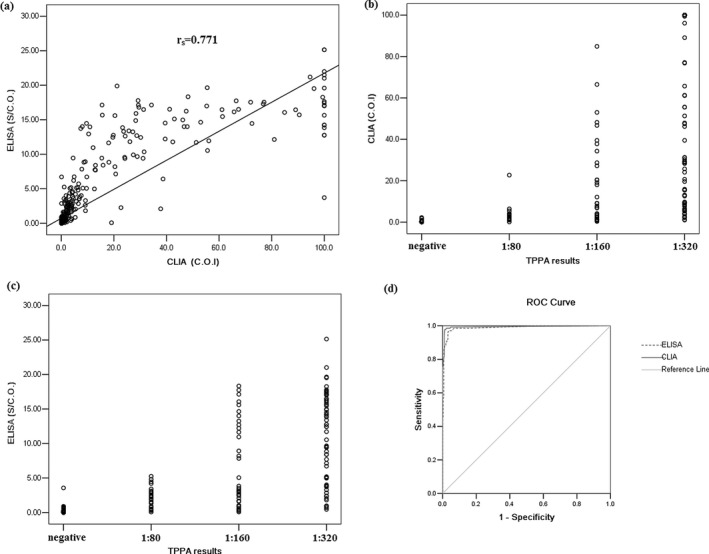
Quantitative analysis of serum T. pallidum specific antibodies detected by ELISA and CLIA . (a) Correlation of serum T. pallidum specific antibodies’ levels between ELISA and CLIA. (b and c) Correlation of serum T. pallidum specific antibodies’ levels between CLIA or ELISA and TPPA. (d) ROC analysis of CLIA and ELISA for serum T. pallidum specific antibodies detection with TPPA as the confirmatory test.
Table 3.
Kappa Test Analysis Between CLIA and ELISA
| CLIA | ||||
|---|---|---|---|---|
| Negative | Gray zone | Positive | ||
| ELISA | Negative | 649 | 6 | 15 |
| Gray zone | 10 | 4 | 7 | |
| Positive | 3 | 5 | 166 | |
| Kappa coefficient | 0.854 (P ≤ 0.001) | |||
Under the manufacturers’ cutoff in 865 samples, there were 30 discrepant results between CLIA and ELISA. And excluding the data in gray zone, in 833 samples 18 sera results were discrepant between CLIA and ELISA (Table 3). TPPA analysis showed that in 18 discrepant samples the consistency rate between CLIA and TPPA was significantly higher than that between ELISA and TPPA (72.22% (13/18) vs. 27.78% (5/18), P = 0.008). Further analysis showed that in 18 discrepant samples the true‐positive consistency rate of CLIA (12/14, 85.71%) was higher than that of ELISA (2/14, 14.29%; P ≤ 0.001), and the true‐negative consistency rate of CLIA (1/4, 25%) was lower than that of ELISA (3/4, 75%; P ≤ 0.001) compared with TPPA.
ROC Analysis of CLIA and ELISA with TPPA as the Confirmatory Test
AUC, specificity and sensitivity of CLIA and ELISA were calculated with TPPA as the confirmatory test. It showed that AUC of CLIA (0.994, P ≤ 0.001) was higher than that of ELISA (0.989, P ≤ 0.001; Fig. 1d). Under the manufactures’ cutoff, the sensitivity of CLIA was higher than that of ELISA (94.07% vs. 82.96%) and the specificity of CLIA was similar to that of ELISA (99.07% vs. 99.38%). In quantitative data, Spearman correlation coefficient between CLIA and TPPA (r s = 0.937, P ≤ 0.001) was higher than that between ELISA and TPPA (r s = 0.806, P ≤ 0.001; Fig. 1b and 1c)
When excluding the gray‐zone data, the sensitivity and specificity of CLIA were 91.11% and 98.45%, and those of ELISA were 87.40% and 99.37%. The consistency analysis of qualitative data showed Kappa coefficient between CLIA and TPPA was higher than that between ELISA and TPPA (0.967 (P ≤ 0.001) vs. 0.897 (P ≤ 0.001), Table 4). The false‐positive rates of CLIA and ELISA were similar (0.94% (3/320) vs. 0.63% (2/318)), and the false‐negative rate of CLIA was lower than that of ELISA (2.38% (3/126) vs. 12.60% (16/127), P = 0.002).
Table 4.
Comparison of ELISA and CLIA with TPPA Using Serum Specimens
| TPPA results (N) | ||||||
|---|---|---|---|---|---|---|
| Assays and results | Negative | Positive | %Sensitivity (95% CI) | %Specificity (95% CI) | %agreement (95% CI) | Kappa values (P‐value) |
| CLIA | 91.11 | 98.45 | 96.28 | 0.967 | ||
| (88.33–93.54) | (97.12–99.38) | (94.35–97.82) | (P ≤ 0.001) | |||
| Negative | 317 | 3 | ||||
| Positive | 3 | 123 | ||||
| Equivocal | 2 | 9 | ||||
| Low valuea | 1 | 0 | ||||
| High valueb | 1 | 9 | ||||
| ELISA | 87.40 | 99.37 | 93.44 | 0.897 | ||
| (84.20–90.28) | (98.44–99.89) | (90.99–95.52) | (P ≤ 0.001) | |||
| Negative | 316 | 16 | ||||
| Positive | 2 | 111 | ||||
| Equivocal | 4 | 8 | ||||
| Low valuea | 4 | 7 | ||||
| High valueb | 0 | 1 | ||||
Value was lower than the manufacturer's cutoff of CLIA or ELISA.
Value was higher than the manufacturer's cutoff of CLIA or ELISA.
Accuracy Analysis in Gray‐Zone Data of ELISA and CLIA
It was hard to define gray‐zone data as true positive or true negative. Here TPPA was as the confirmatory test to detect serum T. pallidum specific antibodies. Compared with TPPA, the consistency rate of CLIA in gray‐zone data was significantly higher than that of ELISA (90.91% (10/11) vs. 41.67% (5/12), P = 0.027); true‐positive rate in gray zone of CLIA also was strikingly higher than that of ELISA (81.82% (9/11) vs. 8.33% (1/12), P = 0.001) and true‐negative rate of CLIA was lower than that of ELISA (9.09% (1/11) vs. 33.33% (4/12), P = 0.317; Table 4). Further analysis showed that 54.50% data in gray zone of CLIA were in TPPA low titer level (1:80), and 50% data in gray zone of ELISA were in TPPA median (1:160) and high titer levels (1:320, Table 5).
Table 5.
ELISA and CLIA Gray Zone Data Analysis Compared with TPPA
| TPPA | ||||
|---|---|---|---|---|
| Positive | ||||
| Negative | 1:80 | 1:160 | 1:320 | |
| ELISA gray zone | 4(33.30%) | 2(16.70%) | 3(25.00%) | 3(25.00%) |
| CLIA gray zone | 2(18.20%) | 6(54.50%) | 2(18.20%) | 1(9.10%) |
DISCUSSION
Serological tests, especially treponemal tests, play an important role in syphilis diagnosis. Reverse sequence screening test for T. pallidum specific antibodies, in which sera are tested first by a treponemal EIA, is widely used for syphilis serodiagnosis to reduce the time and labor required for syphilis screening. And EIA, especially ELISA and CLIA, will gradually become the critical screening treponemal test. ELISA, an important assay of EIAs, is considered as a traditional assay to detect serum T. pallidum specific antibodies, and especially based on its high sensitivity and specificity, it has been widely used since 1980s 13. CLIA, a newly developed method, can allow for rapid detection of T. pallidum specific antibodies on random‐access analyzers with higher precision, sensitivity and specificity 6, 7. And TPPA, as the confirmatory test, can ensure its sensitivity and specificity for diagnosing syphilis infection by using T. pallidum (Nichols Strain) as antigen. Table 1 presented the characteristics of ELISA, TPPA, and CLIA, which indicated that CLIA was less time consuming and easy to handle compared with ELISA and TPPA. It also indicated that different assays applied different antigens and analysis systems to detect serum T. pallidum specific antibodies. CLIA and ELISA just used some T. pallidum antigen peptides, while in TPPA coated‐antigen was the Nichols strain T. pallidum. As it was known that the difference of coated antigen could influence sensitivity and specificity of assays. Here we expected to make the performance evaluation of CLIA in detecting T. pallidum specific antibodies compared with ELISA and TPPA.
Because treponemal tests were used to diagnose syphilis, the test stability was important. Table 2 demonstrated that CLIA was a more stable assay in detecting different T. pallidum specific antibodies levels (all CVs (%) < 5%), and ELISA results were diverse, especially in low‐level detection (which was close to background level and easily influenced by many factors), and CVs of ELISA in median or high level were still higher than 5%.
Some reports showed that in the population with low prevalence of syphilis there was higher discordant percentage and false‐positive percentage among different tests 3. So in our subjects the suspected syphilis patients and preoperative patients were included to avoid the different prevalence of syphilis in different population.
CLIA and ELISA were both EIAs, and ELISA was the traditional test. Here we made a consistency analysis between ELISA and CLIA in a prospective study with 865 sera. Because some data of CLIA were more than its upper limit, scatter figure was limited to show the correlation between CLIA and ELISA in abnormal distributed data, and Spearman correlation analysis would be a more proper method to evaluate their correlation. Here Kappa test (Kappa = 0.854) and Spearman correlation analysis (r s = 0.771) both indicated that there was a good consistency between CLIA and ELISA.
Further, we used TPPA as the confirmatory test to assess CLIA and ELISA performance characteristics. In quantitative and qualitative analysis, CLIA showed higher consistency with TPPA compared with ELISA; especially as a serodiagnosis test CLIA had low false‐negative rate (2.38%) and false‐positive rate (0.94%) to guarantee the lower misdiagnosis and missed diagnosis rates. Figure 1d indicated that with TPPA as the confirmatory test both CLIA and ELISA had good diagnosis efficiency (all the AUCs ≥ 0.95), and CLIA was better. Compared with other studies 5, 6, 14, 15, 16, 17, the sensitivity and specificity of CLIA and ELISA in our study were close or similar to others’—which indicated that the difference of reference/confirmatory tests may not be the critical factor to influence the sensitivity and specificity of evaluated assays. Wellinghausen N et al. and we both appraised diagnostic efficiency of CLIA with different confirmatory tests (FTA‐ABS in Wellinghausen N et al.'s study and TPPA in our study). We have drawn a common conclusion that CLIA was a good screening test for syphilis diagnosis with good sensitivity and specificity (more than 90%). Here we speculated that the differences of coated antigens and analyzers or detection systems between ELISA and CLIA could be important factors to influence the sensitivity of ELISA. Some study demonstrated that CLIA might have a role either as a diagnostic screening test or as a confirmatory test following a nontreponemal screening test (6). However, we thought CLIA may be more appropriate for a screening test, not a perfect confirmatory test for syphilis diagnosis due to the coated nonintact T. pallidum antigen in CLIA. The nonintact T. pallidum antigen theoretically could cause false‐positive results and influence the specificity and sensitivity of CLIA, which is shown in Table 4.
Gray‐zone definition and interpretation were always problems for diagnostic test. Here Table 4 showed that CLIA had a higher accuracy and sensitivity with TPPA in all data analysis or excluding gray‐zone data analysis than ELISA. And discrepant results’ analysis demonstrated that CLIA had higher diagnostic accuracy rate (72.22%) and positive consistency rate (85.71%) with TPPA than ELISA. Table 5 shows that CLIA and ELISA gray‐zone data mainly distributed in low titer of TPPA and high titer of TPPA, respectively. Our study indicated that ELISA had lower sensitivity and higher false‐negative rate as a screening test compared with CLIA. Meanwhile, Table 4 shows that excluding the gray‐zone data the sensitivity of ELISA could increase, and the sensitivity and specificity of CLIA were not influenced, which indicated that the data in ELISA gray zone should be paid more attention, and samples in ELISA gray zone had better be repeated with other treponemal test.
CONCLUSIONS
Compared with ELISA, CLIA is more reliable, sensitive and accurate to detect serum T. pallidum specific antibodies. In the future it may be an alternative test with higher sensitivity to ELISA as a screening test for syphilis diagnosis.
DISCLOSURE
The authors have declared no conflicts of interest.
ACKNOWLEDGMENTS
We thank Fujirebio Diagnostics company for generous donation of their assays and Mr. Qingfei Zeng for his hard work on data collection.
REFERENCES
- 1. Neuza SS. Laboratorial diagnosis of syphilis In: Neuza SS, editor. Syphilis – Recognition, Description and Diagnosis, Croatia: InTech Inc; 2011. p 87–108. [Google Scholar]
- 2. Seña AC, White BL, Sparling PF. Novel Treponema pallidum serologic tests: A paradigm shift in syphilis screening for the 21st century. Clin Infect Dis 2010;51(6):700–708. [DOI] [PubMed] [Google Scholar]
- 3. Radolf JD, Bolan G, Park IU, et al. Discordant results from reverse sequence syphilis screening – five laboratories, United States, 2006—2010. MMWR Morb Mortal Wkly Rep 2011;60(5):133–137. [PubMed] [Google Scholar]
- 4. Marangoni A, Sambri V, Storni E, D'Antuono A, Negosanti M, Cevenini R. Treponema pallidum surface immunofluorescence assay for serologic diagnosis of syphilis. Clin Diagn Lab Immunol 2000;7:417–421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cole MJ, Perry KR, Parry JV. Comparative evaluation of 15 serological assays for the detection of syphilis infection. Eur J Clin Microbiol Infect Dis 2007;26:705–713. [DOI] [PubMed] [Google Scholar]
- 6. Knight CS, Crum MA, Hardy RW. Evaluation of the LIAISON chemiluminescence immunoassay for diagnosis of syphilis. Clin Vaccine Immunol 2007;14(6):710–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mo X, Jin Y, Yang Y, Hu W, Gu W. Evaluation of a new chemiluminescence immunoassay for diagnosis of syphilis. Eur J Med Res 2010;15:66–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Yoshioka N, Deguchi M, Kagita M, et al. Evaluation of a chemiluminescent microparticle immunoassay for determination of Treponema pallidum antibodies. Clin Lab 2007;53(9–12):597–603. [PubMed] [Google Scholar]
- 9. Schmidt BL, Edjlalipour M, Luger A. Comparative evaluation of nine different enzyme‐linked immunosorbent assays for determination of antibodies against Treponema pallidum in patients with primary syphilis. J Clin Microbiol 2000;38(3):1279–1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Viriyataveekul R, Laodee N, Potprasat S, Piyophirapong S. Comparative evaluation of three different treponemal enzyme immunoassays for syphilis. J Med Assoc Thai 2006;89(6):773–779. [PubMed] [Google Scholar]
- 11. Binnicker MJ, Jespersen DJ, Rollins LO. Treponema‐specific tests for serodiagnosis of syphilis: Comparative evaluation of seven assays. J Clin Microbiol 2011;49(4):1313–1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wellinghausen N, Dietenberger H. Evaluation of two automated chemiluminescence immunoassays, the LIAISON Treponema Screen and the ARCHITECT Syphilis TP, and the Treponema pallidum particle agglutination test for laboratory diagnosis of syphilis. Clin Chem Lab Med 2011;49(8):1375–1377. [DOI] [PubMed] [Google Scholar]
- 13. Young H, Moyes A, McMillan A, Robertson DH. Screening for treponemal infection by a new enzyme immunoassay. Genitourin Med 1989;65:72–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Young H, Pryde J, Duncan L, Dave J. The Architect Syphilis assay for antibodies to Treponema pallidum automated screening assay with high sensitivity in primary syphilis. Sex Transm Infect 2009;85:19–23. [DOI] [PubMed] [Google Scholar]
- 15. Tsang RS, Martin IE, Lau A, Sawatzky P. Serological diagnosis of syphilis; comparison of the Trep‐Chek IgG enzyme immunoassay with other screening and confirmatory tests. FEMS Immunol Med Microbiol 2007;51:118–124. [DOI] [PubMed] [Google Scholar]
- 16. Ebel A, Bachelart L, Alonso JM. Evaluation of a new competitive immunoassay (Bio Elisa Syphilis) for screening for Treponema pallidum antibodies at various stages of syphilis. J Clin Microbiol 1998;36:358–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Halling VW, Jones MF, Bestrom JE, et al. Clinical comparison of the Treponema pallidum CAPTIA Syphilis‐G enzyme immunoassay with the fluorescent treponemal antibody absorption immunoglobulin G assay for syphilis testing. J Clin Microbiol 1999;37:3233–3234. [DOI] [PMC free article] [PubMed] [Google Scholar]


