Abstract
Background
Toxocariasis is the clinical term that is applied to infection in the human host with Toxocara species larvae. Serological tests are important tools for the diagnosis of toxocariasis. The aim of this study was to evaluate the excretory–secretory (ES) antigens of T. cati larvae using enzyme‐linked immunosorbent assay (ELISA) and also Western blotting for serodiagnosis of human toxocariasis.
Method
The ES antigens were prepared from T. cati third‐stage larvae. Serum samples were obtained from 33 confirmed cases of toxocariasis, 35 patients infected with other parasitic diseases, and 30 from healthy individuals tested with ELISA and immunoblotting.
Results
The ELISA showed appropriate performance in term of specificity (96.7%) and sensitivity (97.0%). Electrophoretic analysis of T. cati ES antigens revealed a range of 20‐ to 150‐kDa fractions. The highest sensitivity was achieved with 42‐ and 50‐kDa fractions.
Conclusion
The ELISA analyses using T. cati ES antigens demonstrated good sensitivity and specificity compared to T. canis ES as antigens for diagnosis of human toxocariasis. Accordingly, application of Western blotting, based on 42‐ and 50‐kDa fractions of ES antigens, can be recommended for the accurate diagnosis of toxocariasis.
Keywords: Toxocara cati, serodiagnosis, excretory–secretory antigens, ELISA, Western blotting
INTRODUCTION
Toxocariasis is a worldwide helminthic zoonosis caused by the ascariid nematode Toxocara species. Dogs and cats are the definitive hosts and act as reservoirs for T. canis and T. cati, respectively 1. Humans are infected by ingestion of embryonated eggs in the soil or through contaminated hands and fomites, and eating the meat of paratenic hosts containing encapsulated larvae 2. The larva hatches in the intestine and migrates into different organs, mainly the liver and lungs. It may occasionally migrate into the kidney, myocardium, and central nervous system 3. There are four clinical forms of toxocariasis including visceral larva migrans (VLM), ocular larva migrans, covert toxocariasis, and neurotoxocariasis 4.
Prevalence of Toxocara infestation of dogs and cats and the resulting contamination of the ground is relatively high in many countries all over the world. The presence of T. canis infected dogs and T. cati infected felines in public spaces, their defecation habits, and their direct contact with humans constitute significant factors that promote the transmission of this zoonosis. A number of studies taking place worldwide have described the extent of environmental contamination with this parasite. It has been determined that in Iran 42.6% of stray cats are infected with T. cati 5.
There is little finding on the location of T. cati larvae in paratenic hosts as causative agents of human toxocariasis 6. The diagnosis of human toxocariasis is mainly based on clinical, epidemiological, and laboratory data, which include imagining exams, blood exams, eosinoplilia, total IgE level, and serological tests 1. Enzyme‐linked immunosorbent assay (ELISA) and Western blotting are two types of tests that are available for the immunodiagnosis of toxocariasis, both using Toxocara excretory–secretory (ES) antigens. The test currently employed for the serodiagnosis of toxocariasis is ELISA using ES antigens from Toxocara larvae 7. Due to the greater sensitivity and specificity of the Toxocara Western blotting, investigations demonstrate that this method is a better technique than ELISA to investigate patients having visceral or ocular involvement consistent with toxocariasis 7, 8, 9, 10. Considering that T. cati is one of common helminths in Iran and that it is a potentially preventable disease and information about the diagnosis of patients with Toxocara infection by using the T. cati ES antigens is scanty 11, we describe the development, sensitivity, and specifity for Western blotting analysis made with ES T. cati antigens.
METHODS
Patients and sera
Serum samples from 33 patients with toxocariasis diagnosed based on clinical (fever, anorexia, cough, nausea, vomiting), hematological (leukocytosis and eosinophilia), and serological findings were used to identify specific Toxocara antibodies. Sera from 30 healthy individuals (with repetitive negative results for intestinal parasites examination) were used as controls.
Cross‐reactivity was assessed by selecting sera from 30 healthy subjects and 35 patients with other proven parasitic infections confirmed by blood smear, stool examination, positive specific serological test results: leishmaniasis (five patients), strongyloidiasis (six patients), hydatidiasis (six patients), toxoplasmosis (seven patients), trichostrongyloidiasis (six patients), and ascariasis (five patients).
Preparation of T. cati ES antigens
Toxocara cati ES antigens were prepared from third‐stage larvae by the method described by de Savigny 12 with some modifications. In brief, T. cati adult worms were collected from the intestines of infected stray cats from different areas of Shiraz, Iran. The T. cati eggs were isolated from uteri of female worms and were decoated by sodium hypochlorite (7% w/v) and embryonated in 2.5% formalin/ringer solution while incubated in an atmosphere of 5% CO2 at 25 °C for 3 weeks 13. The embryonated eggs suspension was washed in sterile distilled water to remove the traces of chlorine. The larvae were collected aseptically in a Baermann's apparatus. The larvae were cultivated in RPMI 1640 (Sigma‐Aldrich Chemie GmbH, Germany) medium supplemented with sodium bicarbonate (Merck, Darmstadt, Germany; 8.5% μg/ml), l‐glutamine (20 mM), HEPES (Merck) penicillin/streptomycin (100 IU, 100 μg/ml), and 1% glucose (Merck, Darmstadt, Germany). Culture supernatant was exchanged weekly and pooled; then dialyzed overnight against 5 mM acetate buffer (pH 5) at 4 °C, protease inhibitors (phenylmethylsulfonyl fluoride) were added, concentrated, and stored at −20 °C. The protein content (120 μg/ml) was measured by the Bradford protein assay 14. The ES antigens’ profile was analyzed using sodium dodecyl sulfate‐polyacrylamide gel electrophoresis (SDS‐PAGE).
Enzyme‐linked immunosorbent assay
ELISA microplates (Nunc, Roskilde, Denmark) were coated with 5 μg/ml of T. cati ES antigens (100 μl/well) in coating buffer (0.05 M carbonate–bicarbonate buffer, pH 9.6) and incubated overnight at 4 °C. Plates were washed five times in phosphate‐buffered saline containing 0.05% Tween 20 (PBST). After removing unbound coating antigen, the excess binding sites were blocked with 3% skimmed milk in PBST and plates incubated for 2 h at room temperature. The wells were washed in PBST and 100 μl of diluted (1:100) serum samples were added to the plates and incubated at room temperature for 1 h. The plates were washed again and 100 μl of diluted anti‐human IgG horseradish peroxidase conjugated (Sigma‐Poole, UK) at a 1,000‐fold dilution in PBST (1:1,000) was added to each well and incubated for 1 h at 37 °C. After washing three times, the plates were incubated with chromogen/substrate (100 μg/well of OPD, 0.025% H2O2 in 0.1 M citrate buffer pH 5) and were stopped by addition of 100 μl of 1 M sulfuric acid after 30 min. The optical density (OD) of samples was monitored at a wavelength of 450 nm using a microplate reader. The cut‐off point was set as the mean optical density of the negative controls plus two standard deviations.
SDS‐PAGE and Western blotting
Toxocara cati ES antigens were diluted in sample buffer and were boiled at 100 °C for 5 min. The prepared antigens along with molecular weight markers (Fermentas, UK) were submitted to electrophoresis in SDS‐PAGE, using a Mini‐PROTEAN III Electrophoresis Cells (Bio‐Rad Laboratories, Hercules, CA) and 12.5% polyacrylamide gel in the presence of 10% sodium dodecyl sulfate at 50 mA/gel for 1 h. The antigens were transferred into nitrocellulose membrane (Schleicher & Schuell BioScience, Hamburg, Germany). Efficacy of transfer was checked by staining the membrane with Ponceau S stain (0.001 g/ml) in 3% trichloroacetic acid. The membranes with blotted antigen were cut into strips and blocked with 5% (w/v) of skimmed milk in washing buffer (10 mM Tris, 150 mM NaCl, and 0.05% Tween 20; pH 7.4) for 2 h at 37 °C. The strips were incubated with test sera (1/100 dilution in washing buffer with 1% bovine serum albumin) for 2 h at room temperature. After washing three times (each for 15 min) in PBST, the strips were incubated with horseradish peroxidase conjugated anti‐human immunoglobulin (Dako) at a dilution of 1:2,000 for 2 h at room temperature. After three washes as before, bound antigens was developed using diaminobanzidine (DAB) substrate (0.1% H2O2 + DAB in 50 mM Tris‐HCl, pH 7.6). Positive reactions were determined by the appearance of clearly defined bands. The relative molecular weight of each recognized band was estimated by comparison with standard marker.
Ethical Considerations
The study was approved by ethical committee of the Shiraz University of Medical Sciences and informed consent was obtained from the participants prior to data collection.
Statistical Analysis
Analyses of differences in sensitivity and specificity of ELISA and Western blotting were performed using 2 × 2 tables.
RESULTS
ELISA
In this study, ELISA and Western blotting were performed with a panel of 98 sera including 33 serologically confirmed toxocariasis (Toxocara infection), 35 patients with parasitic disease other than toxocariasis, and 30 healthy individuals. Of the 33 serum samples from positively diagnosed toxocariasis patients, one serum sample was negative using the ELISA based on T. cati antigens. In these patients, the diagnosis of toxocariasis was confirmed by Western blotting using T. cati ES antigens. The ELISA yielded one false‐positive result with sera from healthy individuals. The sensitivity and specificity of ELISA for diagnosis of human toxocariasis were found to be 97.0% and 96.7%, respectively.
SDS‐PAGE and Western blotting
Using the SDS‐PAGE and Western blotting, fractions of 20, 28, 32, 42, 50, 70, and 150 kDa were detected in sera of serologically confirmed patients. Frequencies of different antigenic fractions reacting with the sera from Toxocara infection were presented in the bands of 20 (n = 20, 60.6%), 28 (n = 18, 54.5%), 32 (n = 24, 72.7%), 42 (n = 33, 100%), 50 (n = 33, 100%), 70 (n = 12, 36.4%), and 150 kDa (n = 14, 42.4%). Samples from patients with leishmaniasis, trichostrongyliasis, hydatidiasis, toxoplasmosis, and ascariasis did not yield any detectable band in the Western blotting (Table 1). The 42‐ and 50‐kDa bands were reactive with all of the sera from 33 toxocariasis patients (Fig. 1). The Western blotting presented good reproducibility. The diagnostic sensitivity and specifity of immunoblotting assay are summarized in Fig. 2. The 42‐ and 50‐kDa fractions have been recognized by all of the patient's sera with toxocariasis, but not by any of sera from nontoxocariasis patients. Accordingly, specificity of the system was found to be 100%.
Table 1.
Frequency of Different Antigenic Fractions Reacting With Sera From Patients With Toxocariasis, Other Parasitic Infection, and Healthy Individuals
| Detection of ES antigen fractions (kDa) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Infection | Sera (n = 98) | 20 | 28 | 32 | 42 | 50 | 70 | 150 | |
| Trichostrongyliasis | 6 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Strongyloidiasis | 6 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Ascariasis | 5 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Hydatidosis | 6 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Leishmaniasis | 5 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Toxoplasmosis | 7 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Healthy control | 30 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Toxocariasis | 33 | 20 (60.6%) | 8 (54.5%) | 24 (72.2%) | 33 (100%) | 33 (100%) | 12 (36.4%) | 14 (42.4%) | |
Figure 1.
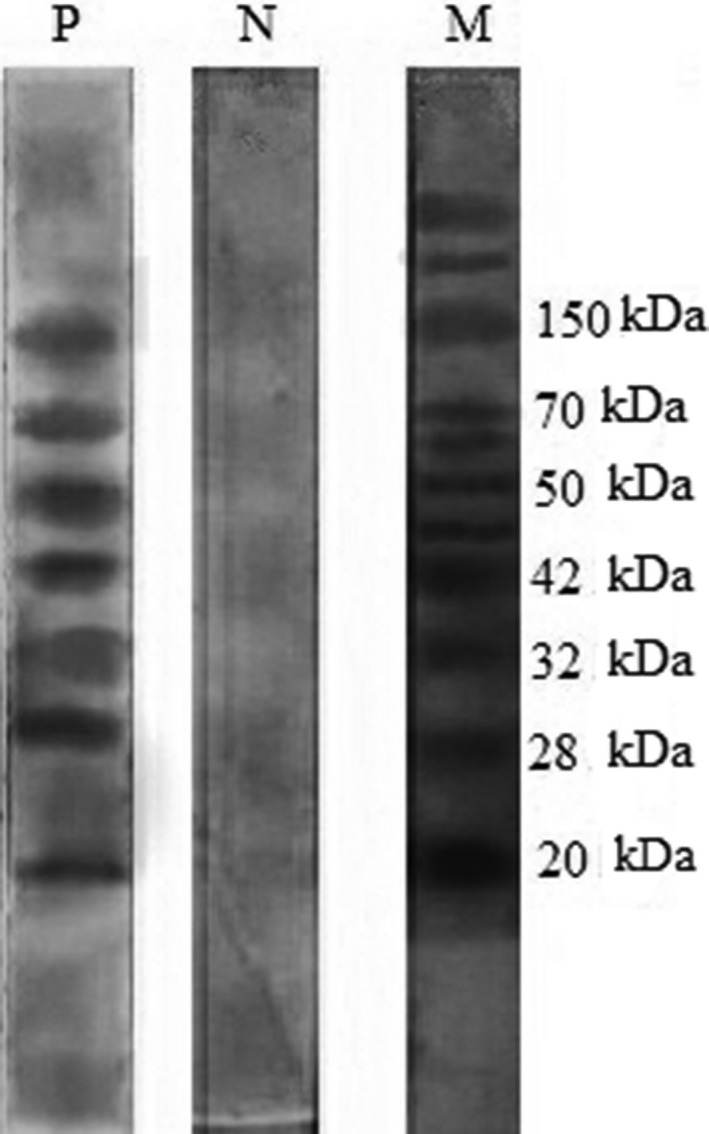
Western blotting with T. cati ES antigens. P, toxocariasis patients; N, toxocariasis‐negative sera; M, molecular weights marker. All sera from healthy individuals and from other parasitic diseases did not react with any band.
Figure 2.
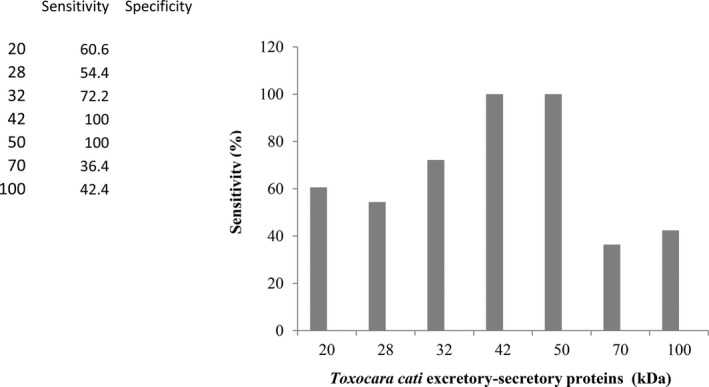
Frequency of immunodominant proteins (kDa) of anti‐T. cati ES antigens recognized by 33 serum samples from toxocariasis.
DISCUSSION
The development of specific, sensitive, and reliable methods to demonstrate of antibodies in Toxocara infection is an important step toward improving diagnosis. Several serodiagnostic techniques, using Toxocara antigens, have been developed to improve the diagnosis of toxocariasis. Sensitivity and specificity of each test are different according to the source of antigens 10, 15. The most common assay format is an indirect antibody ELISA using T. canis ES antigens secreted from larvae maintained in culture 16. Serological tests are also useful for seroepidemiological studies of prevalence of human toxocariasis 4.
Variations in the sensitivity and specificity of Toxocara (canis) serodiagnostic ELISAs may be due to differences in ES antigens preparation, also disparity in methods between laboratories 15, 16. Glickman et al. 17 reported a sensitivity and specificity of 73% and 92%, respectively, for an indirect ELISA system, using T. canis ES antigens. In a relatively similar study by Magnaval et al. 7, a sensitivity of 62.9% has been reported for sera from 66 patients with toxocariasis when tested independently by ELISA using T. canis ES antigens. In a study has been demonstrated the development of a highly specific recombinant T. canis antigen that yields 100% specificity as compared to the conventional ES antigens 18. Jin et al. 19 reported that the in‐house ELISA using crude antigen of T. canis larvae is useful for serodiagnosis of human toxocariasis with 92.2% sensitivity and 86.6% specificity. However, these and other similar studies have evaluated the efficacy of ES antigens derived from the third‐stage larvae of T. canis not T. cati.
Using sera from 33 patients with Toxocara infection from Iran, where a relatively common of human toxocariasis was reported 4, we evaluated and compared the negative and positive predictive values for ELISA using T. cati ES antigens for identifying those individuals that were exposed to infection. The ELISA analyses using T. cati ES antigens demonstrated good sensitivity and specificity compared to T. canis ES as antigens for diagnosis of human toxocariasis. One of 33 individuals seropositive by employing T. canis ES as antigens was seronegative by T. cati ES antigens. The ELISA of the current study presented good sensitivity (97%) and specificity (96.7%). In a study by Roldan et al. 10, sensitivity and specificity were 100% and 95.5%, respectively, considering healthy individuals, and 67.9% also considering sera from patients with other helminthiasis. In another study of Roldan et al. 20, sensitivity and specificity were 100% and 90%, respectively, in Toxocara infection. This result shows that T. canis ES antigens provided higher sensitivity for the immunodiagnosis of human toxocariasis.
Previous studies have reported that Western blotting technique has greatly decreased the risk of cross‐reaction in studies carried out in humans with toxocariasis 7, 21. We found a characteristic pattern of five antigenic bands that were recognized by sera from clinically and serologically confirmed human toxocariasis. Our findings revealed that reactivity towards the 42‐ and 50‐kDa was not obtained with sera from human infected with other parasitic diseases, indicating that these bands can be used as specific bands for diagnosis of human toxocariasis.
Kennedy et al. 22 studied the immune responses of mice to surface component and ES glycoprotein of infective larvae of T. cati and determined that three bands of 32, 120, and 400 kDa obtained from surface antigens and five bands of 28, 32, 60, 120, and 400 kDa obtained from ES glycoproteins could be used for diagnosis of toxocariasis. Two of these antigens were correlated with the bands present in our study. Magnaval et al. 7 obtained sera from human patients infected with Toxocara and performed Western blotting assay following SDS‐PAGE with T. canis ES antigens in order to evaluate their immunodiagnostic potential. They reported that serum samples with toxocariasis recognized two groups of antigenic polypeptides: the first included bands of 24, 28, 30, and 35 kDa (low molecular weight) and other consisted bands of 132, 147, and 200 kDa (high molecular weight). These authors found that the low molecular weight fractions were specific to T. canis infection. Nunes et al. 23 observed numerous bands in patients with signs of VLM, among which five principal components can be distinguished—above 205, around 205, 116–97, 55–50, 35–29 kDa, and agree in that the low molecular weight fractions do not present any cross‐reactivity with other ascariid antigens. The authors have found that the 55–50 kDa antigenic band had cross‐reactions mainly with sera from patients with ascariasis (Ascaris suum).
In summary, we have demonstrated that T. cati ES antigens can be used for sensitive and specific diagnosis of toxocariasis using an ELISA. However, the ELISA using T. canis ES antigens is more sensitive for diagnosis of human toxocariasis than T. cati ES antigens. The work has clearly demonstrated that Western blotting using the 42‐ and 50‐kDa bands is a useful tool and reliable method for confirmatory diagnosis of human toxocariasis.
ACKNOWLEDGMENTS
We thank the Parasitology Laboratory, Faculty of Health Sciences, Kobe University Graduate School of Health Sciences, Japan, for providing some chemical and instrumental facilities. The authors thank the staff, Parasitology Laboratory, Shiraz University of Medical Sciences for technical assistance and Prof. Fasihi‐Harandi from Kerman University of Medical Sciences, Iran, for providing positive serum samples. This study was supported by grant number 2893 from SUMS. The authors declare that there is no conflict of interest statement.
REFERENCES
- 1. Despommier D. Toxocariasis: Clinical aspects, epidemiological, medical ecology and molecular aspects. Clin Microbiol Rev 2005;16:265–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Azizi S, Oryan A, Sadjjadi SM, Zibaei M. Histopathologic change and larval recovery of Toxocara cati in experimentally infected chickens. Parasitol Res 2007;102:47–52. [DOI] [PubMed] [Google Scholar]
- 3. Rubinsky‐Elefant G, Hirata CE, Yamamoto JH, Ferreira MU. Human toxocariasis: Diagnosis, worldwide seroprevalences and clinical expression of the systemic and ocular forms. Ann Trop Med Parasitol 2010;104:3–23. [DOI] [PubMed] [Google Scholar]
- 4. Sadjjadi SM, Khosravi M, Mehrabani D, Oryan A. Seroprevalence of Toxocara infection in school children in Shiraz, Southern Iran. J Trop Pediatrics 2000;46:327–330. [DOI] [PubMed] [Google Scholar]
- 5. Zibaei M, Sadjjadi SM, Sarkari B. Prevalence of Toxocara cati and other intestinal helminths in stray cats in Shiraz, Iran. Trop Biomed 2007;24:39–43. [PubMed] [Google Scholar]
- 6. Strube C, Heuer L, Janecek E. Toxocara spp. infections in paratenic hosts. Vet Parasitol 2013;193:375–389. [DOI] [PubMed] [Google Scholar]
- 7. Magnaval JF, Faber R, Maurieres P, Charlet JP, de Larrad B. Application of the Western blotting procedure for the immunodiagnosis of human toxocariasis. Parasitol Res 1991;77:697–702. [DOI] [PubMed] [Google Scholar]
- 8. Ishiyama S, Ono K, Rai SK, Uga S. Method for detecting circulating Toxocara canis antigen and its application in human serum samples. Nepal Med Coll J 2009;11:9–13. [PubMed] [Google Scholar]
- 9. Maraghi S, Rafiei A, Hajihosseini R, Sadjjadi SM. Seroprevalence of toxocariasis in hypereosinophilic individuals in Ahwaz, south‐western Iran. J Helminthol 2012;86:241–244. [DOI] [PubMed] [Google Scholar]
- 10. Roldan WH, Espinoza YA. Evaluation of an enzyme‐linked immunoelectrotransfer blot test for the confirmatory serodiagnosis of human toxocariasis. Mem Inst Oswaldo Cruz 2009;104:411–418. [DOI] [PubMed] [Google Scholar]
- 11. Fillaux U, Magnaval J. Laboratory diagnosis of human toxocariasis. Vet Parasitol 2013;193:327–336. [DOI] [PubMed] [Google Scholar]
- 12. de Savigny DH. In vitro maintenance of Toxocara canis larvae and a simple method for the production of Toxocara ES antigens for use in serodiagnosis tests for visceral larva migrans. J Parasitol 1975;61:781–782. [PubMed] [Google Scholar]
- 13. Zibaei M, Sadjjadi SM, Jahadi Hosseini SH, Sarkari B. A method for accelerating the maturation of Toxocara cati eggs. Iran J Parasitol 2007;2:39–42. [Google Scholar]
- 14. Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein‐dye binding. Anal Biochem 1976;72:248–254. [DOI] [PubMed] [Google Scholar]
- 15. Elefant GR, Shimizu SH, Sanchez MC, Jacob CM. A serological follow‐up of toxocariasis patients after chemotherapy based on the detection of IgG, IgA, and IgE antibodies by enzyme‐linked immunosorbent assay. J Clin Lab Anal 2006;20:164–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Watthanakulpanich D, Smith HV, Hobbs G, Whalley AJ, Billington D. Application of Toxocara canis excretory‐secretory antigens and IgG subclass antibodies (IgG‐4) in serodiagnostic assays of human toxocariasis. Acta Trop 2006;106:90–95. [DOI] [PubMed] [Google Scholar]
- 17. Glickman L, Schantz P, Dombroske R, Cypess R. Evaluation of serodiagnostic tests for visceral larva migrans. Am J Trop Med Hyg 1978;27:492–498. [DOI] [PubMed] [Google Scholar]
- 18. Yamasaki H, Araki K, Lim PKC, Zasmy N, Mak JW, Taib R, et al. Development of highly specific recombinant Toxocara canis second‐stage larvae excretory‐secretory antigen for immunodiagnosis of human toxocariasis. J Clin Microbiol 2000;38:1409–1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Jin Y, Shen C, Huh S, Sohn WM, Choi MH, Hong ST. Serodiagnosis of Toxocariasis by ELISA using crude antigen of Toxocara canis larvae. Korean J Parasitol 2013;51:433–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Roldan WH, Cornejo W, Espinoz YA. Evaluation of the dot‐enzyme‐linked immunosorbent assay in comparison with standard ELISA for the immunodiagnosis of human toxocariasis. Mem Inst Oswaldo Cruz 2006;101:71–74. [DOI] [PubMed] [Google Scholar]
- 21. Magnaval JF, Glickman LT, Dorchies P, Morassin B. Highlights of human toxocariasis. Korean J Parasitol 2001;39:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kennedy MW, Maizels RM, Meghji M, Yong L, Qureshi F, Smith HV. Species‐specific and common epitopes on the secreted and surface antigens of Toxocara canis and Toxocara cati infective larvae. Parasite Immunol 1987;9:407–420. [DOI] [PubMed] [Google Scholar]
- 23. Nunes CM, Tundisi RN, Garcia JF, Heinemann MB, Ogassawara S, Richtzenhain LJ. Cross reactions between Toxocara canis and Ascaris suum in the diagnosis of visceral larva migrans by Western blotting technique. Rev Ins Med Trop Sao Paulo 1997;39:253–256. [DOI] [PubMed] [Google Scholar]


