Abstract
Background
Cyclooxygenase‐2 (Cox‐2) is frequently overexpressed in cervical carcinoma, but little is known about its altered serum concentration. Hence, this study evaluates clinical utility of cellular and serum level of Cox‐2 enzyme in cervical cancer.
Methods
The expression of Cox‐2 was evaluated in cervical tissues and serum samples collected from normal controls (n = 100; n = 68), cervical intraepithelial neoplasia patients (CIN, n = 67; n = 12), and invasive squamous cell carcinoma patients (SCCs; n = 153; n = 127) by immunohistochemical and enzyme‐linked immunosorbent assay (ELISA) analyses.
Results
The significant cytoplasmic overexpression of Cox‐2 was noted in 50.7% of CIN and 69.9% of SCCs as compared with normal (P = 0.0001). Serum level of Cox‐2 was also found to be elevated both in CIN (median 4.35 ng/ml) and in SCCs (median 19.39 ng/ml) with respect to normal (median 0.44 ng/ml; P = 0.0001), respectively. The ROC analysis revealed the potential of serum Cox‐2 over its cellular expression to distinguish CIN and SCCs from normal.
Conclusion
Augmented Cox‐2 activity is implicated in the pathogenesis of cervical cancer, and its serum level could serve a potential to distinguish this malignancy. Therefore, it is suggested that serum Cox‐2 may be useful in monitoring the diagnosis and treatment outcome of patients.
Keywords: cervical cancer, cervical intraepithelial neoplasia, Cox‐2, invasive squamous cell carcinoma
Introduction
Extensive research is going on to find out the exact cause of cervical cancer, which is one of the serious gynecologic malignancy worldwide as well as in India 1, 2. Among the various predisposing factors, the inflammation caused by high‐risk‐human papilloma virus (HR‐HPV) infection is considered as the important contributing factor for this malignancy 3. It is reported that the microenvironment of HPV‐associated infection is mostly harbored with the deregulated levels of pro‐ and anti‐inflammatory molecules, such as chemokines, neutrophils, eosinophils, and cytokines 4. Hence, the detailed study of molecular basis of inflammation and its underlying pathways are highly obligatory for understanding the final outcome of this disease.
For the activation of inflammatory responses in cancer, cyclooxygenase‐2 (Cox‐2) has been considered as one of the master switch molecule. This inducible enzyme is located on human chromosome 1 and primarily involved in the production of prostaglandin E2 (PGE2) 5. It is reported that Cox‐2‐mediated activated PGE2 signaling is involved in the stimulation of angiogenesis, cell proliferation, establishment of cell invasiveness, inhibition of immune responses, and apoptosis, respectively 6, 7, 8. Kim et al. 9 have suggested that the persistent chronic inflammation intervened by Cox‐2 enhances the activity of various cellular signalings having oncogenic potential. A large number of reports are available regarding the increased expression of Cox‐2 in transformed cells leading to the malignant behavior of various tumors 10, 11, 12. In cervical cancer also, overexpression of Cox‐2 is strongly correlated with its development and progression 13, 14, 15. The intensity of Cox‐2 overexpression is considerably higher in the cervical cancer patients with lymphatic and parametrium invasion 16. Moreover, it is also observed that patients harboring enhanced Cox‐2 activity exert significant reduced response to the treatment 17, 18, but its inhibition increases the sensitivity of cervical tumor cells to the radiation therapy 19. In addition to the cellular role, the activity of this enzyme is also regulated through its circulatory form. The altered serum level of Cox‐2 has been noted in systemic sclerosis patients (SSc) with arthritis, suggesting its essentiality for the manifestations of SSc 20.
Although extensive studies are available on the subcellular localization of Cox‐2, very few reports highlight the oncogenic role of its circulatory form in cervical cancer. As Cox‐2 is the main transducer for generating inflammation, the detailed study of serum Cox‐2 in addition to its tissue expression will be more helpful in understanding the molecular etiology of this cancer. Therefore, this study has been focused to evaluate the biological relevance of both cellular and circulatory activity of Cox‐2 in cervical cancer.
Material and Methodology
Patients
The patients diagnosed with cervical intraepithelial neoplasia (CIN, n = 67) and invasive squamous cell carcinoma (SCCs; n = 153) at Department of Obstetrics and Gynecology, Safdarjung Hospital, New Delhi, India, were recruited in the study. After obtaining informed written consent, the punch biopsy/or surgically resected cervical tissues were collected from all the enrolled patients during their diagnostic/treatment procedures. In addition, blood samples (by vena puncture) were also obtained from the same cohort of CIN (n = 12) and SCCs (n = 127). The demographic and clinicopathological characteristics were recorded from clinical data and from in‐person interview. The clinical staging and pathological evaluation were done according to standard criterion of International Federation of Gynecology and Obstetrics and World Health Organization 21, 22. Of 67 CIN patients, 13 cases were histopathologically diagnosed with cervical intraepithelial neoplasia grade 1 (CIN‐1), 19 cases with cervical intraepithelial neoplasia grade 2 (CIN‐2), and 35 cases were with cervical intraepithelial neoplasia grade 3 (CIN‐3). The median age of CIN patients was 47 years (range 21–72 years), while 30 women were premenopausal and 37 were postmenopausal. The median age of SCCs patients was 55 years (range 23–85 years). Clinically 32 (20.9%) patients were diagnosed with stage I, 68 (44.4%) were with stage II, while 49 (32.02%) and 4 (2.6%) patients were diagnosed with stage III and stage IV, respectively. Twenty‐nine (18.9%) patients were premenopausal and 124 (81.0%) were postmenopausal. Lymph node positivity was noted in 54.9% (84/153) cases, while 69 (45.0%) patients were free from lymphatic involvement. In addition, the tissue (n = 100) and blood (n = 68) samples were also collected from normal control patients to compare Cox‐2 expression. The patients advised for hysterectomies for uterovaginal prolapse were considered as control. All the cervix tissues collected from control patients showed the normal features of stratified squamous epithelium on histopathological examination.
Processing of tissue and blood samples
The tissue samples were fixed in 10% buffered formalin and then embedded in paraffin to prepare tissue blocks. The 5‐μ sections were cut on poly‐L‐lysin coated glass slides. Tissue architecture and usefulness of sections for immunohistochemical analysis were initially evaluated by routine histological staining. The H&E confirmed slides were further processed for immunohistochemical analysis (IHC) of Cox‐2. Collected blood samples were centrifuged at 1,500g for 10 min to separate serum. Serum samples were then aliquoted & stored at −70°C for ELISA analysis.
Immunohistochemistry
The polyclonal anti‐Cox‐2 (ab15191; Abcam, Inc., Cambridge, UK) primary antibody was used to evaluate the expression profile of Cox‐2 protein in cervix tissues. The IHC was performed according to the standard protocol as mentioned in previous publication 23. The activity of endogenous peroxidase was inhibited by keeping slides in 3% H2O2 in Leishman for 45 min. The treatment of antigen retrieval was given in Tris EDTA buffer [(0.1 M) pH 9.0] at 900 W‐15 min/360 W‐5 min followed by three consecutive wash in Tris buffer saline (TBS; 0.1 M; pH: 7.4). After antigen retrieval, the sections were incubated overnight (at 4°C) with anti‐Cox‐2 primary antibody (dilution 1:250) in humidified chamber. On the next day, the sections were incubated with secondary antibody (polymer based; Envision™; DakoCytomation, Glostrup, Denmark) for 1 hr at room temperature. The development of color was done with the help of DAB (3, 3‐diaminobenzidine hydrochloride) and Mayer's Hematoxylin. Slides were mounted with DPX and observed under light microscope (Olympus BX‐51; Tokyo, Japan). Confirmed ovarian carcinoma tissues were used as a positive control, while negative staining (negative control) was achieved by replacing primary antibody with isotype‐specific immunoglobulin G (Dako, Cytomation).
Assessment of immunohistochemical staining
Immunohistochemical results of Cox‐2 were independently studied by two observers including one pathologist. Only cytoplasmic immunopositivity was considered for positive Cox‐2 expression. The scoring criterion of semi‐quantification was based on the multiplication score of staining intensity [negative (0), mild (+1), moderate (+2), intense (+3)] and percentage (%) of positive stained cells [negative (0), <5% positive stained cells (+1), 5%–20% cells positivity (+2), 21%–50% cells positivity (+3), (>50% cells positivity (+4)] as described by us in previous report 23. On the basis of multiplication score of IHC, the cases were further divided in to mild positive (IHC score 1 and 2), moderate positive (IHC score 3, 4, and 6), and intense positive (IHC 8, 9, and 12), respectively.
ELISA
The serum concentration of Cox‐2 enzyme was measured by quantitative sandwich enzyme immunoassay with commercially available ELISA kit (cat no: CSB‐E‐10103 h; Cusabio Biotech Co., Ltd, Wuhan, China) according to the manufacturer's instructions. The detection range for serum Cox‐2 was 1.25–80 ng/ml with minimum detectable dose <0.312 ng/ml. The assay was performed in a 96‐well pate precoated with Cox‐2 specific antibody. The equal amount of standards and samples (100 μl) were added to each well and incubated at 37°C for 2 hr. The liquid was removed from well and 100 μl of Biotin‐antibody (1X) was added followed by incubation at 37°C for 1 hr. Then, each well was aspirated and washed for three times in washing buffer. The 1X HRP‐avidin (100 μl) was added to the wells and again incubated for 1 hr at 37°C followed by aspiration and washing (5 times). Later, each well was reacted with 90 μl TMB substrate, for 15–30 min at 37°C (reaction was protected from light). Finally, the reaction was stopped by adding stop solution (50 μl). The absorbance was measured at 450 nm using spectrophotometric microtiter plate reader (Powerwave XS, MQX200R; BioTek Instruments, Inc., Winooski, VT). The final concentration of Cox‐2 was determined by comparing a calibration curve built using reference standards.
Statistical analysis
Both immunohistochemical and ELISA data were subjected to statistical analysis. Chi‐square test and chi‐square trend analysis were performed to determine change in Cox‐2 immunoexpression among normal, CIN, and SCCs. The serum concentration of Cox‐2 among study groups was compared by Wilcoxon W‐test/Mann–Whitney U‐test (two‐tailed). ROC curve analysis was carried out to determine sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) of Cox‐2. The relationship between endogenous and serum Cox‐2 with clinicopathological parameters were tested by chi‐square and Mann–Whitney U‐test (two‐sided), respectively. The test was considered significant when P value was <0.05. Software packages SPSS (version 18.0 SPSS Inc., Chicago, IL) and MedCalc (version 15.4; MedCalc software, Acacialaan, Belgium) were used to perform statistical analysis.
Results
Immunoexpression of Cox‐2 in cervix tissues
The immunoexpression and subcellular localization of Cox‐2 protein was evaluated in different phases of cervical carcinogenesis (normal, CIN, and SCCs), and results are presented in Table 1. The endogenous expression of Cox‐2 was absent in majority of normal cervix tissues (Fig. 1A) except only seven cases that showed its very mild but detectable reactivity. In contrast, strong cytoplasmic immunopositivity of the protein was noticed in both CIN and SCCs as compared with normal (P = 0.0001; P = 0.0001). Out of 67 CIN, 34 (50.7%) patients showed cytoplasmic overexpression of Cox‐2 protein (Fig. 1B). Among these, expression was intense in 20.5% of cases (IHC score 8, 9, and 12), moderate in 41.1% of cases (IHC score 3, 4, and 6), and mild in 38.2% of cases (IHC score 1 and 2). Similarly, in SCCs overall, 107/153 (69.9%) patients were harbored with significant overexpression of Cox‐2 enzyme (Fig. 1C). Out of these 107 cases, mild Cox‐2 staining was detected in 24 cases (IHC score 1, 2), moderate staining was detected in 50 cases (IHC score 3, 4, 6), and intense staining was observed in 33 cases (IHC score 8, 9, and 12), respectively. The overall expression of Cox‐2 was significantly correlated with the disease progression from normal to CIN to SCCs (0.31 ± 0.12, 2.26 ± 0.39, 3.81 ± 0.28, Chi tends P < 0.0001; Fig. 1E; Table 1).
Table 1.
Immunoexpression of Cox‐2 in Normal, CIN, and SCCs Tissues
| Cases (N) | Cox‐2 (Cyto) n (%) Mean ± SE | P value | |
|---|---|---|---|
| + | − | ||
| Normal (N = 100) | 07 (7.0) | 93 (93.0) |
0.0001
a
(P < 0.001) |
| 0.31 ± 0.12 | |||
| CIN (N = 67) | 34 (50.7) | 33 (49.2) |
0.0001
b
(P < 0.001) |
| 2.26 ± 0.39 | |||
| SCCs (N = 153) | 107 (69.9) | 46 (30.0) |
0.0001
c
(P < 0.001) |
| 3.81 ± 0.28 | |||
| P value (normal vs. CIN vs. SCCs) |
0.0001
*
(P < 0.001) |
||
Chi‐square test showing significance tested against a, normal vs. CIN; b, CIN vs. SCCs; c, normal vs. SCCs.
*Significance tested against normal vs. CIN vs. SCCs by chi‐square trend analysis. P ≤ 0.05 was considered significant; Cyto, cytoplasm; CIN, cervical intraepithelial neoplasia; SCCs, squamous cell carcinoma. Bold values represent statistical significance.
Figure 1.
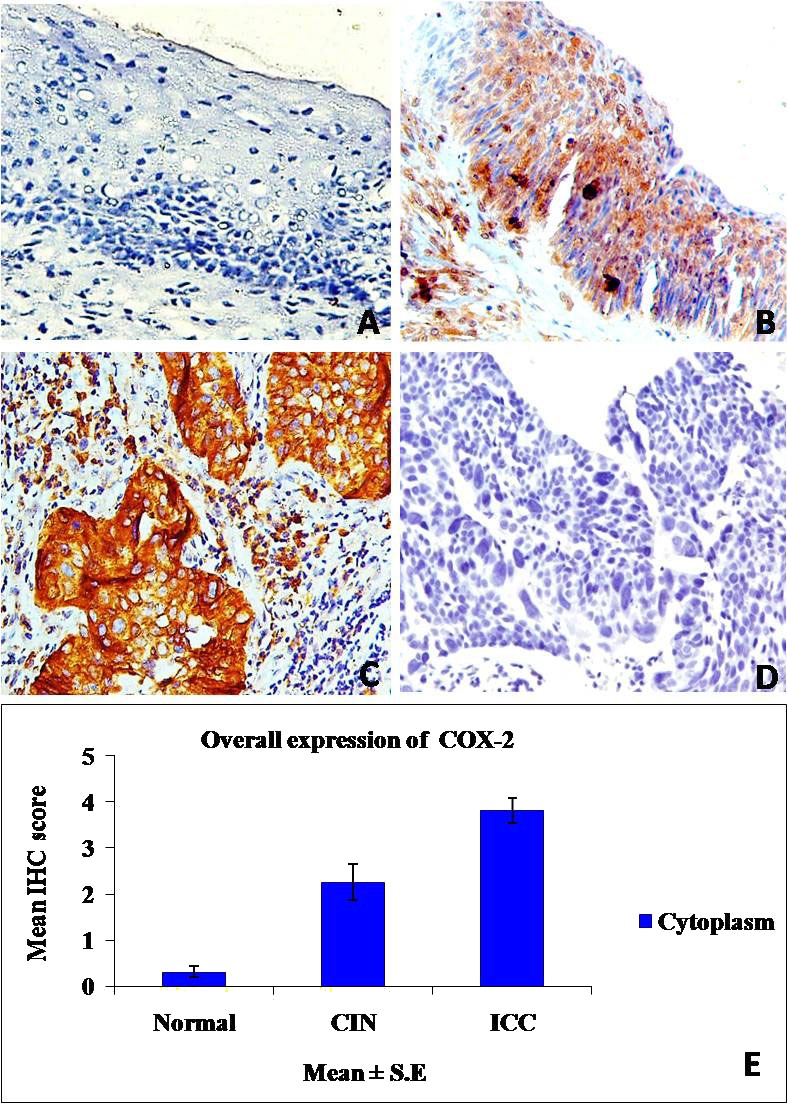
Immunohistochemical expression of Cox‐2. (A) Stratified squamous epithelium of normal cervix without Cox‐2 immunostaining. (B) CIN‐III showing strong cytoplasmic Cox‐2 expression. (C) SCCs depicting intense cytoplasmic overexpression of Cox‐2 in tumor cells. (D) Negative control in which primary antibody replaced with nonspecific IgG. (E) Bar graph demonstrating overall distribution of IHC score of Cox‐2 in normal, CIN, and SCCs. (A–D) Magnification: 200×.
Serum concentration of Cox‐2
Very low Cox‐2 serum positivity was measured in histologically normal patients (0.468 ± 0.012; median 0.44 ng/ml). In contrast, the serum level of Cox‐2 was significantly increased in both CIN (7.976 ± 1.749; median 4.35 ng/ml; P = 0.0001) and SCCs patients (22.186 ± 1.155; median 19.39 ng/ml; P = 0.0001) as compared with normals. Overall, the serum level of Cox‐2 was significantly elevated with respect to the progression of disease from normal to precancer to invasive cancer (P = 0.0001; Fig. 2; Table 2).
Figure 2.
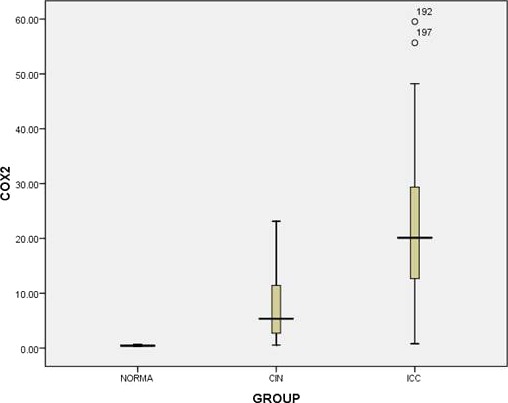
Box plot analysis showing distribution of serum concentration (ng/ml) of Cox‐2 in normal, CIN, and SCCs patients. The vertical axis demonstrates the total Cox‐2 concentration and bold line indicates median score. Upper and lower limits of plot at 75th and 25th percentiles, respectively.
Table 2.
Overall Serum Concentration of Cox‐2 in Normal, CIN, and SCCs
| Study groups | Cox‐2 concentration (ng/ml) (mean ± SE) median | P value |
|---|---|---|
| Normal (N = 68) | 0.468 ± 0.012 | 0.0001 * |
| 0.44 | (P < 0.001) | |
| CIN (N = 12) | 7.976 ± 1.749 | 0.0001 ** |
| 4.35 | (P < 0.001) | |
| SCC (N = 127) | 22.186 ± 1.155 | 0.0001 *** |
| 19.39 | (P < 0.001) |
Mann–Whitney U‐test and Wilcoxon W‐test {Asymp. Sig. (2‐tailed)}; *normal vs. CIN; **CIN vs. SCCs; ***normal vs. SCCs; Bold values represent statistical significance.
Biomarker potential of Cox‐2 to distinguish CIN and SCCs from normal
In normal vs. CIN group, with regard to the Cox‐2 IHC score, the ROC curve analysis showed lower sensitivity (58.2%), specificity (65.0%), AUC (0.610), PPV (52.5%), and NPV (69.0%) values. However, the serum level of Cox‐2 in this group showed significant higher sensitivity (91.6%), specificity (98.4%), AUC (0.980), PPV (90.0%), and NPV (69.0%) values, respectively. On comparing both the analysis, serum Cox‐2 was found to be most significant than that of cellular Cox‐2 [P < 0.0001; Table 3; Fig. 3 A (a and b)].
Table 3.
Analysis of Biomarker Potential of Tissue and Serum Cox‐2 level in CIN and SCCs
| Performance test | Cox‐2 | Sensitivity % | Specificity % | Cut‐Off | AUC | PPV (%) (95% CI) | NPV (%) (95% CI) | P value |
|---|---|---|---|---|---|---|---|---|
| Normal vs. CIN | Cellular expression | 58.2 | 65.0 | ≥1 | 0.610 | 52.5 (40.6–64.3) | 69.0 (59.6–79.0) | 0.0001 * <0.001 |
| Serum level | 91.6 | 98.4 | >0.65 | 0.980 | 90.9 (60.8–99.7) | 69.0 (92.0–99.9) | ||
| Normal vs. SCCs | Cellular expression | 68.3 | 90.0 | ≥1 | 0.844 | 90.6 (86.2–94.2) | 62.5 (56.0–71.9) | 0.0001 ** |
| Serum level | 89.0 | 98.0 | >0.67 | 0.985 | 98.6 (94.4–99.0) | 83.8 (74.2–90.5) |
AUC; area under curve, PPV; positive predictive value, NPV; negative predictive value, 95% CI; 95% confidence interval, *comparison between tissue and serum Cox‐2 ROC curves in normal vs. CIN group, **comparison between tissue and serum Cox‐2 of ROC curves in normal vs. SCC group. Significance at P < 0.05; Bold values represent statistical significance.
Figure 3.
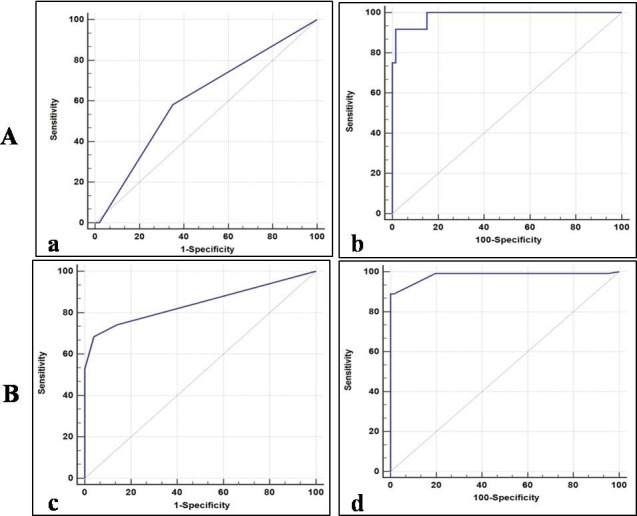
Biomarker ability of Cox‐2 in cervical cancer. (A) ROC curve analysis of tissue (a) and serum Cox‐2 (b) in CIN as compared with normal. (B) ROC curve analysis of tissue (c) and serum (d) Cox‐2 in SCCs as compared with normal. The curves represent sensitivity, specificity, and area under curve calculated on the basis of respective cut‐off values.
In normal vs. SCCs group, the sensitivity, specificity, AUC, PPV, and NPV values of Cox‐2 immunostaining were 68.3%, 90.0%, 0.844, 90.6%, and 62.5%. While for serum Cox‐2, these values were 89.0%. 98.0%, 0.985, 98.6%, and 83.8%, respectively. Similarly, in this group also, serum Cox‐2 was found to be the most significant as compared with cellular Cox‐2 [P = 0.0001; Table 3; Fig. 3 B (c and d)].
Association of Cox‐2 with clinicopathological parameters of SCCs patients
We further investigated the association of both tissues and serum level of Cox‐2 with established risk factors as well as clinicopathological parameters of cervical cancer, and results are presented in Table 4.
Table 4.
Association of Both Cellular and Serum Levels of Cox‐2 with Clinicopathological Parameters of SCCs Patients
| Clinicopathological parameters | Cellular expression of Cox‐2 | Serum level of Cox‐2 | |||||
|---|---|---|---|---|---|---|---|
| Cases N = 153 | Cox‐2 Cyto +ve n (%) | P value | Cases N = 127 | Cox‐2 level (ng/ml) Median values | P value | ||
| +107 (69.9) | −46 (30.0) | ||||||
| Age (median 55 years, 23–85 years) | |||||||
| ≤50 | 35 | 30 (85.7) | 05 (14.2) | 0.512 | 34 | 15.76 | 0.352 |
| >50 | 118 | 77 (65.2) | 41 (34.7) | 93 | 20.10 | ||
| Menopausal status | |||||||
| Pre | 29 | 21 (72.4) | 08 (27.5) | 0.700 | 18 | 19.23 | 0.488 |
| Post | 124 | 86 (69.3) | 38 (30.6) | 109 | 20.45 | ||
| Gravida (median 4; 1–11) | |||||||
| <4 | 36 | 25 (69.4) | 11 (30.5) | 0.313 | 29 | 15.11 | 0.165 |
| ≥4 | 117 | 82 (70.0) | 35 (29.9) | 98 | 20.72 | ||
| Parity (median 4; 1–9) | |||||||
| <4 | 78 | 53 (67.9) | 25 (32.0) | 0.265 | 63 | 19.23 | 0.836 |
| ≥4 | 75 | 54 (72.0) | 21 (28.0) | 64 | 19.74 | ||
| Contraceptiona | |||||||
| Yes | 21 | 11 (52.3) | 96 (72.7) | 0.400 | 16 | 15.84 | 0.527 |
| No | 132 | 10 (47.6) | 36 (27.2) | 111 | 20.19 | ||
| Habitsb | |||||||
| Yes | 40 | 12 (30.0) | 28 (70.0) | 0.744 | 28 | 21.92 | 0.181 |
| No | 113 | 95 (84.0) | 18 (15.9) | 99 | 19.00 | ||
| Tumor size (cm) | |||||||
| ≤4 | 68 | 31 (45.5) | 37 (54.4) | 0.002 | 51 | 11.79 | 0.0001 |
| >4 | 85 | 76 (89.4) | 09 (10.5) | 76 | 23.91 | ||
| Histopathological grade | |||||||
| G1 | 64 | 52 (81.2) | 12 (18.7) | 0.168 | 54 | 20.98 | 0.467 |
| G2 | 44 | 29 (65.9) | 15 (34.0) | 36 | 19.23 | ||
| G3 | 45 | 26 (57.7) | 19 (42.2) | 35 | 19.19 | ||
| Lymphatic involvement | |||||||
| Yes | 84 | 62 (73.8) | 22 (26.1) | 0.032 | 74 | 23.34 | 0.0001 |
| No | 69 | 45 (65.2) | 24 (34.7) | 53 | 13.44 | ||
| FIGO stage | |||||||
| I + II | 100 | 62 (62.0) | 38 (38.0) | 0.001 | 83 | 14.11 | 0.0001 |
| III + IV | 53 | 45 (84.9) | 08 (15.0) | 44 | 30.01 | ||
Chi‐square or Fisher exact test wherever applicable (for cellular expression of Cox‐2); Mann–Whitney U or Kruskal–Wallis test wherever applicable (for serum level of Cox‐2); P ≤ 0.05 is considered as significant; bold values indicate statistically significant.
aContraception includes only the use of oral contraceptive pills.
bHabits include tobacco chewing, smoking, and/or alcohol consumption; FIGO, International Federation of Gynecology and Obstetrics; G1, well differentiated; G2, moderately differentiated; G3, poorly differentiated.
Both cellular and serum level of Cox‐2 were found to be associated with increased tumor size (P = 0.002; P = 0.0001), indicating that the patients with greater tumor size than 4 cm also represents significant higher expression of Cox‐2 in both tumor cells and circulation. The Cox‐2 level in both tumor cell and circulation was also significantly elevated in SCCs patients with advanced FIGO stage (III + IV) than that of early stage SCCs patients (I + II; P = 0.001; P = 0.0001). Furthermore, Cox‐2 was found to be significantly upregulated in patients showing lymph node metastasis as compared with lymph node negative patients (P = 0.032; P = 0.0001). In addition, the Cox‐2 expression and levels were not associated with other risk factors, such as age, gravida, parity, menopausal status, contraception, and habits.
Discussion
In recent years, it has become clear that multiple neoplastic conditions are originated from persistent chronic irritation and inflammation. Hence, much attention has been focused on understanding the role of inflammation in tumor biology. Among handful of cellular factors, Cox‐2 plays pivotal role for the induction of inflammation either individually or through sustained production of PGE2 6, 7, 8. The aberrant overexpression of Cox‐2 has been reported in various human malignancies, including colon, breast, prostate, and lung 24. Similarly, various immunohistochemical investigations are available, denoting frequent overexpression of Cox‐2 in cervical cancer. The altered expression of this enzyme has been noted in all the pathological subtypes of cervical malignancy, including cervical intraepithelial neoplasia, adenocarcinoma, and squamous cell carcinoma. However, the frequency of Cox‐2 expression noted in these studies was 7.4% in CIN, 13% in adenocarcinoma, and 28.8% in SCCs 13, 14, 15. In addition, recent study again signifies the oncogenic role of Cox‐2 in cervical cancer with 56.52% positivity in tumors 25. In concordance with these findings, our present immunohistochemical analysis also showed significant overexpression of Cox‐2 both in CIN and SCCs as compared with normal (P = 0.0001, P = 0.001). However, the frequency of Cox‐2 positivity was quite higher in our study as compared with previous reports, as our results provide 50.7% and 69.9% positivity in precancerous and cancerous lesions, respectively. This significant high proportion of Cox‐2 suggests that this protein may be the principal transducer for generation of inflammatory responses during development and progression of cervical cancer. Apart from this, very mild cytoplasmic positivity of Cox‐2 noticed in control group (7/100) may be due to presence of other unidentified inflammatory conditions.
It is reported that in cervical cancer, the MAPK‐associated transcription of Cox‐2 is facilitated by HPV‐16 E6 and E7 oncoproteins induced amphiregulin 8. Moreover, another oncoprotein of HPV‐16 like E5 also activates epidermal growth factor receptor (EGFR) and MAPK signaling to induce transcription of Cox‐2 26, 27. HPV‐mediated abrogated Cox‐2 activity is found to be involved in the upregulation of VEGF preferably by deregulating MEK/ERK 1/2 and PI3K/Akt signaling pathways and downregulation of membrane‐bound E‐cadherin 26, 28, 29. In addition, there are reports available regarding the regulation of Cox‐2 by certain oncogenic cellular pathways. The role of canonical Wnt/β‐catenin in the activation of Cox‐2 is well explained in gastric cancer 30. It has been also observed that in Wnt‐stimulated Min/+ tumors, the upregulated PGE2 transactivates EGFR for the stimulation of Cox‐2 by negative feedback loop 31. Our previous reports also suggest the activation of Wnt/β‐catenin signaling in cervical cancer 23, 32. However, in another part of this study, we have observed significant positive association of Cox‐2 with nuclear β‐catenin (data not shown). This highlights the augmented activity of Cox‐2, which is under the influence of activated Wnt signaling in cervical cancer. Moreover, we recently showed the high HPV‐16 positivity in the SCCs patients 32. On the chi‐square analysis, the significant positive association of Cox‐2 was observed with HPV‐16, suggesting that oncogenic HPV may enhance the cellular activity of Cox‐2 probably by upregulating Wnt signaling to limit host immune responses during progression of disease (data not shown).
In addition to the cellular expression, the serum level of Cox‐2 is also found to be elevated in inflammation‐associated diseases. Bassyouni et al. 20 observed the increased serum concentration of Cox‐2 in systemic sclerosis (SSc) complicated with arthritis as well as digital ulcers and opined that it is an essential step for the manifestations of SSc. Konturek et al. 33 on RIA analysis of gastrin/progastrin (upstream regulators of Cox‐2) in sera of gastric cancer patients proposed that these proinflammatory cytokines are the prime mediators for the upregulation of Cox‐2. However, another study by Hanbek et al. 34 also showed the elevated PGE2 serum level in patients with head and neck carcinoma and correlated this with increased activity of Cox‐2. Although the clinical efficacy of serum Cox‐2 is demonstrated in cancer, but to the best of our knowledge, no studies that symbolize the role of circulating Cox‐2 in cervical cancer are available. Hence, this report appears to be the first study to determine the concentration of circulating level of Cox‐2 in the serum of CIN and SCCs patients. On the ELISA analysis, the mean concentration of Cox‐2 was found to be elevated in both CIN (7.976 ng/ml) and SCCs (22.186 ng/ml) as compared with normal (0.468 ng/ml), suggesting the regulatory role of serum Cox‐2 in development of cervical lesions. Therefore, it is hypothesized that tumor cell may be the source for synthesis and secretion of Cox‐2 in cervical cancer, which may be essential for further interaction with surrounding environment during proliferation and acceleration of disease. The ROC curves analysis also indicates higher potential of serum Cox‐2 in differentiating CIN and SCCs from normal. Hence, it is recommended that analysis of serum level of Cox‐2 in cervical cancer patients may be the more beneficial in risk prediction. In addition, significant positive association of Cox‐2 with increased tumor size (P = 0.002, P = 0.0001), lymphatic involvement (P = 0.032, P = 0.0001), and progressed tumor stage (P = 0.001, P = 0.0001) underscores its involvement in progression of disease. Our results are in agreement with the other studies on cervical cancer, which demonstrated the link between Cox‐2 overexpression and lymph node metastasis 14, 16, 35, 36. In consideration of the above facts, it is suggested that Cox‐2 may perform critical role in disease advancement by interfering with aggressive behavior of tumors. It has been observed that cervical cancer patients harboring Cox‐2 overexpression showed significant reduced survival period when treated with radiation therapy 17, 18, 37, 38. In this respect, the potential of various Cox‐2 inhibitors is studied in cervical cancer, among them the role of celecoxib is well explained 17, 39. The studies based on inhibition of Cox‐2 demonstrated that its cellular level gets drastically reduced in cancer patients especially when treated with celecoxib‐associated CCRT regimens 39. Moreover, blocking of this protein with celecoxib was also shown to inhibit the proliferation of tumor cells through the suppression of VEGF and reduction of serum level of Cox‐2 in gastric cancer 40, 41. Although the effect of celecoxib on the modulation of Cox‐2 level was not examined in this study, ELISA result of Cox‐2 may be helpful for monitoring the treatment response in cervical cancer patients undergoing neoadjuvant chemoradiation including selective Cox‐2 inhibitors.
In conclusion, this study signifies the biological and clinical importance of Cox‐2 overexpression in cervical cancer. The increased serum activity of Cox‐2 may be the secondary consequences of interaction of tumor cells with adjacent environment during circumvention of immune responses. However, this requires a strong drive for in‐depth analysis to understand the underlining molecular mechanism involved in the Cox‐2‐mediated cervical carcinogenesis. Last but not the least considering the clinical utility of Cox‐2, this study also provides the new insight toward the potential of this enzyme as surrogate serum biomarker for early detection and prognosis prediction of patients with cervical cancer.
Source of support: Indian Council of Medical Research (ICMR), New Delhi, India (grant 5/13/71/2008‐NCD‐III).
References
- 1. Ferlay J, Soerjomataram I, Ervik M, et al. GLOBOCAN 2012, v 1.0, Cancer Incidence and Mortality Worldwide. IARC Cancer Base;11. Lyon, France: International Agency for Research on Cancer; 2013. Available at: http://globocan.iarc.fr. Accessed February 14,2015. [Google Scholar]
- 2. Panday S, Mishra M, Chandrawati. Human Papilloma virus screening in North Indian women. Asian Pac J Cancer Prev 2012;13:2643–2646. [DOI] [PubMed] [Google Scholar]
- 3. zur Hausen H. Papilloma viruses causing cancer: Evasion from host cell control in early events in carcinogenesis. J Natl Cancer Inst 2000;92:690–698. [DOI] [PubMed] [Google Scholar]
- 4. Caruso C, Lio D, Cavallone L, Franceschi C. Aging, longevity, inflammation, and cancer. Ann N Y Acad Sci 2004;1028:1–13. [DOI] [PubMed] [Google Scholar]
- 5. Tay A, Squire JA, Goldberg H, Skorecki K. Assignment of the human prostaglandin‐endoperoxide synthase 2 (PTGS2) gene to 1q25 by fluorescence in situ hybridization. Genomics 1994;23:718–719. [DOI] [PubMed] [Google Scholar]
- 6. Sheng H, Shao J, Washington MK, DuBois RN. Prostaglandin E2 increases growth and motility of colorectal carcinoma cells. J Biol Chem 2001;276:18075–18081. [DOI] [PubMed] [Google Scholar]
- 7. Dohadwala M, Luo J, Zhu L, et al. Non‐small cell lung cancer cyclooxygenase‐ 2‐dependent invasion is mediated by CD44. J Biol Chem 2001;276:20809–20812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Subbaramaiah K, Dannenberg AJ. Cyclooxygenase‐2 transcription is regulated by human papillomavirus 16 E6 and E7 oncoproteins: Evidence of a corepressor/coactivator exchange. Cancer Res 2007;67:3976–3985. [DOI] [PubMed] [Google Scholar]
- 9. Kim HS, Kim T, Kim MK, Suh DH, Chung HH, Song YS. Cyclooxygenase‐1 and ‐2: Molecular Targets for Cervical Neoplasia. J Cancer Prev 2013;18:123–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Subbaramaiah K, Telang N, Ramonetti JT, et al. Transcription of cyclooxygenase‐2 is enhanced in transformed mammary epithelial cells. Cancer Res 1996;56:4424–4429. [PubMed] [Google Scholar]
- 11. Chi‐Man Tang T, Tung‐Ping Poon R, Fan ST. The significance of cyclooxygenase‐2 expression in human hepatocellular carcinoma. Biomed Pharmacother 2005;59(Suppl 2):S311–S316. [DOI] [PubMed] [Google Scholar]
- 12. Zhang J, Ji J, Yuan F, et al. Cyclooxygenase‐2 expression is associated with VEGF‐C and lymph node metastases in gastric cancer patients. Biomed Pharmacother 2005;59(Suppl 2):S285–S288. [DOI] [PubMed] [Google Scholar]
- 13. Kim MH, Seo SS, Song YS, et al. Expression of cyclooxygenase‐1 and ‐2 associated with expression of VEGF in primary cervical cancer and at metastatic lymph nodes. Gynecol Oncol 2003;90:83–90. [DOI] [PubMed] [Google Scholar]
- 14. Kang S, Kim MH, Park IA, et al. Elevation of cyclooxygenase‐2 is related to lymph node metastasis in adenocarcinoma of uterine cervix. Cancer Lett 2006;237:305–311. [DOI] [PubMed] [Google Scholar]
- 15. Kulkarni S, Rader JS, Zhang F, et al. Cyclooxygenase‐2 is overexpressed in human cervical cancer. Clin Cancer Res 2001;7:429–434. [PubMed] [Google Scholar]
- 16. Ryu HS, Chang KH, Yang HW, Kim MS, Kwon HC, Oh KS. High cyclooxygenase‐2 expression in stage IB cervical cancer with lymph node metastasis or parametrial invasion. Gynecol Oncol 2000;76:320–325. [DOI] [PubMed] [Google Scholar]
- 17. Ferrandina G, Lauriola L, DiStefano MG, et al. Increased cyclooxygenase‐2 expression in associated with chemotherapy resistance and poor survival in cervical cancer patients. J Clin Oncol 2002;20:973–981. [DOI] [PubMed] [Google Scholar]
- 18. Gaffney DK, Holden J, Davis M, Zempolich K, Murphy KJ, Dodson M. Elevated cyclooxygenase‐2 expression correlates with diminished survival in carcinoma of the cervix treated with radiotherapy. Int J Radiat Oncol Biol Phys 2001;49:1213–1217. [DOI] [PubMed] [Google Scholar]
- 19. Gallo O. Re: Enhancement of tumor response to gammaradiation by an inhibitor of cyclooxygenase‐2 enzyme. J Natl Cancer Inst 2000;92:346–347. [DOI] [PubMed] [Google Scholar]
- 20. Bassyouni IH, Talaat RM, Salem TA. Serum concentrations of cyclooxygenase‐2 in patients with systemic sclerosis: Association with lower frequency of pulmonary fibrosis. J Clin Immunol 2012;32:124–130. [DOI] [PubMed] [Google Scholar]
- 21. Shepherd JH. Cervical and vulva cancer: Changes in FIGO definitions of staging. Br J Obstet Gynaecol 1996;103:405–406. [DOI] [PubMed] [Google Scholar]
- 22. Tavassoli FA, Devilee P. Pathology and genetics of tumours of the breast and female genital organs, World Health Organization Classification of Tumours, Lyon: IARC Press; 2003. [Google Scholar]
- 23. Jawanjal P, Salhan S, Dhawan I, Tripathi R, Rath G. Peptidyl‐prolyl isomerase Pin1‐mediated abrogation of APC‐β‐catenin interaction in squamous cell carcinoma of cervix. Rom J Morphol Embryol 2014;55:83–90. [PubMed] [Google Scholar]
- 24. Harris RE. Cyclooxygenase‐2 (cox‐2) blockade in the chemoprevention of cancers of the colon, breast, prostate, and lung. Inflammopharmacology 2009;17:55–67. [DOI] [PubMed] [Google Scholar]
- 25. Mandić A, Ušaj‐Knežević S, Kapicl TI, Ninčić D, Malenković G. Cyclooxygenase‐2 expression in cervical cancer. Vojnosanit Pregl 2014;71:997–1005. [DOI] [PubMed] [Google Scholar]
- 26. Kim SH, Juhnn YS, Kang S, et al. Human papillomavirus 16 E5 up‐regulates the expression of vascular endothelial growth factor through the activation of epidermal growth factor receptor, MEK/ERK1,2 and PI3K/Akt. Cell Mol Life Sci 2006;63:930–938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Fehrmann F, Laimins LA. Human papillomaviruses: Targeting differentiating epithelial cells for malignant transformation. Oncogene 2003;22:5201–5207. [DOI] [PubMed] [Google Scholar]
- 28. Dai Y, Zhang X, Peng Y, Wang Z. The expression of cyclooxygenase‐ 2, VEGF and PGs in CIN and cervical carcinoma. Gynecol Oncol 2005;97:96–103. [DOI] [PubMed] [Google Scholar]
- 29. Kim YM, Park JY, Lee KM, et al. Does pretreatment HPV viral load correlate with prognosis in patients with early stage cervical carcinoma? J Gynecol Oncol 2008;19:113–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Nuñez F, Bravo S, Cruzat F, Montecino M, De Ferrari GV. Wnt/β‐Catenin signaling enhances cyclooxygenase‐2 (COX2) transcriptional activity in gastric cancer cells. PLoS ONE 2011;6:e18562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Moran AE, Hunt DH, Javid SH, Redston M, Carothers AM, Bertagnolli MM. APC deficiency is associated with increased EGFR activity in the intestinal enterocytes and adenomas of C57BL/6J‐Min/+ mice. J Biol Chem 2004;279:43261–43272. [DOI] [PubMed] [Google Scholar]
- 32. Rath G, Jawanjal P, Salhan S, Nalliah M, Dhawan I. Clinical significance of inactivated glycogen synthase kinase 3β in HPV associated cervical cancer: Relationship with Wnt/β‐catenin pathway activation. Am J Reprod Immunol 2015;73:460–478. [DOI] [PubMed] [Google Scholar]
- 33. Konturek PC, Konturek SJ, Bielanski W, et al. Influence of COX‐2 inhibition by rofecoxib on serum and tumor progastrin and gastrin levels and expression of PPARgamma and apoptosis‐related proteins in gastric cancer patients. Dig Dis Sci 2003;48:2005–2017. [DOI] [PubMed] [Google Scholar]
- 34. Hambek M, Baghi M, Wagenblast J, Schmitt J, Baumann H, Knecht R. Inverse correlation between serum PGE2 and T classification in head and neck cancer. Head Neck 2007;29:244–248. [DOI] [PubMed] [Google Scholar]
- 35. Yamamoto K, Arakawa T, Taketani Y, et al. TNF alpha‐dependent induction of cyclooxygenase‐2 mediated by NF kappa B and NF‐IL6. Adv Exp Med Biol 1997;407:185–189. [PubMed] [Google Scholar]
- 36. Surh YJ, Chun KS, Cha HH, et al. Molecular mechanisms underlying chemopreventive activities of anti‐inflammatory phytochemicals: Down‐regulation of COX‐2 and iNOS through suppression of NF‐kappa B activation. Mutat Res 2001;480–481:243–268. [DOI] [PubMed] [Google Scholar]
- 37. Noriyuki M, Sumi T, Zhi X, et al. Vascular endothelial growth factor, matrix metalloproteinases, and cyclooxygenase‐2 influence prognosis of uterine cervical cancer in young women. Int J Oncol 2007;31:531–536. [PubMed] [Google Scholar]
- 38. Kim YB, Kim GE, Cho NH, et al. Overexpression of cyclooxygenase‐2 is associated with a poor prognosis in patients with squamous cell carcinoma of the uterine cervix treated with radiation and concurrent chemotherapy. Cancer 2002;95:531–539. [DOI] [PubMed] [Google Scholar]
- 39. Harris RE, Casto BC, Harris ZM. Cyclooxygenase‐2 and the inflammogenesis of breast cancer. World J Clin Oncol 2014;10:677–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Liu H, Huang P, Xu X, Liu J, Guo C. Anticancer effect of celecoxib via COX‐2 dependent and independent mechanisms in human gastric cancers cells. Dig Dis Sci 2009;54:1418–1424. [DOI] [PubMed] [Google Scholar]
- 41. Han X, Li H, Su L, et al. Effect of celecoxib plus standard chemotherapy on serum levels of vascular endothelial growth factor and cyclooxygenase‐2 in patients with gastric cancer. Biomed Rep 2014;2:183–187. [DOI] [PMC free article] [PubMed] [Google Scholar]


