Abstract
Background
Mycoplasma pneumoniae (M. pneumoniae, MP) is recognized globally as a significant cause of primary atypical pneumonia in humans, particularly in children. Overzealous host immune responses are viewed as key mediators of the pathogenesis of M. pneumoniae infection. Although Th17 cells have been identified as key modulators in the clearance of pathogens and induction of autoimmunity caused by excessive immune responses, little is known about the role of Th17 cells in patients with M. pneumoniae infection.
Methods
The percentages of T cells, CD4+ T cells and Th17 cells in children with M. pneumoniae infection were measured by flow cytometry.
Results
We documented an increased frequency of Th17 cells in children with M. pneumoniae infection. Furthermore, we found a significantly higher percentage of Th17 cells in M. pneumoniae‐infected children with extrapulmonary manifestations, compared with children without extrapulmonary manifestations. In addition, patients who experienced a short course of Mycoplasma pneumoniae pneumonia (MPP) showed an increase in the percentage of Th17 cells.
Conclusion
Our findings suggest that Th17 cells may be involved in the clearance of M. pneumoniae during an acute infection. Excessive Th17 cell responses may also contribute to the immuno‐pathological damage observed during persistent infection.
Keywords: children, Mycoplasma Pneumoniae, respiratory infection, Th17 cells
Introduction
Mycoplasma pneumoniae (M. pneumoniae) is recognized globally as a significant cause of upper and lower respiratory tract infections in humans, particularly in children, and accounts for over 40% of community‐acquired pneumonia in children admitted to the hospital 1, 2, 3, 4, 5. Although M. pneumoniae infection is rarely fatal, patients of every age can develop severe and fulminant disease. Moreover, M. pneumoniae can also cause extrapulmonary manifestations in almost every compartment, including the hepatic, cardiovascular, skin, hematologic, musculoskeletal, and nervous system 6. The pathophysiologic mechanism underlying the diverse symptoms associated with M. pneumoniae‐induced human diseases is thought to result from indirect tissue injury caused by an overzealous host immune responses 7, 8.
Substantial evidence supports a key role of Th17 cells in the development and pathogenesis of various excessive immune response‐induced diseases 9. Indeed, Th17 cells are often associated with the recruitment and activation of inflammatory myeloid cells that cause severe local tissue injury 10, 11. However, Th17 cells are not designed to elicit autoimmune responses but are rather intended to provide an effective host defense against pathogens. Accumulating evidence has shown that Th17 cell‐deficient mice have an increased susceptibility to infection from a wide variety of microorganisms, including bacteria, parasites, fungi and viruses 12. Previous studies in which levels of IL‐17 in the peripheral blood of children infected by M. pneumoniae were measured 13, 14. Furthermore, M. pneumoniae antigens have been shown to enhance Th17 cell response in an experimental mice model 15. However, little is known about the role of Th17 cells in mycoplasma pneumonia and its extrapulmonary complications in humans, particularly in children.
Here, we document for the first time that the frequency of Th17 cells was significantly higher in M. pneumoniae‐infected patients with a short course of MPP and in patients with extrapulmonary manifestations. Our results indicate that Th17 cells may be involved in the clearance of M. pneumoniae during an acute infection. However, excessive Th17 cell responses may result in immuno‐pathological damage to organs in cases of persistent infection in which clearance does not occur in a timely fashion. Taken together, these data suggest that appropriate Th17 cell responses are required and influence the control and clinical outcome of M. pneumoniae infection.
Materials and Methods
Ethics statement
Ethical clearance for this study was obtained from the Institutional Review Board of Nanjing Medical University, Nanjing, China (Permit Number: 2008NMUIEC130). The aims and objectives of the study were explained to each participant and written informed consent was obtained.
Patients and healthy controls
The study involved a total of 51 subjects. The subjects included 30 children with MPP from Nanjing Children's Hospital and 21 healthy controls. In all cases, pneumonia was diagnosed by the presence of consolidation on a chest X‐ray confirmed by two pediatric radiologists at the time of admission. Infection with M. pneumoniae was confirmed by an initial MP‐IgM titer greater than 1:40. The MP‐IgM titer was measured using a diagnostic kit for the measurement of antibodies to M. pneumoniae (FUJIREBIO INC, Tokyo, Japan). The sputum and blood from all children who were diagnosed with MPP were examined to rule out the presence of coinfection with other bacteria or viruses. Peripheral blood was collected from each fasting participant after admission in the morning. All patients were in the acute stage of MPP and had not taken any medication prior to the blood collection.
We classified pneumonia as long or short course, namely 14 days or longer and fewer than 14 days, respectively. A white blood cell (WBC) number greater than 10 × 109/l was considered a high level of WBCs. Elevated levels of ALT (normal value, <40 U/l) and CK‐MB (normal value, <24 U/l) were considered diagnostic of liver and myocardial injury (extra‐pulmonary manifestations), respectively. Serum ALT and CK‐MB levels were assayed using a HITACHI 7600‐020 Chemical Analyzer (HITACHI, Tokyo, Japan).
Flow cytometry (FCM) analysis
Human peripheral blood mononuclear cells (PBMCs) were collected into sodium heparin tubes (BD Biosciences, San Diego, CA) and purified by Ficoll‐paque plus (GE healthcare, Uppsala, Sweden) density gradient centrifugation. Cells recovered from the gradient interface were washed twice and stained for 30 min at 4°C with the following antibodies or isotype‐matched controls: CD3‐APC (eBioscience, San Diego, CA), CD4‐FITC (eBioscience). Then, cells were stimulated for 4 h at 37°C in a humidified atmosphere of 10% CO2 in culture medium containing PMA (25 ng/ml; Sigma, St. Louis, MO), ionomycin (1 μg/ml; Sigma), and monensin (Golgi Stop; 1 μg/ml; BD Biosciences, San Jose, CA). After stimulation, cells were fixed and made permeable with Cytofix/Cytoperm and Perm/Wash buffer according to the manufacturer's instructions (BD Biosciences), then labeled with IL‐17‐PE (eBioscience). Cells were incubated for 30 min at 4°C and washed twice in Perm/Wash before analysis. Cell acquisition was performed using a FACSCalibur cytometer (BD Biosciences). Data were analyzed with FlowJo (version 10.0.7; Tree Star, Ashland, OR).
Statistical analyses
All data were analyzed using SPSS software (version 22; IBM, Armonk, NY). Significant differences between specimens were determined using Mann–Whitney U test or Fisher's Exact test according to the characteristics of the variables. A P value <0.05 was considered to be statistically significant.
Results
Increased frequency of Th17 cells in patients with MPP
To determine whether the percentage of Th17 cells was increased in patients with MPP, a total of 30 patients and 21 healthy controls were recruited. There was no significant difference in the distribution of age or gender between patients and healthy controls (Table 1). The frequency of total T cells and T cells among total lymphocytes was comparable between patients and healthy controls (Fig. 1a–c). Th17 cells were defined as CD3+CD4+IL‐17+, as shown in the gate strategy in Figure 1a. Results demonstrated an increased frequency of Th17 cells among CD3+ T in patients with MPP when compared with healthy controls (Fig. 1d, e).
Table 1.
The Demographic and Clinical Characteristics of Subjects
| Parameters | HC | MPP | ||||||
|---|---|---|---|---|---|---|---|---|
| Long course | Short course | With EPM | Without EPM | Wbc ≤ 10 × 109/l | Wbc > 10 × 109/l | |||
| CK‐MB > 24 U/l | ALT > 40 U/l | |||||||
| Number | 21 | 9 | 21 | 4 | 5 | 21 | 22 | 8 |
| Age(months) | 25.7 ± 13.1 | 26.9 ± 13.1 | 28.9 ± 9.4 | 35.0 ± 10.7 | 25.8 ± 9.1 | 29.9 ± 11.5 | 24.9 ± 5.0 | |
| Male | 14 | 4 | 14 | 3 | 1 | 14 | 13 | 5 |
| Female | 7 | 5 | 7 | 1 | 4 | 7 | 9 | 3 |
HC, healthy control; MPP, mycoplasma pneumoniae pneumonia.
Figure 1.
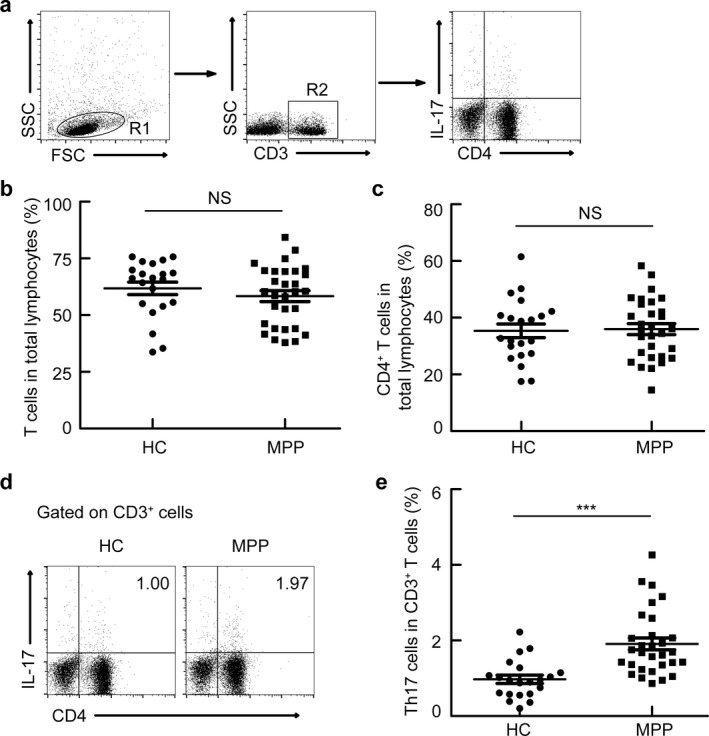
Increased frequency of Th17 cells in patients with MPP. PBMCs were isolated from healthy controls (HC, n = 21) and patients with MPP (MPP, n = 30) and analyzed. (a) Gating schemes for analysis of the percentage of Th17 (CD3+ CD4+ IL‐17+). PBMCs were stained with CD3, CD4, and IL‐17 antibodies. (b, c) Percentages of T cells (b) and CD4+ T cells (c) in total lymphocytes; (d, e) Representative FACS plots and statistics show Th17 cells. ***P < 0.001, NS indicating not significant.
Subjects with a short course of MPP had an increase in peripheral blood Th17 cells
To investigate whether the frequency of Th17 cells correlated with the time course of MPP, we next examined the frequency of Th17 cells in patients with a short or long course of MPP. We found a significantly higher percentage of Th17 cells in patients with a short course of MPP than in patients with a long course of MPP (Fig. 2), suggesting that Th17 cells may be involved in the control of M. pneumoniae infection.
Figure 2.
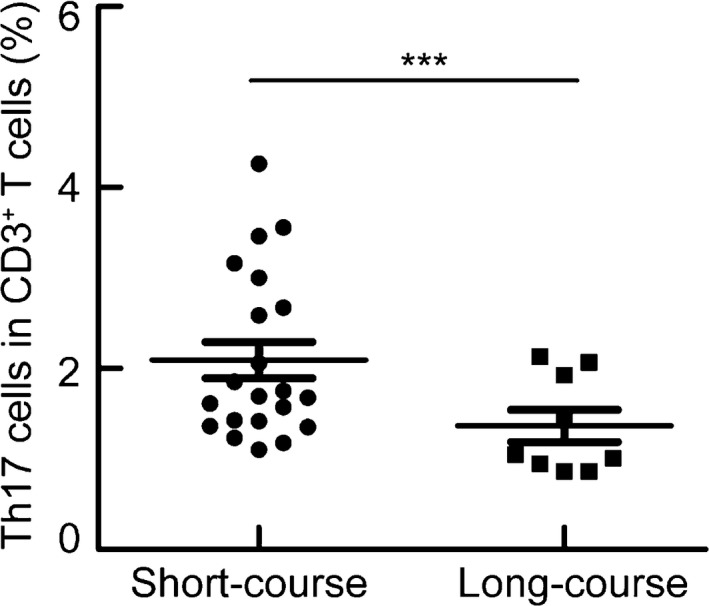
Patients with a short course of MPP had an increase in peripheral blood Th17 cells. PBMCs were isolated from patients with a short (n = 21) or long course of MPP (n = 9) and analyzed. PBMCs were stained with CD3, CD4, and IL‐17 antibodies. Frequencies of Th17 within CD3+ T cells in patients with a short or long course of MPP. ***P < 0.001.
The percentage of Th17 cells was comparable between study subjects with a high level of WBCs and those with a normal level of WBCs
No significant difference in the percentage of Th17 cells was found between patients with a high level of WBCs and those with a normal level of WBCs (Fig. 3).
Figure 3.
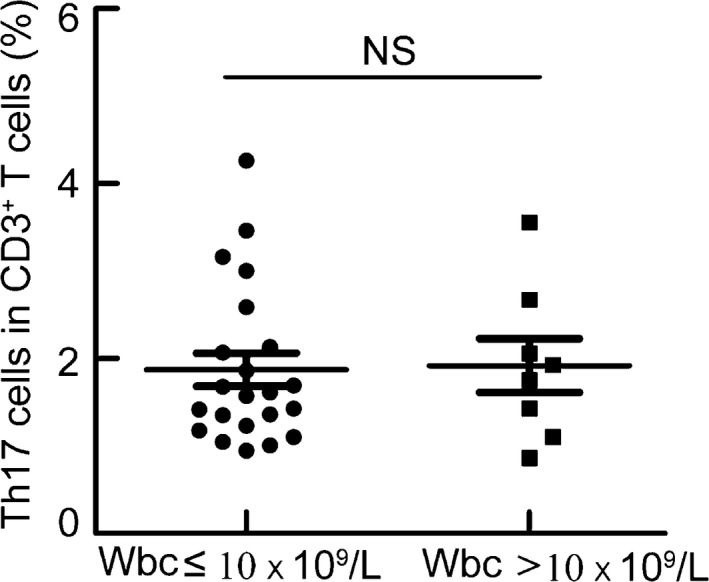
The percentages of Th17 cells were comparable between study subjects with a high level of WBCs and those with a normal level of WBCs. PBMCs were isolated from patients with high (n = 8) or normal (n = 22) levels of WBCs and analyzed. PBMCs were stained with CD3, CD4, and IL‐17 antibodies. Frequencies of Th17 cells within CD3+ T cells in patients with high or normal levels of WBCs. NS indicating not significant.
The frequency of Th17 cells was increased in patients with extrapulmonary manifestations
Patients with hepatic and cardiovascular extrapulmonary manifestations tended to have a higher percentage of Th17 cells compared with patients without extrapulmonary manifestations (Fig. 4). These results suggest that Th17 cells may be important players in the pathogenesis of MPP.
Figure 4.
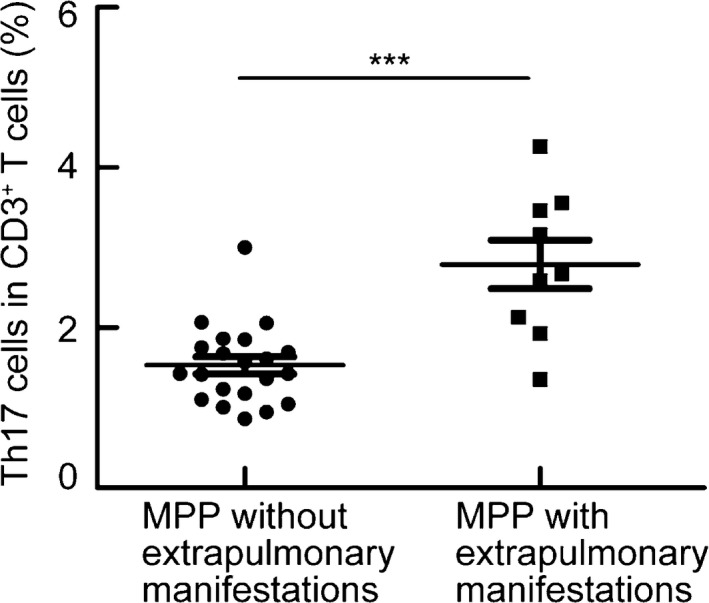
The frequency of Th17 cells was increased in patients with extrapulmonary manifestations. PBMCs were isolated from patients with (n = 9) or without (n = 21) extrapulmonary manifestations and analyzed. PBMCs were stained with CD3, CD4, and IL‐17 antibodies. Frequencies of Th17 with CD3+ T cells in patients with or without extrapulmonary manifestations. ***P < 0.001.
Discussion
Mycoplasma pneumoniae is recognized globally as a significant cause of primary atypical pneumonia 3. M. pneumoniae can cause not only severe respiratory symptoms but is also associated with extrapulmonary manifestations 3. However, the exact mechanisms underlying the pathogenesis of M. pneumoniae infection remain unclear. In this study, we observed an increase in the percentage of Th17 cells in M. pneumoniae‐infected patients. Furthermore the percentage of Th17 cells was significantly higher in M. pneumoniae‐infected patients with extrapulmonary manifestations or with a short course of MPP. Our results suggest that Th17 cells may play a potential role in both the control and pathogenesis M. pneumoniae infection.
Pathogens as diverse as gram‐positive Propionibacterium acnes, gram‐negative Citrobacter rodentium, Klebsiella pneumoniae, Bacteroides spp., and Borrelia spp., the acid‐fast Mycobacterium tuberculosis, and fungi‐like Pneumocystis carinii and Candida albicans can trigger a strong Th17 response 16, 17, 18, 19, 20, 21, 22. Consistent with published data showing that Th17 cells are induced by M. pneumoniae antigens in mice 15, we demonstrate for the first time that the percentage of Th17 cells are significantly increased in M. pneumoniae‐infected patients.
On one hand, Th17 cells constitute a branch of the adaptive immune system whose function is to clear specific types of pathogens 9. Th17 cells are clearly involved in the development of mucosal immunity and the granulopoietic response to extracellular pathogens, including bacteria and fungi 23, 24. In agreement with published data from mice, our data show that patients with a short course of MPP have a higher percentage of Th17 cells, indicating a role of Th17 cells in the control of M. pneumoniae infection.
However, Th17 cells are also potent inducers of tissue inflammation and have been linked to the pathogenesis of human inflammatory conditions and many experimental autoimmune diseases 9. For instance, Th17 responses have been associated with tissue injury in atherosclerotic artery disease 25 and biliary cirrhosis 26 in humans, as well as experimental autoimmune encephalomyelitis 27 and collagen‐induced arthritis 28 in mice. Similarly, our data showed an increase in the frequency of Th17 cells in patients with extrapulmonary manifestations, suggesting that Th17 cells may contribute to the pathogenesis of extrapulmonary organ damage, such as that observed in the liver and myocardium. Thus, when manipulating Th17 cells to eliminate the M. pneumoniae in patients, it is very important to consider their adverse effects in triggering Th17‐mediated extrapulmonary organ damage.
Previous studies have demonstrated that Th17 cells are converted to Th1 cells in vitro 29, 30. More importantly, IL17+IFNγ+ T cells have been described and Th17 cells have been shown to develop into Th1 cells in vivo 31. In addition, Th17 cells also produce IL‐10 32, IL‐21 32, and IL‐22 33. Although the precise pathogenic mechanism by which Th17 cells contribute to MPP is poorly understood, these cells may well enhance disease progression by producing various cytokines such as IL‐17 and IFNγ.
In summary, our study is the first to report an increase in the percentage of Th17 cells in M. pneumoniae‐infected patients with a short course of MPP or who exhibit extrapulmonary manifestations. Our study indicates that Th17 cells may be involved in the clearance of M. pneumoniae during an acute infection. Excessive Th17 cell responses may also contribute to the immuno‐pathological damage to organs in cases of persistent infection in which clearance of the pathogen does not occur in a timely fashion. Taken together, these data suggest that appropriate Th17 cell responses are required and influence the control and clinical outcome of M. pneumoniae infection.
Author contributions
XM, XC, HT, JZ, SZ, and ZX performed the experiments; XM XC, and CS analyzed the data; XM, FL, and CS designed the study; and XM, XC, and CS wrote the paper.
Acknowledgments
This work was supported by grants from the National Natural Science Foundation of China (No. 81271861 and No. 81430052) and the Research Foundation of Jiangsu Province, China (12KJA310001) to Chuan Su, and the Priority Academic Program for Development of Jiangsu Higher Education Institutions (PAPD). We thank the patients and control subjects for their participation in this study.
References
- 1. Jain S, Self WH, Wunderink RG, et al. Community‐Acquired Pneumonia Requiring Hospitalization among US Adults. N Engl J Med 2015;373:415–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Jain S, Williams DJ, Arnold SR, et al. Community‐acquired pneumonia requiring hospitalization among US Children. N Engl J Med 2015;372:835–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Atkinson TP, Balish MF, Waites KB. Epidemiology, clinical manifestations, pathogenesis and laboratory detection of Mycoplasma pneumoniae infections. FEMS Microbiol Rev 2008;32:956–973. [DOI] [PubMed] [Google Scholar]
- 4. Michelow IC, Olsen K, Lozano J, et al. Epidemiology and clinical characteristics of community‐acquired pneumonia in hospitalized children. Pediatrics 2004;113:701–707. [DOI] [PubMed] [Google Scholar]
- 5. Baer G, Engelcke G, Abele‐Horn M, Schaad UB, Heininger U. Role of Chlamydia pneumoniae and Mycoplasma pneumoniae as causative agents of community‐acquired pneumonia in hospitalised children and adolescents. Eur J Clin Microbiol Infect Dis 2003;22:742–745. [DOI] [PubMed] [Google Scholar]
- 6. Narita M. Pathogenesis of extrapulmonary manifestations of Mycoplasma pneumoniae infection with special reference to pneumonia. J Infect Chemother 2010;16:162–169. [DOI] [PubMed] [Google Scholar]
- 7. Waites KB, Talkington DF. Mycoplasma pneumoniae and its role as a human pathogen. Clin Microbiol Rev 2004;17:697–728, table of contents. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Fernald GW, Collier AM, Clyde WA Jr. Respiratory infections due to Mycoplasma pneumoniae in infants and children. Pediatrics 1975;55:327–335. [PubMed] [Google Scholar]
- 9. Korn T, Bettelli E, Oukka M, Kuchroo VK. IL‐17 and Th17 cells. Annu Rev Immunol 2009;27:485–517. [DOI] [PubMed] [Google Scholar]
- 10. Stark MA, Huo Y, Burcin TL, Morris MA, Olson TS, Ley K. Phagocytosis of apoptotic neutrophils regulates granulopoiesis via IL‐23 and IL‐17. Immunity 2005;22:285–294. [DOI] [PubMed] [Google Scholar]
- 11. Sherlock JP, Joyce‐Shaikh B, Turner SP, et al. IL‐23 induces spondyloarthropathy by acting on ROR‐gammat+ CD3+ CD4‐CD8‐ entheseal resident T cells. Nat Med 2012;18:1069–1076. [DOI] [PubMed] [Google Scholar]
- 12. Milner JD, Holland SM. The cup runneth over: Lessons from the ever‐expanding pool of primary immunodeficiency diseases. Nat Rev Immunol 2013;13:635–648. [DOI] [PubMed] [Google Scholar]
- 13. Michelow IC, Katz K, McCracken GH, Hardy RD. Systemic cytokine profile in children with community‐acquired pneumonia. Pediatr Pulmonol 2007;42:640–645. [DOI] [PubMed] [Google Scholar]
- 14. Shao L, Cong Z, Li X, Zou H, Cao L, Guo Y. Changes in levels of IL‐9, IL‐17, IFN‐gamma, dendritic cell numbers and TLR expression in peripheral blood in asthmatic children with Mycoplasma pneumoniae infection. Int J Clin Exp Pathol 2015;8:5263–5272. [PMC free article] [PubMed] [Google Scholar]
- 15. Kurata S, Osaki T, Yonezawa H, Arae K, Taguchi H, Kamiya S. Role IL‐17A and IL‐10 in the antigen induced inflammation model by Mycoplasma pneumoniae. BMC Microbiol 2014;14:156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mangan PR, Harrington LE, O'Quinn DB, et al. Transforming growth factor‐beta induces development of the T(H)17 lineage. Nature 2006;441:231–234. [DOI] [PubMed] [Google Scholar]
- 17. Infante‐Duarte C, Horton HF, Byrne MC, Kamradt T. Microbial lipopeptides induce the production of IL‐17 in Th cells. J Immunol 2000;165:6107–6115. [DOI] [PubMed] [Google Scholar]
- 18. Ye P, Rodriguez FH, Kanaly S, et al. Requirement of interleukin 17 receptor signaling for lung CXC chemokine and granulocyte colony‐stimulating factor expression, neutrophil recruitment, and host defense. J Exp Med 2001;194:519–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chung DR, Kasper DL, Panzo RJ, et al. CD4+ T cells mediate abscess formation in intra‐abdominal sepsis by an IL‐17‐dependent mechanism. J Immunol 2003;170:1958–1963. [DOI] [PubMed] [Google Scholar]
- 20. Khader SA, Bell GK, Pearl JE, et al. IL‐23 and IL‐17 in the establishment of protective pulmonary CD4+ T cell responses after vaccination and during Mycobacterium tuberculosis challenge. Nat Immunol 2007;8:369–377. [DOI] [PubMed] [Google Scholar]
- 21. Rudner XL, Happel KI, Young EA, Shellito JE. Interleukin‐23 (IL‐23)‐IL‐17 cytokine axis in murine Pneumocystis carinii infection. Infect Immun 2007;75:3055–3061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Huang W, Na L, Fidel PL, Schwarzenberger P. Requirement of interleukin‐17A for systemic anti‐Candida albicans host defense in mice. J Infect Dis 2004;190:624–631. [DOI] [PubMed] [Google Scholar]
- 23. Schwarzenberger P, La Russa V, Miller A, et al. IL‐17 stimulates granulopoiesis in mice: Use of an alternate, novel gene therapy‐derived method for in vivo evaluation of cytokines. J Immunol 1998;161:6383–6389. [PubMed] [Google Scholar]
- 24. McAleer JP, Kolls JK. Mechanisms controlling Th17 cytokine expression and host defense. J Leukoc Biol 2011;90:263–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Eid RE, Rao DA, Zhou J, et al. Interleukin‐17 and interferon‐gamma are produced concomitantly by human coronary artery‐infiltrating T cells and act synergistically on vascular smooth muscle cells. Circulation 2009;119:1424–1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Rong G, Zhou Y, Xiong Y, et al. Imbalance between T helper type 17 and T regulatory cells in patients with primary biliary cirrhosis: The serum cytokine profile and peripheral cell population. Clin Exp Immunol 2009;156:217–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Komiyama Y, Nakae S, Matsuki T, et al. IL‐17 plays an important role in the development of experimental autoimmune encephalomyelitis. J Immunol 2006;177:566–573. [DOI] [PubMed] [Google Scholar]
- 28. Nakae S, Nambu A, Sudo K, Iwakura Y. Suppression of immune induction of collagen‐induced arthritis in IL‐17‐deficient mice. J Immunol 2003;171:6173–6177. [DOI] [PubMed] [Google Scholar]
- 29. Bending D, De la Pena H, Veldhoen M, et al. Highly purified Th17 cells from BDC2.5NOD mice convert into Th1‐like cells in NOD/SCID recipient mice. J Clin Invest 2009;119:565–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lee YK, Turner H, Maynard CL, et al. Late developmental plasticity in the T helper 17 lineage. Immunity 2009;30:92–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Shi G, Cox CA, Vistica BP, Tan C, Wawrousek EF, Gery I. Phenotype switching by inflammation‐inducing polarized Th17 cells, but not by Th1 cells. J Immunol 2008;181:7205–7213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Zhou L, Chong MMW, Littman DR. Plasticity of CD4+ T Cell lineage differentiation. Immunity 2009;30:646–655. [DOI] [PubMed] [Google Scholar]
- 33. Duhen T, Geiger R, Jarrossay D, Lanzavecchia A, Sallusto F. Production of interleukin 22 but not interleukin 17 by a subset of human skin‐homing memory T cells. Nat Immunol 2009;10:857–863. [DOI] [PubMed] [Google Scholar]


