Abstract
Background
Liver dysfunction is common and often unrecognized. Liver biopsy is the gold standard in the assessment of liver fibrosis, but has disadvantages. We assessed the diagnostic accuracy of serum prolidase enzyme activity (SPA) in predicting the presence and degree of liver fibrosis, as compared with liver biopsy. Further, we evaluated the effect of hemolysis on measured SPA levels.
Methods
We undertook a prospective case control study. Thirty eight outpatients without apparent liver illness and 20 patients with liver pathology scheduled to undergo liver biopsy had their SPA levels measured.
Results
Patients undergoing liver biopsy had higher SPA levels (361 (268) IU/l [median (interquartile range)]) compared with controls (169 (160) (P < 0.001)). A SPA cutoff value of 200 IU/l yielded a sensitivity of 89%, specificity of 59%, an odds ratio of 11.5, negative predictive value of 92%, and a positive predictive value of 50%. Hemolysis causes an apparent increase in SPA levels.
Conclusion
Higher SPA levels in patients undergoing liver biopsies compared with controls may reflect the presence of liver fibrosis. SPA levels could not be used to stage the degree of fibrosis. SPA measurement may be useful in the diagnostic workup of suspected liver disease.
Keywords: diagnostic accuracy, liver pathology, receiver‐operator curve, screening, prolidase
INTRODUCTION
Liver disease is common and is showing increasing prevalence with many patients going onto develop advanced disease 1, 2. In suspected liver disease, liver biopsy is the reference standard for investigation but 3, 4 owing to its invasive nature 5, it has potential pitfalls and may be inaccurate 6, 7, 8, 9. Other investigative methods include clinical imaging techniques 10, measures of liver stiffness using transient elastography 11, 12, 13, individual serum biomarkers and combinations thereof. However, all have various shortcomings 1, 2, 5, 6, 8, 9, 10, 14, 15.
Prolidase is a cytosolic enzyme that cleaves collagen breakdown products and recycles collagen 16 and is the rate limiting step in fibrosis 17. Its activity is induced in periods of fibrosis 17. Serum levels are low 16, 18, but induction of irreversible liver injury in rats reveals a strong correlation between the degree of resulting fibrosis and the serum prolidase enzyme activity (SPA) levels 18, 19.
Prolidase has been measured in various clinical conditions 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29. However, there is a paucity of studies in the setting of liver fibrosis. In one study 30 although elevated plasma prolidase was found in cirrhosis, there was no correlation between plasma prolidase activity and the degree of liver fibrosis. In another study, levels of SPA correlated with both cirrhosis and alcoholic hepatitis, but it could not be used to distinguish between early fibrosis and more severe pathology 31. A prospective study of steatosis and steatohepatitis (NASH) in patients with nonalcoholic fatty liver disease 32 showed that SPA levels were significantly higher in patients with NASH 33. In view of the relative uncertainty about SPA, we undertook a study to evaluate the usefulness of SPA compared with liver biopsy and to determine whether it could be used as a biomarker to assess the potential presence and degree of liver fibrosis in our local setting.
MATERIALS AND METHODS
Patient Recruitment
Patients were recruited from the inpatient population, which included patients with suspected liver disease. A total of 64 patients were enrolled in the study, which included 28 people undergoing liver biopsy at Groote Schuur Hospital, a tertiary hospital in Cape Town, South Africa. All inpatients scheduled to have liver biopsy from October 2009 to August 2010 were considered. Patients were excluded if the liver biopsy was cancelled or was done as an outpatient procedure. Samples were rejected if patients did not provide informed consent (8 of the 28 patients were thus excluded). Serum and liver biopsy samples were analyzed on the patients undergoing biopsy.
Control Patients
A control group of serum samples from 38 outpatients at the same hospital were used (Fig. 1)34. These were patients who were attending hospital for nonliver related pathology and did not have liver related tests requested.
Figure 1.
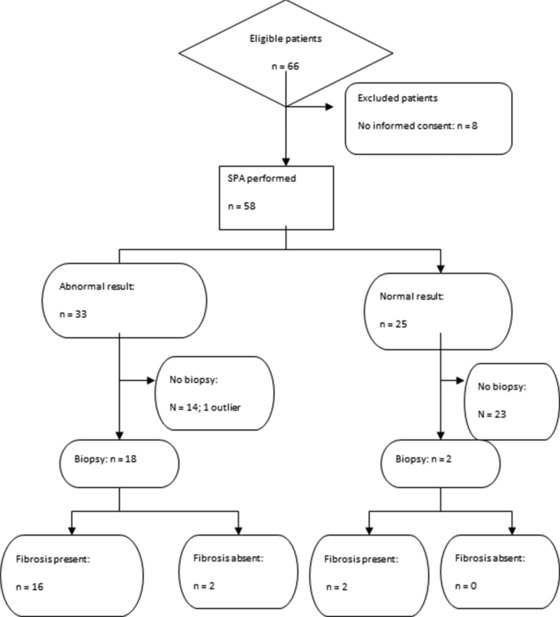
STARD flow diagram illustrating the testing process.
Ethics Committee Permission
Demographic data to ensure that the samples were from adults were checked, but no other data were captured. This study was approved by the UCT Research Ethics Committee (Rec Ref: 435/2009).Written informed consent to be included in the study was obtained from all participants undergoing liver biopsy. All samples were anonymized and confidentiality was maintained.
Prolidase Assay
Serum samples were taken as part of the routine in‐patient work‐up for liver biopsy; all patients therefore had blood drawn on the day prior to, or on the day of the biopsy. Informed consent to use this left over serum for SPA analysis was obtained from patients. Serum was stored at −80°C prior to analysis. SPA was assayed using a spectrophotometric method based on the reaction of proline with Chinard's reagent 30. Five microliters of serum was incubated for 2 hr with 15 μl 5 mmol/l MnCl2 in 100 mmol/l Tris HCl (pH 8.0) in a 37°C water bath. Thereafter, 60 μl 100 mmol/l glycyl‐proline (Sigma‐Aldrich), 80 μl 100 mmol/l Tris (pH 8.0), and 40 μl water was added and the mixture was incubated for 30 min in a 37°C water bath. The reaction was stopped by the addition of 100 μl 20% w/v trichloroacetic acid (TCA). The mixture was centrifuged for 5 min and 200 μl supernatant fluid was mixed with 400 μl glacial acetic acid and 400 μl Chinard's reagent (2.5 g of ninhydrin dissolved at 70°C in 60 ml glacial acetic acid, 23.7 ml water, and 16.3 ml 85% orthophosphoric acid (d = 1.7) and heated for 10 min at 90°C. Color development at 515 nm was read against a blank treated similarly, except for the addition of TCA prior to substrate. Proline concentration was calculated using a standard curve of increasing concentrations of proline (Sigma‐Aldrich) in 6.7% w/v TCA. SPA levels were reported as micromole proline formed per minute per liter serum (IU/l). Quality control was performed via frozen aliquots of a single serum analyzed in each run. The person performing the SPA assay was blinded as to the results of the liver biopsy.
The Effect of Hemolysis on the Prolidase Assay
The effect of hemolysis was assessed on a pooled serum sample using a published modified osmotic shock method 35. Briefly, a hemolysate was made by centrifuging whole blood obtained from a volunteer after which the plasma was removed. The red cells were washed five times with an equal volume of 0.9% saline, and then stored at −20°C overnight with an equal volume of distilled water. Thereafter, this was centrifuged and the hemoglobin (Hb) in the supernatant measured. A range of hemolysates were then prepared using dilutions of this stock solution in saline to provide a range of Hb 0–100 g/l in increments of 5 g/l. A 100 μl of increasing hemolysate (100 μl saline was used as a blank) was added to 900 μl pooled serum.
Histological Assessment of Liver Biopsy
The liver biopsies were stained with hematoxylin and eosin, and further sections were stained with bile sirius red stains. The degree of fibrosis at biopsy was assessed using a well‐characterized scoring system by one pathologist (ML) who was blinded to the results of SPA analysis but who had access to the patient's records if required (ML)36.
Statistical Analysis
Statistical analysis was undertaken on Microsoft Excel and Statistica 9.0. Data showed a Gaussian distribution after being transformed by taking square roots. The F‐test of variance was used on the square‐root transformed data to establish that variances were equal, and then Student's unpaired t‐test assuming equal variances was used. Back‐transformed data are shown and scatter plots drawn. P < 0.05 was taken as indicative of statistical significance.
RESULTS
Prolidase Assay
Linearity and precision of the prolidase assay concurred with published reports (data not shown)30, 32. Hemolysis was a significant interferent in the prolidase assay and even low levels of hemolysis affected the results obtained (Fig. 2). Therefore, hemolyzed samples should not be used.
Figure 2.
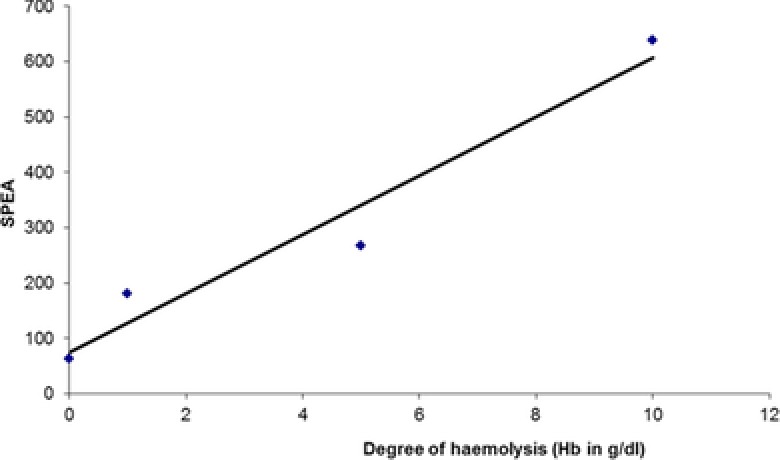
Effect of hemolysis on serum prolidase activity.
Liver Biopsy and SPA Assay
No adverse events were reported in any of the patients. Some patients did not have all tests performed during this admission. In such instances, all available data were analyzed.
Two patients tested positive for hepatitis B virus (HBV) infection and two for human immunodeficiency virus (HIV) infection. Two further patients tested positive for two infectious agents: one for HBV and HIV, and the other for HIV and syphilis. Thus, in total six (30%) of patients who had a liver biopsy tested positive for HIV, hepatitis B and/or syphilis. None of the patients tested positive for hepatitis A or C infection.
The patients who underwent liver biopsy had higher SPA levels than the controls that did not (P < 0.001) (Fig. 3). SPA values were as follows: control group 166 IU/l (22–504) (mean (mean ± 2SD)), the entire fibrosis group 376 (P < 0.001 vs. controls), minimal fibrosis group 392 (P < 0.001 vs. controls), extensive fibrosis group 335 (P < 0.001 vs. controls). There was no significant difference in SPA levels between the groups with minimal and extensive fibrosis (P = 0.59) (Fig. 3).
Figure 3.
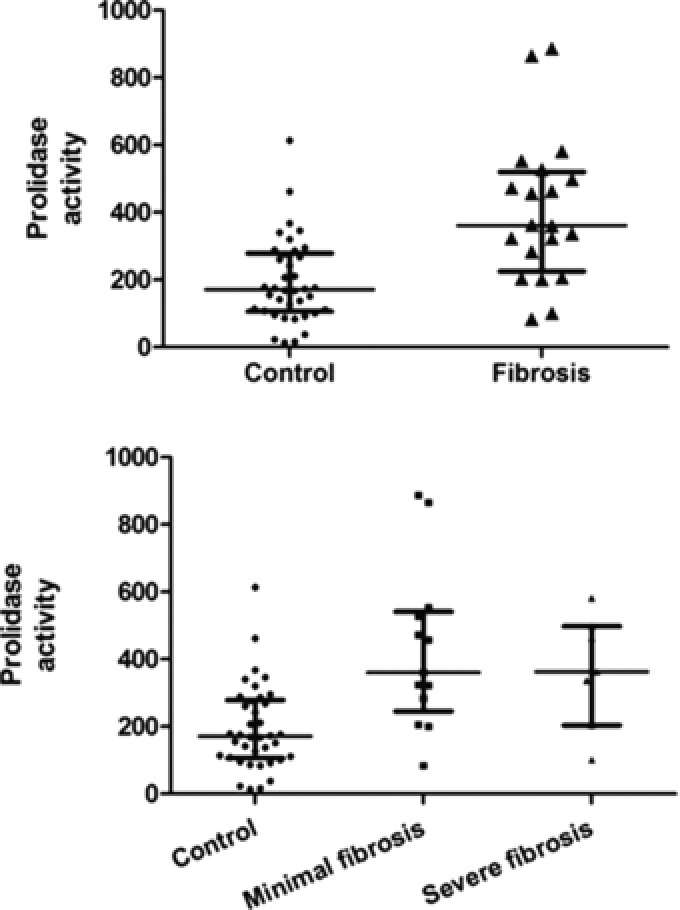
(A) Scatter plots for serum prolidase enzyme activity (SPA) in the control group versus the patients undergoing liver biopsy. The horizontal lines represent the median and the interquartile range. (B) Scatter plots for serum prolidase enzyme activity (SPA) stratified on the basis of increasing degree of fibrosis. The horizontal lines represent the median and the interquartile range.
Receiver operating characteristics curve analysis revealed an optimum cut‐off of 200 IU/l for the detection of fibrosis (Table 1 and Fig. 4).
Table 1.
Results of Serum Prolidase Enzyme Activity (SPA) Discriminatory Power at a SPA Cut‐Off Level of 200 IU/l
| SPA | ||||
|---|---|---|---|---|
| Positive | Negative | Total | ||
| Liver biopsy | ||||
| Positive | 16 | 23 | 39 | |
| Negative | 16 | 23 | 39 | |
| Total | 32 | 25 | 57 | |
Figure 4.
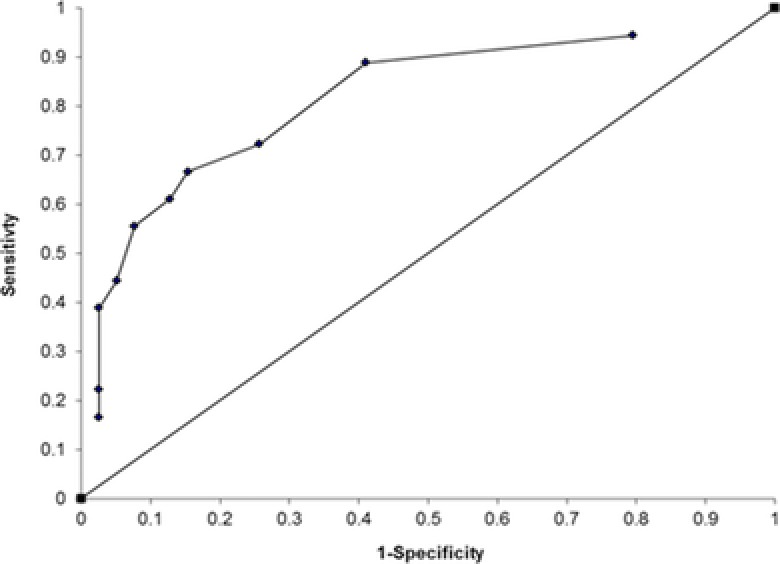
Receiver operating characteristics curve analysis showing serum prolidase activity cut‐off levels of 529, 500, 463, 450, 360, 330, 321, 280, 200, and 100 IU/l.
This SPA cut‐off value yielded a sensitivity of 89%, specificity of 59%, and a diagnostic odds ratio of 11.5. This assay had a negative predictive value (NPV) of 92% and a positive predictive value (PPV) of 50%. The area under the ROC curve was 0.96. If these results were translated into a screening test in a general unselected population, then using a quoted population prevalence of liver fibrosis of 2.8% 14, 16 this would translate into a NPV of 99.5% and a PPV of 5.9%.
DISCUSSION
This study has demonstrated that in patients undergoing liver biopsy, SPA levels were significantly higher than in control patients who were attending the outpatient clinic and who were assumed to be free of liver disease. The lack of liver biopsies from the group of controls is problematic, but owing to ethical considerations, it would not be possible to have liver histology data from disease‐free individuals. Given the prevalence of liver disease, there may well have been individuals in the control group with unsuspected liver fibrosis. If so, this would have reduced the apparent discriminatory power of SPA analysis. Furthermore, the fact that this study was performed in a referral hospital will bias the findings because of the selected population 12. The small size and the case control design are limitations of the study.
Fibrosis is often, but not always, found in chronic liver injury and this may reflect the apparent lack of resolving power of SPA when examining the sensitivity, specificity, and related data 2. At a cut‐off SPA value of 200 IU/l a negative predictive value of 92% can be obtained but is at the expense of multiple false positives as the specificity is 59%. This concurs with previous findings 32, 37. A negative likelihood ratio at this cut‐off is 0.19 which is above the suggested value <0.1 for an excellent test 12.
There was no significant difference in SPA in the group with minimal compared with extensive fibrosis as determined by liver biopsy. This contrasts with a previous study however, there were patients with moderate to severe fibrosis included 32. In another study, plasma prolidase activity was measured in 338 patients with various disorders 30. The authors found elevated levels of plasma prolidase in 5 out of 27 patients known to have cirrhosis. However, there was no correlation between plasma prolidase activity and the degree of liver fibrosis determined at liver biopsy in 13 patients in this study. In another study, plasma prolidase enzyme activity and liver histology were compared in 53 adult patients who had alcohol intake in excess of 60 g/day. In this study, levels of SPA correlated with both cirrhosis and alcoholic hepatitis, but it could not be used to distinguish between early fibrosis and more severe pathology 31. A prospective study of 54 patients and 17 healthy controls used SPA to distinguish between NASH in patients with nonalcoholic fatty liver disease (P < 0.001)32. This showed that SPA levels were significantly higher in 36 consecutive patients with NASH, compared with controls. However in the present study, there appears to be an increase in SPA with mild‐moderate fibrosis, with a reduction once fibrosis becomes more severe. This trend is not statistically significant but has been observed 18, 21, 22. More advanced fibrosis may be characterized by a slower turn‐over of collagen than states of moderate fibrosis and this may lessen the induction of prolidase activity 30.
We also demonstrate that hemolysis is a positive interferent in SPA because prolidase levels are high in erythrocytes. No hemolyzed samples were used in this study. It is not clear how any hemolyzed samples, if any, were handled in previous studies 21, 30, 32, 38.
Future areas of research should include more diagnostic accuracy studies and analysis of SPA levels in other conditions such as Paget's disease and an examination of the biological variation in healthy people including pregnant women and children. In view of the HIV epidemic in South Africa and reports that liver fibrosis is more common in HIV infection, SPA should be investigated in this setting 39. Tuberculosis remains a significant source of morbidity and pathology locally and early reports that SPA can be used to distinguish pleural effusions of tuberculous from nontuberculous origin should be investigated 27.
The values that were obtained for SPA in this study were lower than those reported in several other studies but this may be due to differences produced by the analytical methods used 21, 30, 32, 38. There is no reference method or standard reference material available. Although the turn‐around time of the prolidase analysis has improved dramatically with a reduction in the length of time for the preincubation step, this remains extended at 2 hr and would not fulfill expectations for a routine screening test. Further refinements of the assay have been described which should be investigated 18. Future advances may involve the use of an artificial substrate which, when cleaved by prolidase, produces a chromogen directly without the need for further manipulation. Immunoassays that measure the prolidase mass rather than activity will be useful. The effects of other possible interferents, including bilirubin, which may be increased in liver disease, remains to be elucidated. We conclude that measurement of SPA may be useful in diagnostic investigation of suspected fibrotic liver disease.
ACKNOWLEDGMENTS
We thank Dr. Judy King for proofreading the article and Dr. David Haarburger for assistance with statistics. J.C.S. and T.S.P. were supported by a grant from National Health Laboratory Services Research Trust.
Grant sponsor: National Health Laboratory Service Research Trust.
REFERENCES
- 1. Castera L. Non‐invasive diagnosis of steatosis and fibrosis. Diabetes Metab 2008;34(6 Pt 2):674–679. [DOI] [PubMed] [Google Scholar]
- 2. Dufour DR, Lott JA, Nolte FS, Gretch DR, Koff RS, Seeff LB. Diagnosis and monitoring of hepatic injury. II. Recommendations for use of laboratory tests in screening, diagnosis, and monitoring. Clin Chem 2000;46(12):2050–2068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Albanis E, Friedman SL. Hepatic fibrosis. Pathogenesis and principles of therapy. Clin Liver Dis 2001;5(2):315–334, v–vi. [DOI] [PubMed] [Google Scholar]
- 4. Bolarin DM, Azinge EC. Biochemical markers, extracellular components in liver fibrosis and cirrhosis. Nig Q J Hosp Med 2007;17(1):42–52. [DOI] [PubMed] [Google Scholar]
- 5. Campbell MS, Reddy KR. Review article: the evolving role of liver biopsy. Aliment Pharmacol Ther 2004;20(3):249–259. [DOI] [PubMed] [Google Scholar]
- 6. Gressner OA, Weiskirchen R, Gressner AM. Biomarkers of liver fibrosis: clinical translation of molecular pathogenesis or based on liver‐dependent malfunction tests. Clin Chim Acta 2007;381(2):107–113. [DOI] [PubMed] [Google Scholar]
- 7. Plebani M, Basso D. Non‐invasive assessment of chronic liver and gastric diseases. Clin Chim Acta 2007;381(1):39–49. [DOI] [PubMed] [Google Scholar]
- 8. Carey E, Carey WD. Noninvasive tests for liver disease, fibrosis, and cirrhosis: Is liver biopsy obsolete? Cleve Clin J Med 77(8):519–527. [DOI] [PubMed] [Google Scholar]
- 9. Oh MK, Winn J, Poordad F. Review article: diagnosis and treatment of non‐alcoholic fatty liver disease. Aliment Pharmacol Ther 2008;28(5):503–522. [DOI] [PubMed] [Google Scholar]
- 10. Browning JD. New imaging techniques for non‐alcoholic steatohepatitis. Clin Liver Dis 2009;13(4):607–619. [DOI] [PubMed] [Google Scholar]
- 11. Andersen ES, Christensen PB, Weis N. Transient elastography for liver fibrosis diagnosis. Eur J Intern Med 2009;20(4):339–342. [DOI] [PubMed] [Google Scholar]
- 12. Guha IN, Rosenberg WM. Noninvasive assessment of liver fibrosis: serum markers, imaging, and other modalities. Clin Liver Dis 2008;12(4):883–900. [DOI] [PubMed] [Google Scholar]
- 13. Foucher J, Castera L, Bernard PH, et al. Prevalence and factors associated with failure of liver stiffness measurement using FibroScan in a prospective study of 2114 examinations. Eur J Gastroenterol Hepatol 2006;18(4):411–412. [DOI] [PubMed] [Google Scholar]
- 14. Poynard T, Lebray P, Ingiliz P, et al. Prevalence of liver fibrosis and risk factors in a general population using non‐invasive biomarkers (FibroTest). BMC Gastroenterol 2010;10:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fryer AA, Hanna FW. Managing demand for pathology tests: financial imperative or duty of care? Ann Clin Biochem 2009;46(Pt 6):435–437. [DOI] [PubMed] [Google Scholar]
- 16. Kurien BT, Patel NC, Porter AC, et al. Prolidase deficiency and the biochemical assays used in its diagnosis. Anal Biochem 2006;349(2):165–175. [DOI] [PubMed] [Google Scholar]
- 17. Galicka A, Surazynski A, Wolczynski S, Palka J, Popko J, Gindzienski A. Phenotype variability in a daughter and father with mild osteogenesis imperfecta correlated with collagen and prolidase levels in cultured skin fibroblasts. Ann Clin Biochem 2005;42(Pt 1):80–84. [DOI] [PubMed] [Google Scholar]
- 18. Tarcin O, Gedik N, Karakoyun B, Tahan V, Sood G, Celikel C, Tozun N. Serum prolidase and IGF‐1 as non‐invasive markers of hepatic fibrosis during four different periods after bile‐duct ligation in rats. Dig Dis Sci 2008;53(7):1938–1945. [DOI] [PubMed] [Google Scholar]
- 19. Abraham P, Wilfred G, Ramakrishna B. Plasma prolidase may be an index of liver fibrosis in the rat. Clin Chim Acta 2000;295(1–2):199–202. [DOI] [PubMed] [Google Scholar]
- 20. Aslan M, Nazligul Y, Horoz M, et al. Serum prolidase activity and oxidative status in Helicobacter pylori infection. Clin Biochem 2007;40(1–2):37–40. [DOI] [PubMed] [Google Scholar]
- 21. Cakmak A, Zeyrek D, Atas A, Celik H, Aksoy N, Erel O. Serum prolidase activity and oxidative status in patients with bronchial asthma. J Clin Lab Anal 2009;23(2):132–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Toy H, Camuzcuoglu H, Arioz DT, Kurt S, Celik H, Aksoy N. Serum prolidase activity and oxidative stress markers in pregnancies with intrauterine growth restricted infants. J Obstet Gynaecol Res 2009;35(6):1047–1053. [DOI] [PubMed] [Google Scholar]
- 23. Demirbag R, Yildiz A, Gur M, Yilmaz R, Elci K, Aksoy N. Serum prolidase activity in patients with hypertension and its relation with left ventricular hypertrophy. Clin Biochem 2007;40(13–14):1020–1025. [DOI] [PubMed] [Google Scholar]
- 24. Erbagci AB, Araz M, Erbagci A, Tarakcioglu M, Namiduru ES. Serum prolidase activity as a marker of osteoporosis in type 2 diabetes mellitus. Clin Biochem 2002;35(4):263–268. [DOI] [PubMed] [Google Scholar]
- 25. Namiduru ES, Binnur Erbagci A, Celik A, Yilmaz M, Tarakcioglu M. Serum prolidase activity in postmenopausal osteoporosis. Minerva Med 2007;98(6):647–651. [PubMed] [Google Scholar]
- 26. Evrenkaya TR, Atasoyu EM, Kara M, Unver S, Gultepe M. The role of prolidase activity in the diagnosis of uremic bone disease. Ren Fail 2006;28(4):271–274. [DOI] [PubMed] [Google Scholar]
- 27. Ipcioglu OM, Ozcan O, Gultepe M, Deniz O, Akgul EO. Prolidase activity in serum and pleural fluids in patients with tuberculous pleural effusion [correction of effussion]. Clin Biochem 2008;41(9):670–675. [DOI] [PubMed] [Google Scholar]
- 28. Kir ZO, Oner P, Iyidogan YO, et al. Serum prolidase I activity and some bone metabolic markers in patients with breast cancer: in relation to menopausal status. Clin Biochem 2003;36(4):289–294. [DOI] [PubMed] [Google Scholar]
- 29. Vural M, Camuzcuoglu H, Toy H, Aksoy N. Amniotic fluid prolidase activity and oxidative status in neural tube defects. Fetal Diagn Ther 2010;28(1):34–39. [DOI] [PubMed] [Google Scholar]
- 30. Myara I, Myara A, Mangeot M, Fabre M, Charpentier C, Lemonnier A. Plasma prolidase activity: a possible index of collagen catabolism in chronic liver disease. Clin Chem 1984;30(2):211–215. [PubMed] [Google Scholar]
- 31. Brosset B, Myara I, Fabre M, Lemonnier A. Plasma prolidase and prolinase activity in alcoholic liver disease. Clin Chim Acta 1988;175(3):291–295. [DOI] [PubMed] [Google Scholar]
- 32. Kayadibi H, Gultepe M, Yasar B, et al. Diagnostic value of serum prolidase enzyme activity to predict the liver histological lesions in non‐alcoholic fatty liver disease: a surrogate marker to distinguish steatohepatitis from simple steatosis. Dig Dis Sci 2009;54(8):1764–1771. [DOI] [PubMed] [Google Scholar]
- 33. Horoz M, Aslan M, Bolukbas FF, et al. Serum prolidase enzyme activity and its relation to histopathological findings in patients with non‐alcoholic steatohepatitis. J Clin Lab Anal 2010;24(3):207–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bruns DE, Huth EJ, Magid E, Young DS. Toward a checklist for reporting of studies of diagnostic accuracy of medical tests. Clin Chem 2000;46(7):893–895. [PubMed] [Google Scholar]
- 35. Dimeski G. Interference testing. Clin Biochem Rev 2008;29(Suppl 1):S43–S48. [PMC free article] [PubMed] [Google Scholar]
- 36. Kleiner DE, Brunt EM, Van Natta M, et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology 2005;41(6):1313–1321. [DOI] [PubMed] [Google Scholar]
- 37. Martinez SM, Crespo G, Navasa M, Forns X. Noninvasive assessment of liver fibrosis. Hepatology 2011;53(1):325–335. [DOI] [PubMed] [Google Scholar]
- 38. Verit FF, Geyikli I, Yazgan P, Celik A. Correlations of serum prolidase activity between bone turnover markers and mineral density in postmenopausal osteoporosis. Arch Gynecol Obstet 2006;274(3):133–137. [DOI] [PubMed] [Google Scholar]
- 39. DallaPiazza M, Amorosa VK, Localio R, Kostman JR, Lo Re V, 3rd . Prevalence and risk factors for significant liver fibrosis among HIV‐monoinfected patients. BMC Infect Dis 2010;10:116. [DOI] [PMC free article] [PubMed] [Google Scholar]


