Abstract
Background
This study compares the diagnostic performance (in routine urinalysis) of three URiSCAN devices and three Roche analyzers to manual microscopy and quantitative assays.
Methods
We analyzed eight dipstick tests using three URiSCAN devices. The results were compared to those of the tests performed using three Roche analyzers. The results of leukocyte and erythrocyte screens were compared to those obtained using manual microscopy. Protein, glucose, pH, and specific gravity (SG) assays performed on the URiSCAN devices were compared with the results of corresponding quantitative assays.
Results
The rates of agreement within one grade difference were found to be more than 94.3%. When compared with manual microscopy, the Optima provided better diagnostic performance for the detection of leukocytes compared with the Urisys 1100. Compared to the Urisys 2400, the Super plus provided better diagnostic performance with regard to both leukocytes and erythrocytes. There was good correlation between the three URiSCAN devices and each quantitative assay, except for SG detection.
Conclusion
There were well correlated results between those of the three URiSCAN devices and those obtained using the corresponding Roche analyzers, quantitative assays, and manual microscopy. URiSCAN series devices are therefore suitable for routine urinalysis in clinical laboratories.
Keywords: urinalysis, urine dipstick analysis, microscopic examination, semiquantitative, quantitative
Abbreviations
- AUC
area under the curve
- ROC
receiver‐operating characteristic
- SG
specific gravity
INTRODUCTION
Urinalysis is an important clinical tool for screening, diagnosis, and follow‐up. Urinalysis also allows for the detection of urogenital or systemic disorders that give rise to chemical and physical abnormalities 1. Urine is evaluated by most urinalysis procedures using diagnostic reagent strips (dipsticks) for urine chemistry and microscopy for cell counts. The traditional microscopic techniques that involve urinary sediment are labor‐intensive, time‐consuming, imprecise, and potentially influenced by interobserver variability. Given these limitations, routine urine testing with multiparameter dipsticks is considered the optimal initial step in analysis 2, 3. When used in combination with a urine analyzer, urinalysis with dipstick analysis is valuable for its convenience, speed, and reproducibility. These microchemistry systems have been available for many years and allow for qualitative and semiquantitative analyses in routine urinalysis. Using semiautomatic or fully automatic instruments to read a dipstick may eliminate interobserver variability and time‐sensitive errors associated with visual interpretation 4.
URiSCAN devices (YD Diagnostics, Yongin‐si, Republic of Korea) are some of the most commonly used semiautomatic and/or automatic urine analyzers in Korea. The Korean Association of Quality Assurance for Clinical Laboratory (2009 KEQAS‐UA) conducted a quality assessment trial and found that 45.8% of the 692 analyzers used by participating institutions were URiSCAN devices 5. Although a prior performance evaluation of the URiSCAN Pro II and a comparative device has been conducted 6, there are no data available comparing the performance of the URiSCAN Optima and the URiSCAN Super plus with comparable devices, or with quantitative assays and microscopic examination.
Therefore, the aim of this study was to compare the diagnostic performances of three URiSCAN devices (the Optima, Pro II, and Super plus) and three Roche urine analyzers (the Urisys 1100, Cobas u411, and Urisys 2400; Roche Diagnostics, Mannheim, Germany). The analyzers in routine urinalysis were also compared with results obtained by manual microscopy and quantitative assays.
MATERIALS AND METHODS
Specimens
Fresh urine samples collected from 1,273 inpatients and outpatients between June 2013 and November 2013 were used in this study. The samples did not contain any preservatives. The urine specimens were transferred to two different test tubes. At least 10 ml was allocated for dipstick analysis, along with sequential microscopic examination. More than 2 ml were allocated for quantitative analysis. The samples were analyzed within 2 hr of arrival to the laboratory. This study was approved by our institutional review board (KBC13073D).
Methods
Dipstick analysis was performed, and analyzed using the six analyzers from URiSCAN and Roche. Eight measurements were made, including protein, blood, glucose, ketone, urobilinogen, bilirubin, nitrite, and leukocytes. Each URiSCAN device was compared to a similar Roche analyzer with regard to size, weight, test velocity, test cycle, and memory capacity. Each specimen was well mixed before it was tested with the dipstick analysis. All tests were performed according to each manufacturer's instructions. Three independent sets of comparison studies were performed sequentially for each specimen. A total of 1,273 noncentrifuged urine samples were analyzed with dipstick analyses and microscopic examination. These included 438 specimens for the Optima versus Urisys 1100 comparison, 437 specimens for the URiSCAN Pro II versus Cobas u411 comparison, and 398 specimens for the URiSCAN Super plus versus Urisys 2400 comparison.
The results obtained in each pairwise comparison were considered concordant if they were within one grade difference of each other. The pairwise concordance rates between each set of analyzers were defined by the percent agreement rate. The differences in grading systems between URiSCAN and Roche analyzers precluded the direct application of statistical evaluation. In order to compensate for the differences in the grading systems used for different instruments, the grading system levels (of each test parameter) were converted to comparable scales. For example, the Pro II has five grades with regard to urobilinogen detection [± (0.1 mg/dl), + (1 mg/dl), ++ (4 mg/dl), +++ (8 mg/dl), ++++ (12 mg/dl)], while the Cobas u411 has five different grades [−, ± (1 mg/dl), + (4 mg/dl), ++ (8 mg/dl), +++ (12 mg/dl)]. In this case (Pro II and Cobas u411), the following result pairs were considered to be comparable: (+/− and −), (+ and +/−), (++ and +), (+++ and ++), (++++ and +++).
Following dipstick analysis, urine specimens were centrifuged at 1800 rpm for 3 min. The remaining 200 μl of sediment was reserved for microscopic examination. A qualified medical technologist performed all of the microscopic examinations with a single microscope under 400× magnification (DMLS2; Leica, Lockbourne, OH). In each sample, erythrocytes and leukocytes were counted in high power fields, and the average numbers (from ten fields) were recorded. A positive finding was defined as the presence of at least three erythrocytes and five leukocytes per high power field 7.
The URiSCAN device results for protein, glucose, pH, and specific gravity (SG) were compared to those of corresponding quantitative assays. These quantitative assays included use of the benzethonium chloride method for protein determination on the Cobas Integra 800 (Roche, Indianapolis, IN), the hexokinase method for glucose measurement on the Advia 1800 (Siemens, Tokyo, Japan), a pH meter (Mettler Toledo S220; Mettler Toledo, Zurich, Switzerland), and a refractometer (UG‐α; Atago, Tokyo, Japan). Other test parameters were not considered, as we were unable to perform the requisite quantitative assays due to laboratory limitations. The principle of the URiSCAN device is based on light reflected from the surface of the urine strip. The light is transferred through an optical fiber to a charge‐coupled device sensor, where the reflected light is analyzed to determine the ratio of the three primary colors. The ratio of the three primary colors and compensation colors is used to calculate the change of reflectance rate (%R). An analog to digital converter is then used to convert this %R to an equivalent grade or concentration 8. The average %R was calculated for each test parameter based on duplicate tests performed on the same dipstick. The average %R values were used in each comparison.
Statistical and Analytical Methods
The agreement rates and within‐one‐grade differences were calculated between the classified grades of the simultaneously evaluated URiSCAN and corresponding Roche devices. The area under the curve (AUC) was calculated from the receiver‐operating characteristic (ROC) according to the trace or positive dipstick grading results using manual microscopic examination (P < 0.05). The correlation coefficient and regression line were obtained to verify the correlations between the change %R (of the URiSCAN devices) and the corresponding assays (P < 0.001). IBM SPSS version 18.0 (IBM, Armonk, New York, NY) and STATA version 13.0 (Stata Corp., College Station, TX) were used for statistical analyses. The correlation coefficients and regression lines were calculated using Pearson correlation and linear regression or Spearman's correlation, respectively.
RESULTS
Table 1 summarizes the agreements between the URiSCAN devices and the Roche analyzers for the same urine specimens. The concordance levels of greater than 94.3% to 100% were obtained for the eight parameters investigated (considering the results within one grade of difference).
Table 1.
Correlation Between URiSCAN Devices and Roche Urine Analyzers Represented as Rate (%) of Results Within One Grading Difference
| Blood | Bil | Urobil | Ket | Pro | NT | Glu | Leu | |
|---|---|---|---|---|---|---|---|---|
| Optima versus Urisys 1100 | 97.5 | 99.8 | 100 | 99.5 | 94.3 | 100 | 96.8 | 98.6 |
| Pro II versus Cobas u411 | 97.5 | 100 | 100 | 99.8 | 95.7 | 100 | 98.2 | 97.5 |
| Super plus versus Urisys 2400 | 99.5 | 100 | 99.7 | 98.5 | 98.2 | 100 | 94.7 | 99.0 |
Bil, bilirubin; Urobil, urobilinogen; Ket, ketones; Pro, protein; NT, nitrite; Glu, glucose; Leu, leukocytes.
The results of leukocyte and erythrocyte screens were compared to those obtained by manual microscopy. The sensitivities and specificities of the URiSCAN devices and the Roche analyzers were then calculated (Table 2). The Super plus device offered better diagnostic performance (AUC 0.883) in erythrocyte detection than did the Urisys 2400 device (AUC 0.870). In the leukocyte screen, the Optima and Super plus units had better diagnostic performance (AUC 0.790 and 0.850, respectively) than did the Urisys 1100 and Urisys 2400 devices (AUC 0.740 and 0.770, respectively; P < 0.05). There were no statistical differences in the performances of the Optima and Urisys 1100 erythrocyte screens, or between the Pro II and Cobas u411 combined screens.
Table 2.
Diagnostic Accuracy of Erythrocyte and Leukocyte Detection Using URiSCAN Devices and Roche Urine Analyzers With Manual Microscopy as the Standard
| Erythrocytes | Leukocytes | |||||||
|---|---|---|---|---|---|---|---|---|
| Sensitivity | Specificity | AUC | P | Sensitivity | Specificity | AUC | P | |
| URiSCAN Optima | 91.4 | 78.1 | 0.848 | 0.931 | 76.6 | 93.1 | 0.790 | 0.003a |
| Urisys 1100 | 86.7 | 82.6 | 0.846 | 64.9 | 97.8 | 0.740 | ||
| URiSCAN Pro II | 79.6 | 85.8 | 0.827 | 0.400 | 72.1 | 93.7 | 0.795 | 0.746 |
| Cobas u411 | 76.8 | 86.4 | 0.816 | 70.9 | 94.6 | 0.790 | ||
| URiSCAN Super plus | 92.9 | 83.8 | 0.883 | 0.018a | 87.5 | 84.8 | 0.850 | 0.008a |
| Urisys 2400 | 92.9 | 81.1 | 0.870 | 95.8 | 61.5 | 0.770 | ||
P < 0.05.
Correlation coefficients and regression lines were obtained by comparing the change %R of the URiSCAN devices and the corresponding quantitative methods (Figs. 1, 2, and 3. The quantitative protein results for each quantitative assay were adjusted by applying logarithmic values. There were good correlations using linear regression between protein and SG, both of which exceeded 0.904 (P < 0.001). In contrast, there was a poor correlation between the SG detection of the Optima and Pro II. Nonparametric and nonlinear scatters were observed for both glucose and pH. Spearman's correlation revealed good correlations, with glucose exceeding 0.712 (P < 0.001) and pH exceeding 0.934 (P < 0.001).
Figure 1.
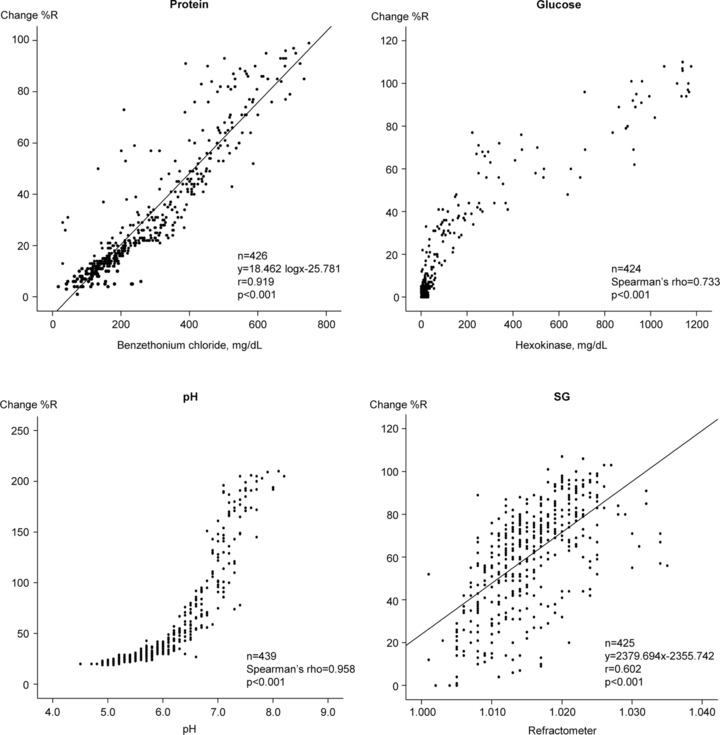
Correlation coefficients between the quantitative assays and URiSCAN Optima results.
Figure 2.
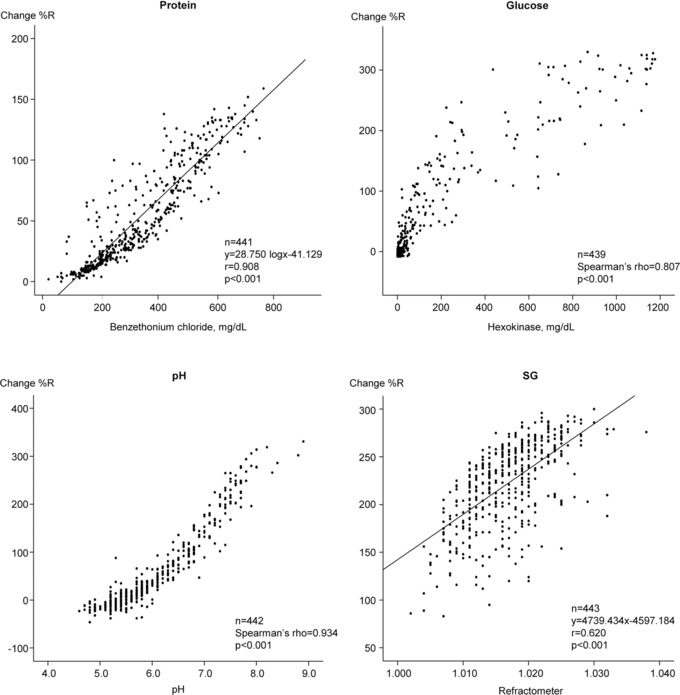
Correlation coefficients between the quantitative assays and URiSCAN Pro II results.
Figure 3.
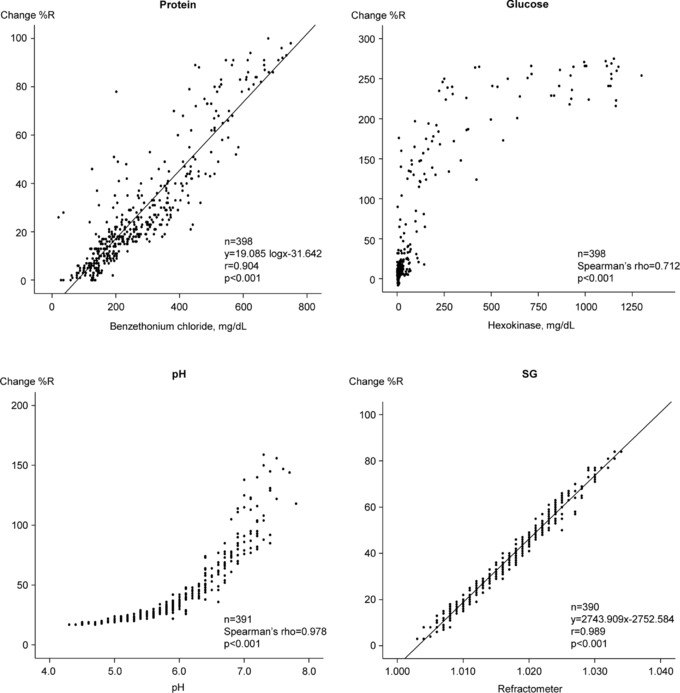
Correlation coefficients between the quantitative assays and URiSCAN Super plus results.
DISCUSSION
As shown in Table 1, there was good overall agreement between the three URiSCAN devices and their corresponding Roche analyzers. The agreement rates between Optima versus Urisys 1100 for protein and glucose were 94.3% and 96.8%, respectively. Among the discrepancy results, 24 of 25 results for protein were only detected by Optima. In contrast, Urisys 1100 demonstrated all 14 glucose results above one‐grading scale. We did not investigate these analyzer discrepancies any further. However, we advise that urinalyses are carefully interpreted to avoid false‐positives readings for protein and/or falsely elevated glucose measurements.
Microscopic urine examination is recommended to clarify false‐negative or false‐positive results on urinalysis. In this study, we compared the URiSCAN and Roche devices based on microscopic examination. In addition, AUCs were calculated according to the devices’ sensitivities and specificities (Table 2). The URiSCAN Optima showed superior diagnostic performance for the detection of leukocytes, whereas we observed no difference in performance for erythrocyte detection. The URiSCAN Pro II showed equivalent performance levels for the detection of both leukocytes and erythrocytes. The URiSCAN Super plus showed the best diagnostic performance for the detection of both leukocytes and erythrocytes. One limitation to this study, however, is that we did not investigate factors that may have influenced false‐negative and/or false‐positive results. According to previous studies, there are numerous factors that influence dipstick‐based urinalysis and can cause false‐negative/false‐positive results. When dipstick analysis is used for leukocyte detection, false‐positive results may be caused by urine contamination by vaginal discharge and bacteriuria. False‐negative results, in contrast, may occur in the setting of elevated SG, glycosuria, ketonuria, proteinuria, oxidizing drugs (cephalexin, nitrofurantoin, tetracycline, and gentamicin), and ascorbic acid. False‐positive erythrocyte detection may occur in the presence of hemoglobinuria, myoglobinuria, menstrual blood, or dehydration. False‐negative erythrocyte detection, in contrast, may occur if the patient takes captopril, or has high SG, acidemia, proteinuria, or ascorbic acid in the urine 1, 9, 10, 11. The sensitivity of leukocyte detection ranged between 64.9% and 95.8%. Previous studies have suggested that leukocyte sensitivity varies according to each urinalysis device. For example, that of the Combostick Reader 720 is 82.4% 6, the URiSCAN Gen 10SGL is 63.6% (8), UriSed is 82% (12), Fus100 is 68% 12, iQ200 is 87% 13, UF‐100 is 71.8% 14, and URiSCAN Super is 87% 15. The differences may be due to varying methodologies used to determine these parameters, such as the strip test analyzers, which measure enzymatic activity rather than counting cells.
The three URiSCAN devices were well correlated with the quantitative assays with regard to four parameters tested (protein, glucose, pH, and SG; Figs. 1, 2, 3. We observed parametric and linear scatters for protein and SG; therefore, linear regression was used to evaluate these two variables. In contrast, we observed nonparametric and nonlinear scatters for glucose and pH. We evaluated these variables by adjusting Spearman's correlation. There was poor correlation between the dipstick analyses with Optima and Pro II instruments and the quantitative assay (with a refractometer) for urine SG. This finding may have resulted from differences in the measurement principles for this assay. Most dipstick analyzers use similar principles for measuring SG. The strip relies on the correlation between the ionic solute concentration and the urine SG to provide an indirect measurement 16. In contrast, the Optima and the Pro II SG tests are based on the apparent pKa change of pretreated polyelectrolytes in relation to the ionic concentration. The presence of cations in urine causes proton release from complex substances, which leads to changes in pH and thus changes the indicator colors of the stick. This method is not affected by nonionic substances. However, the presence of moderate quantities of protein may cause false elevation, while highly buffered alkaline urine samples may cause false‐negative results 16, 17, 18. Refractometry is based on light refraction and can be affected by nonionic substances in the urine. When light passes from one medium into another, the light beam changes its direction at the surface boundary if its speed in the second medium is different from that in the first. The angle created by the light bending is called the critical angle and the ability of a substance to bend light is its refractivity. Therefore, the refractivity of a solution is an indirect measurement of the total solute concentration 7. In previous studies that compared the dipstick method, which uses ionic environmental alteration as a measure, and the refractometer method for detection of urine SG, low correlations were reported 19, 20, 21. Urine SG measurements by the dipstick method has been used as an easy, rapid, noninvasive, and inexpensive way to interpret a patient's hydration status. However, these results should be carefully interpreted given the discrepancy that may exist between the dipstick and refractometer results 22, 23, 24. Ideally, the dipstick SG results should be confirmed using a refractometer before a final diagnosis is made. The URiSCAN Super plus contains a built‐in refractometer unlike the Optima or Pro II. Therefore, the URiSCAN Super plus may be more useful for measuring urine SG than are the Optima or Pro II 25. As might be expected, therefore, the URiSCAN Super plus results were more highly correlated with those of the quantitative assay (r = 0.989, P < 0.001) than were the results obtained using the Optima (r = 0.602) and Pro II (r = 0.620).
In conclusion, the three URiSCAN devices (Optima, Pro II, and Super plus) are well correlated with the corresponding Roche analyzers when used for dipstick analysis. The results obtained using these machines were also comparable to those obtained with quantitative assays. The three URiSCAN devices were comparable to or better than were the Roche analyzers at detecting erythrocytes and leukocytes. This URiSCAN series will likely be valuable for routine urinalysis in clinical laboratories.
CONFLICT OF INTEREST
There are no conflicts of interest regarding the publication of this article.
Supporting information
Disclaimer: Supplementary materials have been peer‐reviewed but not copyedited.
Table S1. Comparison of URiSCAN Optima and Roche Urisys 1100
Table S2. Comparison of URiSCAN Pro II and Roche Cobas u411
Table S3. Comparison of URiSCAN Super plus and Roche Urisys 2400
Point‐by‐Point Replies to Reviewer's Comments
ICMJE Form for Disclosure of Potential Conflicts of Interest
REFERENCES
- 1. Simerville JA, Maxted WC, Pahira JJ. Urinalysis: A comprehensive review. Am Fam Physician 2005;71:1153–1162. [PubMed] [Google Scholar]
- 2. Lamchiagdhase P, Preechaborisutkul K, Lomsomboon P, et al. Urine sediment examination: A comparison between the manual method and the iQ200 automated urine microscopy analyzer. Clin Chim Acta 2005;358:167–174. [DOI] [PubMed] [Google Scholar]
- 3. Ben‐Ezra J, Bork L, McPherson RA. Evaluation of the Sysmex UF‐100 automated urinalysis analyzer. Clin Chem 1998;44:92–95. [PubMed] [Google Scholar]
- 4. Chien TI, Lu JY, Kao JT, et al. Comparison of three automated urinalysis systems—Bayer Clinitek Atlas, Roche Urisys 2400 and Arkray Aution Max for testing urine chemistry and detection of bacteriuria. Clin Chim Acta 2007;377:98–102. [DOI] [PubMed] [Google Scholar]
- 5. Kim KD, Koo SH, Kim EC, et al. Annual report on external quality assessment in urinalysis in Korea (2009). J Lab Med Qual Assur 2010;32:69–93. [Google Scholar]
- 6. Kwon MJ, Lee HW, Kim GY, Nam MH, Lee CK, Kim YK. Laboratory evaluation of automated urine analyzer ComboStick Reader 720(R) and reagent strip ComboStick 10. J Lab Med Qual Assur 2009;31:215–223. [Google Scholar]
- 7. McPherson RA, Pincus MR. Henry's Clinical Diagnosis and Management by Laboratory Methods. Philadelphia: Saunders company; 2011. p 464–465. [Google Scholar]
- 8. Yun KA, Han TJ, Chun S, Min WK. The performance evaluation of Yeongdong URiSCAN GEN 10SGL urine dipstick strip using other quantitative, microscopic, and culture methods. Korean J Clin Pathol 2001;21:471–479. [Google Scholar]
- 9. Eigbefoh JO, Isabu P, Okpere E, Abebe J. The diagnostic accuracy of the rapid dipstick test to predict asymptomatic urinary tract infection of pregnancy. J Obstet Gynaecol 2008;28:490–495. [DOI] [PubMed] [Google Scholar]
- 10. Memişoğulları R, Yüksel H, Yıldırım HA, Yavuz Ö. Performance characteristics of dipstick and microscopic urinalysis for diagnosis of urinary tract infection. Eur J Gen Med 2010;7:174–178. [Google Scholar]
- 11. Rao PK, Gao T, Pohl M, Jones JS. Dipstick pseudohematuria: Unnecessary consultation and evaluation. J Urol 2010;183:560–564. [DOI] [PubMed] [Google Scholar]
- 12. Yuksel H, Kilic E, Ekinci A, Evliyaoglu O. Comparison of fully automated urine sediment analyzers H800‐FUS100 and LabUMat‐UriSed with manual microscopy. J Clin Lab Anal 2013;27:312–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. van den Broek D, Keularts IM, Wielders JP, Kraaijenhagen RJ. Benefits of the iQ200 automated urine microscopy analyser in routine urinalysis. Clin Chem Lab Med 2008;46:1635–1640. [DOI] [PubMed] [Google Scholar]
- 14. Lee AJ, Jeon CH, Kim SG, Suh HS, Bae YC. Comparison of analytical performance between the Sysmex UF‐100 flow cytometer and the Iris iQ200 urine microscopy system. J Lab Med Qual Assur 2010;32:181–188. [Google Scholar]
- 15. Shin SY, Kwon MJ, Woo HY, Park H, Kim YJ. Preliminary evaluation of the URiSCAN SUPER and usefulness of a new urine reagent strip to detect ascorbic acid. J Lab Med Qual Assur 2011;33:63–69. [Google Scholar]
- 16. Burkhardt AE, Johnston KG, Waszak CE, Jackson CE, Shafer SR. A reagent strip for measuring the specific gravity of urine. Clin Chem 1982;28:2068–2072. [PubMed] [Google Scholar]
- 17. Kirschbaum BB. Evaluation of a colorimetric reagent strip assay for urine specific gravity. Am J Clin Pathol 1983;79:722–725. [DOI] [PubMed] [Google Scholar]
- 18. Oregon Health & Science University . Hospitals and Clinics Point of Care Urine Dipstick by Clinitek Status+. Available at: http://www.ohsu.edu/pathology/POC/procedures/urinalysis.pdf. Accessed on July 20, 2015.
- 19. Adams LJ. Evaluation of ames Multistix‐SG for urine specific gravity versus refractometer specific gravity. Am J Clin Pathol 1983;80:871–873. [DOI] [PubMed] [Google Scholar]
- 20. Dorizzi RM, Caputo M. Measurement of urine relative density using refractometer and reagent strips. Clin Chem Lab Med 1998;36:925–928. [DOI] [PubMed] [Google Scholar]
- 21. Siegrist D, Hess B, Montandon M, Takkinen R, Lippuner K, Jaeger P. Urinary specific gravity—Comparative measurements using reagent strips and refractometer in 340 morning urine samples. Schweiz Rundsch Med Prax 1993;82:112–116. [PubMed] [Google Scholar]
- 22. Edijanto SP. Evaluation of urine blood cells and urine specific gravity examination using the dipstick method. Folia Med Indones 2000;36:41–52. [Google Scholar]
- 23. Chadha V, Garg U, Alon US. Measurement of urinary concentration: A critical appraisal of methodologies. Pediatr Nephrol 2001;16:374–382. [DOI] [PubMed] [Google Scholar]
- 24. de Buys Roessingh AS, Drukker A, Guignard JP. Dipstick measurements of urine specific gravity are unreliable. Arch Dis Child 2001;85:155–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. YD‐diagnostics . URiSCAN super. Available at: http://www.yd‐diagnostics.com/new/pdf/uriscan_super.pdf. Accessed on July 20, 2015.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Disclaimer: Supplementary materials have been peer‐reviewed but not copyedited.
Table S1. Comparison of URiSCAN Optima and Roche Urisys 1100
Table S2. Comparison of URiSCAN Pro II and Roche Cobas u411
Table S3. Comparison of URiSCAN Super plus and Roche Urisys 2400
Point‐by‐Point Replies to Reviewer's Comments
ICMJE Form for Disclosure of Potential Conflicts of Interest


