Abstract
Background
The most important proinflammatory cytokine is interleukin (IL)‐1β, however its precursor, prointerleukin‐1β (proIL‐1β), can also potentiate inflammatory state. The aim of this study was to explore the involvement of proIL‐1β in pathogenesis of endometriosis. For this purpose, we evaluated concentrations of proIL‐1β, IL‐1β, and soluble IL‐1 receptor type 2 (sIL‐1R2) in peritoneal fluid (PF) and macrophage culture medium of women with endometriosis.
Methods
PF from 55 women with and without endometriosis was collected during laparoscopy. Peritoneal macrophages were cultured in basal and stimulated with lipopolysaccharide (LPS) conditions. Concentrations of cytokines were measured with enzyme‐linked immunosorbent assays (ELISA).
Results
PF proIL‐1β and IL‐1β levels in endometriosis women were higher than in the control. Higher basal and stimulated macrophage secretion of cytokines in endometriosis patients than in the control was observed. However, in endometriosis, there was a higher level of proIL‐1β than for the mature molecule. Additionally, lower PF and macrophages culture medium sIL‐1R2 levels were observed in women with endometriosis.
Conclusions
Abnormal proIL‐1β concentration in PF and higher macrophage secretion can escalate peritoneal inflammation and endometriosis formation. The results are presented as a total IL‐1β, which is a sum of proIL‐1β and IL‐1β, and we believe that it reflects the actual cytokine production. The imbalance among all studied cytokines in endometriosis may be linked with an ability to transform acute inflammation to the chronic inflammation.
Keywords: IL‐1β, proIL‐1β, endometriosis, macrophages, peritoneal fluid
Background
The interleukin (IL)‐1 cytokine family is associated with the immune response and inflammation. It consists of the following distinct molecular forms: intercellular IL‐1α (IL‐1F1), extracellular IL‐1β (IL‐1F2), IL‐1 receptor antagonist (IL‐1Ra, IL‐1F3), and also IL‐18 (IL‐1F4), IL‐33 (IL‐1F11), IL‐37 (IL‐1F7), and IL‐38 (L‐1F10) 1, 2. The most important proinflammatory cytokine among them is IL‐1β. It is mainly generated by monocytes and macrophages as an inactive 31 kDa precursor, prointerleukin‐1β (proIL‐1β). It requires cleavage by the cysteine protease IL‐1‐converting enzyme (ICE) to generate a 17 kDa mature and biologically active protein 3. Cell activation in response to IL‐1 is mediated via two receptors: the functional signaling receptor type 1 (IL‐1R1) and “decoy” receptor type 2 (IL‐1R2) 4, 5. Moreover, IL‐1R2 could be shed from the cell surface as a soluble form (sIL‐1R2) that captures IL‐1β and inhibits it from binding to IL‐1R1 6.
Recent studies have shown the important role of the IL‐1 cytokine family in endometrium‐related disorders, including endometriosis 7. This is a chronic estrogen‐dependent disorder of an unclear pathogenesis, defined as a result of implantation of endometrial tissue outside the uterine cavity, primarily into the peritoneal cavity. The peritoneal fluid (PF) of women with endometriosis creates a specific microenvironment, which contains immune cells and their secretory products, and succours the growth and development of the ectopic endometrial tissue. Peritoneal macrophages in endometriosis patients play a crucial role in the maintenance of the immune response and inflammation. Those cells secrete a variety of local products, such as the members of the IL‐1 cytokine family 8, 9. In this study, we investigated the endometriosis and nonendometriosis PF levels of IL‐1β and proIL‐1β, presented also as total IL‐1β (tIL‐1β). Moreover, we evaluated the secretion of the studied parameters from the peritoneal macrophages of the endometriosis and control women. To this end, both basal (constitutive) and lipopolysaccharide (LPS) stimulated (induced) syntheses of IL‐1β and proIL‐1β were evaluated. As both studied parameters depend on sIL‐1R2, we also measured concentration of this soluble receptor in PF and macrophage culture media.
Materials and Methods
Study Population
A total of 55 women aged between 23 and 39 (mean age: 28.4 ± 4.3 years) undergoing laparoscopy for infertility were included in this study. All patients were admitted to the Clinic of Gynecology and Obstetrics of the Medical University of Silesia, in the period of 2009–2011, for diagnostic or therapeutic laparoscopy for infertility. Clinical data regarding menstrual cycle, obstetrical history, previous surgical procedures, and history of hormone use were also obtained by means of an appropriate questionnaire. All diagnostic and laparoscopic procedures were performed during the proliferative phase of menstrual cycle. Women included in the study had normal menstrual cycles (28 ± 4 days) and no other pelvic disorders, chronic circulatory, autoimmune, or neoplastic diseases and had not been taking any anti‐inflammatory or immunomodulatory medications in the preceding 2 months.
Endometriosis was confirmed histologically in 30 women, aged 23–35 (mean age ± SD: 29.3 ± 4.7 years). The studied group, including early (stages I and II, n = 18) and advanced (stages III and IV, n = 12) endometriosis, were scored with the revised American Fertility Society (rAFS) classification 10. The control group included 25 women, aged 25–39 (mean age: 30.5 ± 4.9 years), who had unexplained infertility but no evidence of endometriosis or inflammation in the peritoneal cavity. The study protocol was approved by the Ethics Committee of the Medical University of Silesia. All patients and control subjects who participated in this study signed an informed consent form.
Collection and Processing of PF Samples
The samples of PF were collected during the laparoscopic procedure from the posterior cul‐de‐sac, under general anesthesia, immediately after the introduction of the second trocar in order to minimize blood contamination. After aspiration, the samples were processed by a centrifuge at 400 × g for 10 min and stored at –85°C until the analysis.
For isolation of macrophages from PF, first, peritoneal mononuclear cells (PMC) were isolated. For this purpose, cells in PF were suspended in phosphate buffer saline (PBS), (The Regional Blood Bank, Katowice, Poland) and then isolated by density gradient centrifuging over lymphocyte separate medium (LSM 1077; PAA Laboratories, Inc., Pasching, Austria), followed by a double wash. Peritoneal macrophages were purified by PMCs for positive selection using MiniMACS magnetic cell separator and conjugated with anti‐CD68 microbeads (Milteny Biotec GmbH, Gladbach, Germany). The purity of the isolated CD68+ macrophages was 98% for the endometriosis patients and 97% for the control group. Cell viability was consistently >99% as determined by Trypan blue (Sigma Chemical Corporation, Saint Louis, MO) staining. The macrophages were resuspended in Dulbecco's modified eagle medium (DMEM) (PAA Laboratories, Inc., Pasching, Austria), supplemented with 10% fetal bovine serum (FBS; PAA Laboratories, Inc.) and with 100 U/ml of penicillin G and 50 mg/ml of streptomycin (Sigma Chemical Corporation) and plated in 4‐well chamber slides (PAA Laboratories, Inc.) at the density of 1 × 106 macrophages/ml under standard conditions of 5% CO2 and 37°C. The isolated cells were cultured for 72 h to assess the basal production of cytokines. To evaluate the stimulated secretion of cytokines, the macrophages were cultured for another 72 h with 2 ng/ml LPS from Escherichia coli 0111:B4 (Sigma Chemical Corporation). After the incubation period, culture supernatants were collected and stored at −85°C for further analysis.
Cytokine Assay
PF levels of IL‐1β, proIL‐1β, and sIL‐1R2 were measured with standard cytokine‐specific enzyme‐linked immunosorbent assay (ELISA) using commercial kits eBioscience (Vienna, Austria) and R&D Systems (Minneapolis, MN). The sensitivity of the kits was approximately 1 pg/ml for IL‐1β, 3.3 pg/ml for proIL‐1β, and 10 pg/ml for sIL‐1R2.
Statistical Analysis
The data are presented as mean and SD. The differences among and within the groups were evaluated using Student's t‐test and one‐way ANOVAS, respectively. The differences between mean values were considered statistically significant at P < 0.05.
Results
proIL‐1β
The mean PF proIL‐1β level was significantly higher in women with endometriosis than in the controls (P < 0.0001). Higher concentration of the studied parameter was found in PF of women with the advanced stage of the disease, as compared to women with early endometriosis (P < 0.001). Increased proIL‐1β production from both basal and stimulated macrophage culture in women with endometriosis compared to the control group was observed (P < 0.0001). ProIL‐1β secretion was higher at the advanced endometriosis in comparison to the early stage of the disease (P < 0.0001).
IL‐1β
The concentration of PF IL‐1β was significantly higher in women with endometriosis than in the controls (P < 0.0001). Increased concentration of the mature molecule was found in PF of women with the advanced endometriosis compared to the early endometriosis (P < 0.0001). The IL‐1β secretion from both basal and stimulated macrophage culture was elevated in women with endometriosis compared to the control group (P < 0.0001). Higher secretion of IL‐1β was observed at the advanced endometriosis in comparison to the early stage of the disease (P < 0.0001).
IL‐1β and proIL‐1β levels in PF and macrophage culture media are shown in Figures 1 and 2. A statistically significant positive correlation between IL‐1β and proIL‐1 β levels (P < 0.0001, r = 0.7919) was observed in the PF of women with endometriosis. We decided to present our results as a total IL‐1β, which is a sum of precursor and mature molecule concentrations. Levels of proIL‐1β and IL‐1β in PF and macrophage culture media are shown in Figures 1, 2, 3, 4.
Figure 1.
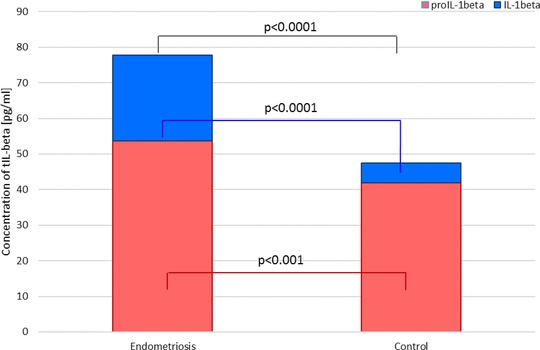
IL‐1β and proIL‐1β levels as a total IL‐1β in peritoneal fluid of women with and without endometriosis.
Figure 2.
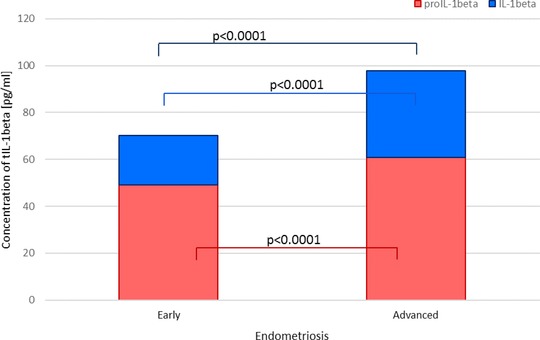
IL‐1β and proIL‐1β levels as a total IL‐1β in peritoneal fluid of women with early and advanced endometriosis.
Figure 3.
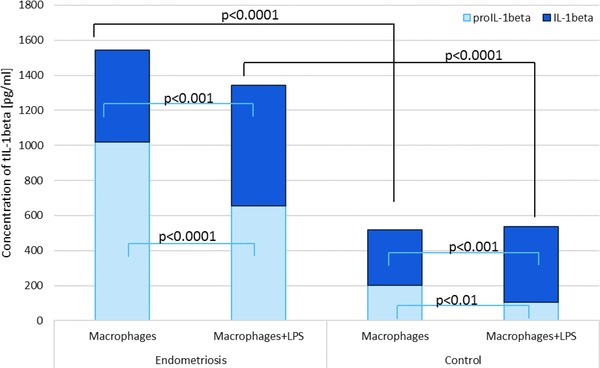
Mean IL‐1β and proIL‐1β levels as a total IL‐1β in macrophage culture media of women with and without endometriosis.
Figure 4.
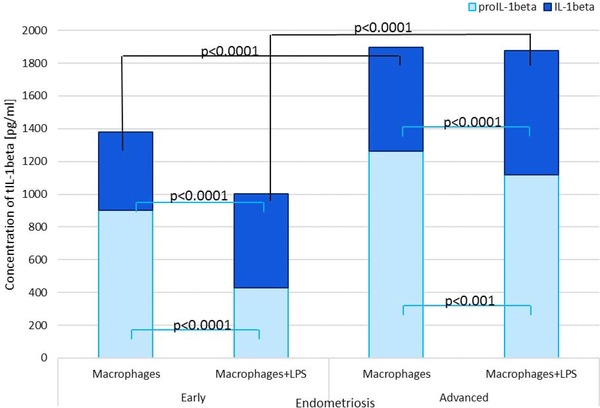
Mean IL‐1β and proIL‐1β levels as a total IL‐1beta in macrophage culture media of women with and without endometriosis.
sIL‐1R2
Concentrations of sIL‐1R2 in PF and macrophage culture medium are presented in Table 1. The mean PF sIL‐1R2 level was statistically significantly lower in women with endometriosis than in the controls (P < 0.0001). Higher concentration of sIL‐1R2 was found in women with the advanced endometriosis, as compared to women with the early disease (P < 0.0001). Decreased sIL‐1R2 production from both basal and stimulated macrophage culture in women with endometriosis compared to the control group was observed (P < 0.0001). There was no statistically significant difference between the group of women with the early endometriosis and advanced endometriosis. After LPS stimulation, only the macrophages of women from the control group produced less sIL‐1R2 (P < 0.001).
Table 1.
sIL‐1R2 Level in PF and Macrophage Culture Media of Women With Endometriosis and Control Group
| Concentration of sIL‐1R2 level (pg/ml) | ||||
|---|---|---|---|---|
| Endometriosis | ||||
| Material | All (n = 30) | Early (n = 18) | Advanced (n = 12) | Control group (n = 25) |
| Peritoneal fluid | 119.08 ± 32.12a | 62.57 ± 11.36a, b | 163.17 ± 29.52a | 217.72 ± 45.77 |
| Basal culture | 24.04 ± 4.29a | 20.75 ± 3.93a, c | 22.16 ± 2.83a | 233.22 ± 28.56 |
| Stimulated culture | 24.85 ± 3.36a | 26.89 ± 2.42a, c | 26.94 ± 3.25a | 146.71 ± 25.70 |
Results are expressed as mean ± SD.
P < 0.0001 compared to control group.
P < 0.0001 compared to advanced endometriosis.
NS compared to advanced endometriosis.
As all studied parameters depend on each other, we evaluated the relations between their concentrations. In the PF of women with endometriosis, we observed a statistically significant positive correlation between IL‐1β and sIL‐1R2 levels (P < 0.001, r = 0.554) and significant positive correlation between proIL‐1β and sIL‐1R2 in PF (P < 0.0001, r = 0.759).
Discussion
The IL‐1 family cytokines are the secretory macrophage products, with IL‐1β being the most important mediator of acute and chronic inflammation and immune response. Defective mechanisms involved in the control of local IL‐1β activity may enhance susceptibility for the adhesion and growth of the ectopic endometrium and it leads to the development of endometriosis 11, 12.
In the present work, for the first time, we measured the concentration of proIL‐1β in PF and macrophage culture media of women with or without endometriosis. Our results showed a higher precursor level in endometriosis patients. It was shaped very similarly to the IL‐1β concentration, so consequently there was a higher tIL‐1β level in endometriosis compared to the tIL‐1β level in healthy women. Peritoneal macrophages of affected women, in contrast to cells from the control group, secreted more proIL‐1β than mature cytokine. After stimulation by LPS, the secretion of IL‐1β was higher in both groups, but the secretion of proIL‐1β was significantly lower. The factor that may have control over the activity of IL‐1 and may be related to proIL‐1β is sIL‐1R2, which prevents IL‐1β from binding to the functional IL‐1R1 and blocks generation effects in target cells. In the present study, we found a decrease in sIL‐1R2 PF level in women with endometriosis compared to the control group. Furthermore, the sIL‐1R2 secretion by macrophages from endometriosis patients was lower compared to the control. LPS stimulation of endometriosis macrophages did not affect the production of sIL‐1R2. It may be due to a lack of culture medium factors that would cause the degradation of the receptor membrane to a soluble form.
The complex formation and secretion of proIL‐1β by macrophages is an important regulatory factor for IL‐1β. So far, there have not been many studies on the participation of the proIL‐1β in the disease pathogenesis. This may be due to the belief that this is only an intracellular factor. It has been proposed that IL‐1β secretion can be through secretory lysosomes called inflammasomes and IL‐1β is linked to ICE activation. The inflammasomes may potentially play an important role in cytokine release 13. The most important inducers of IL‐1β secretion are a reduced intracellular potassium level, adenosine triphosphate, and its purinergic receptor P2X‐7R on macrophages, extracellular LPS, and TNF‐α 14, 15. However, studies suggest that released proIL‐1β may also be secreted and activated extracellularly. Moreover, proIL‐1β can differ significantly from cytosolic proIL‐1β in its ability to be recognized by IL‐1R2 16.
In the available literature, there is no information about the role of proIL‐1β in the formation and development of endometriosis. However, our results showed that proIL‐1β is an important factor involved in the pathogenesis of the disease, and its presence in the PF might be increasing inflammation. Moreover, the overstimulation can cause not only increased synthesis but also the proIL‐1β secretion in endometriosis patients. These observations may be correlated to studies where ICE was found in the PF of women with endometriosis 17. Perhaps, the presence of ICE in PF could lead to the precursor transformation to mature IL‐1β. It may convert inflammation from the acute to the chronic form. The peritoneal environment of women with endometriosis can lead to the accumulation of the immune and inflammatory factors, which then can promote the precursor transformation into mature IL‐1β. Thus, it is an initial assumption that the presentation of our results as tIL‐1β can reflect real peritoneal production and secretion of IL‐1β. It still requires additional studies and it will allow us to confirm our main theory.
The role of sIL‐1R2 in the pathogenesis of endometriosis has been tested in recent years and most authors agree and indicate the decrease in the concentration of this parameter in the fluid from endometriosis patients 18. The reduction of IL‐1R2 expression may be the cause of increased endometrial cell responsiveness to IL‐1β 19, 20. Therefore, in this study we only wanted to show the relationship between the precursor and receptor in the examined women. This is due to the fact that sIL‐1R2 can bind with high‐affinity extracellular proIL‐1β, and in consequence the receptor is protected from proteolysis 21. However, sIL‐1R2 blocks the processing of proIL‐1β and inhibits its maturation, loses affinity for IL‐1Ra, and does not, therefore, interfere with the IL‐1Ra‐mediated inhibition of IL‐1β effects 22. It might suggest that lower sIL‐1R2 levels in PF of endometriosis patient upregulated IL‐1β maturation. However, it should be taken into account that the factors of the same family of IL‐1, as a proIL‐1β, may significantly affect the IL‐1R2 and modify its activity, which requires confirmation in future studies.
Conclusions
Disorders of the mechanisms involved in the secretion of IL‐1β may be an important factor in the development of endometriosis, and the changes in the network of cytokines may promote the development of the disease. The increased secretion of IL‐1β and proIL‐1β may lead to the development of inflammation in the peritoneal cavity of women with endometriosis. The presentation of the results as tIL‐1β can show real production of the cytokine and its participation in disease development. The absence of control over the activity of IL‐1β promotes the secretion of other cytokines and growth factors that do not participate in the removal of ectopic endometrial tissue but also rather support the implantation and growth of the ectopic foci. The imbalance between proIL‐1β, mature molecule, and sIL‐1R2 in the PF of women with endometriosis may be enhancing the ability to transform acute inflammation to the chronic inflammation.
Conflict of Interest
None of the authors has any proprietary, financial, professional, or other personal interest of any nature or kind in any product, service, and/or company.
References
- 1. Garlanda C, Dinarello CA, Mantovani A. The interleukin‐1 family: Back to the future. Immunity 2013;39:1003–1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Sims JE, Smith DE. The IL‐1 family: Regulators of immunity. Nat Rev Immunol 2010;10:89–102. [DOI] [PubMed] [Google Scholar]
- 3. Dinarello CA. Immunological and inflammatory functions of the interleukin‐1 family. Annu Rev Immunol 2009;27:519–550. [DOI] [PubMed] [Google Scholar]
- 4. Boraschi D, Tagliabue A. The interleukin‐1 receptor family. Semin Immunol 2013;25:394–407. [DOI] [PubMed] [Google Scholar]
- 5. Thomas C, Bazan JF, Garcia KC. Structure of the activating IL‐1 receptor signaling complex. Nat Struct Mol Biol 2012;19:455–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Garlanda C, Riva F, Bonavita E, et al. Negative regulatory receptors of the IL‐1 family. Semin Immunol 2013;25:408–415. [DOI] [PubMed] [Google Scholar]
- 7. Akoum A, Al‐Akoum M, Lemay A, et al. Imbalance in the peritoneal levels of interleukin 1 and its decoy inhibitory receptor type II in endometriosis women with infertility and pelvic pain. Fertil Steril 2008;89:1618–1624. [DOI] [PubMed] [Google Scholar]
- 8. Lousse JC, Van Langendonckt A, Defrere S, et al. Peritoneal endometriosis is an inflammatory disease. Front Biosci 2012;4:23–40. [DOI] [PubMed] [Google Scholar]
- 9. Portelli M, Pollacco J, Sacco K, et al. Endometrial seedlings. A survival instinct? Immunomodulation and its role in the pathophysiology of endometriosis.Minerva Ginecol 2011;63:563–570. [PubMed] [Google Scholar]
- 10. American Society for Reproductive Medicine . Revised American Society for Reproductive Medicine classification of endometriosis: 1996. Fertil Steril 1997;67:817–821. [DOI] [PubMed] [Google Scholar]
- 11. Gupta S, Goldberg JM, Aziz N, et al. Pathogenic mechanisms in endometriosis‐associated infertility. Fertil Steril 2008;90:247–257. [DOI] [PubMed] [Google Scholar]
- 12. Khan KN, Kitajima M, Hiraki K, et al. Immunopathogenesis of pelvic endometriosis: Role of hepatocyte growth factor, macrophages and ovarian steroids. Am J Reprod Immunol 2008;60:383–404. [DOI] [PubMed] [Google Scholar]
- 13. Apte RN, Voronov E. Is interleukin‐1 a good or bad guy in tumor immunobiology and immunotherapy? Immunol Rev 2008;222:222–241. [DOI] [PubMed] [Google Scholar]
- 14. Lister MF, Sharkey J, Sawatzky DA, et al. The role of the purinergic P2X7 receptor in inflammation. J Inflamm 2007;4:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wiley JS, Sluyter R, Gu BJ, et al. The human P2×7 receptor and its role in innate immunity. Tissue Antigens 2001;78:321–332. [DOI] [PubMed] [Google Scholar]
- 16. Wewers MD, Winnard AV, Dare HA. Endotoxin‐stimulated monocytes release multiple forms of IL‐1 beta, including a proIL‐1 beta form whose detection is affected by export. J Immunol 1999;162:4858–4863. [PubMed] [Google Scholar]
- 17. Sikora J, Mielczarek‐Palacz A, Kondera‐Anasz Z. Imbalance in cytokines from interleukin‐1 family‐role in pathogenesis of endometriosis. Am J Reprod Immunol 2012;68:138–145. [DOI] [PubMed] [Google Scholar]
- 18. Hou Z, Zhou J, Ma X, et al. Role of interleukin‐1 receptor type II in the pathogenesis of endometriosis. Fertil Steril 2008;89:42–51. [DOI] [PubMed] [Google Scholar]
- 19. Lawson C, Bourcier N, Al‐Akoum M, et al. Abnormal interleukin 1 receptor types I and II gene expression in eutopic and ectopic endometrial tissues of women with endometriosis. J Reprod Immunol 2008;77:75–84. [DOI] [PubMed] [Google Scholar]
- 20. Bellehumeur C, Collette T, Maheux R, et al. Increased soluble interleukin‐1 receptor type II proteolysis in the endometrium of women with endometriosis. Hum Reprod 2005;20:1177–1184. [DOI] [PubMed] [Google Scholar]
- 21. Hailey KL, Li S, Andersen MD, et al. Pro‐interleukin (IL)‐1beta shares a core region of stability as compared with mature IL‐1beta while maintaining a distinctly different configurational landscape: A comparative hydrogen/deuterium exchange mass spectrometry study. J Biol Chem 2009;284:26137–26148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Dunne A, O'Neill LA. The interleukin‐1 receptor/Toll‐like receptor superfamily: Signal transduction during inflammation and host defense. Sci STKE 2003;2003:re3. [DOI] [PubMed] [Google Scholar]


