Abstract
Background
Prolidase is a cytosolic exopeptidase that plays a pivotal role in collagen turnover. Diabetic nephropathy (DN) is associated with structural changes in glomerular basement membrane accompanied with increased amounts of collagen. Prolidase is known to be abundant in kidney and collagen accumulation is increased in DN, so we aimed to determine the value of serum prolidase activity (SPA) in predicting the progression of nephropathy in type 2 diabetes mellitus (DM).
Methods
Thirty type 2 DM patients having microalbuminuria (microalbuminuric group), 30 type 2 DM patients without albuminuria (normoalbuminuric group), and 28 healthy controls (control group) were enrolled. Study groups had similar age, sex distribution, and body mass index (BMI).
Results
Metabolic parameters, SPA and urinary microalbumin were determined. SPA was significantly higher in microalbuminuric group when compared with normoalbuminuric and control groups (P = 0.05 and P < 0.001, respectively). Triglyceride levels were significantly higher and high density lipoprotein cholesterol (HDL‐C) levels were significantly lower in microalbuminuric group compared to control group (Both P < 0.05). SPA showed a negative correlation with HDL‐C level and a positive correlation with urinary albumin excretion (r = –0.219, P < 0.05 and r = 0.39, P < 0.001 respectively). In regression analysis, albumin excretion was the sole parameter influencing SPA.
Conclusion
SPA appears to be higher in type 2 DM patients having microalbuminuria compared to patients without microalbuminuria and healthy controls. The pathophysiological role and the significance of SPA in predicting DN need to be further evaluated.
Keywords: prolidase activity, type 2 diabetes mellitus, diabetic nephropathy, microalbuminuria, high‐density lipoprotein cholesterol
INTRODUCTION
Diabetes mellitus (DM) is the most important leading cause of end‐stage renal disease. Structural and functional changes occur due to chronic hyperglycemia in diabetic nephropathy (DN) and result in increased urinary albumin excretion and decreased glomerular filtration 1, 2. These structural abnormalities include glomerular hypertrophy, thickening of glomerular basement membrane, tubular atrophy, and interstitial fibrosis 1, 3.
Prolidase is a cytosolic exopeptidase that cleaves imidodi‐ and imidotripeptides that have C‐terminal proline or hydroxyproline, and also plays an important role in collagen metabolism, matrix remodeling, and cell growth 4. It is widely distributed in human tissues and the activity has shown to be relatively higher in kidney, intestinal mucosa, and erythrocytes; and lower in liver and plasma 5. One of the major components of glomerular basement membrane is type 4 collagen. Prolidase plays an important role in turnover of type 4 collagen, which contains proline and is mainly enriched with hydroxyproline 6. Several studies have shown that peripheral prolidase activity may reflect the severity of some pathological conditions 7, 8, 9, 10, 11, 12, 13, 14, 15, 16.
Based on the facts that the highest amount of prolidase exists in kidney and collagen content of kidney increases in DN, we hypothesized that measurement of serum prolidase activity (SPA) may reflect the presence of microalbuminuria in DN. Although SPA has been evaluated in several pathological conditions in which collagen turnover increases such as liver fibrosis and malignancy, to our knowledge, SPA has not been evaluated in the early stages of DN.
The exact pathogenesis of DN is still obscure, but microalbuminuria is thought to be one of the possible factors in the pathogenesis. Therefore, we investigated SPA in type 2 DM patients having microalbuminuria and compared it with type 2 DM patients having microalbuminuria and normal controls.
MATERIALS AND METHODS
Study Population
The study was conducted in a prospective manner between June 2008 and January 2010 in endocrinology outpatient unit. Thirty type 2 DM patients having microalbuminuria (albumin‐to‐creatinine ratio (ACR) ranging from 30 to 299 μg/mg; microalbuminuric group) and thirty type 2 DM patients without microalbuminuria (ACR<29 μg/mg) (normoalbuminuric group) were randomly included. Healthy volunteers without renal disease, hepatic diseases, cardiac disease, hypertension, or diabetes were enrolled as a control group (n = 28). These volunteers were chosen by the hospital staff. All groups were similar in terms of age, sex distribution, weight, and body mass index (BMI). Antidiabetic regimens of the two patient groups were similar. Informed consent was obtained from all the participants. Study protocol was approved by the local Ethical Committee.
Presence of chronic diseases (known as malignancy and rheumatological disease), cardiovascular disease, suspicious ischemia on 12‐lead electrocardiography, hematuria, acute febrile diseases, pregnancy, obesity, regular alcohol intake, and smoking were used as exclusion criteria. Since angiotensin‐converting enzyme inhibitors or angiotensin receptor blockers have a potential role in the pathogenesis of DN, patients having controlled blood pressure with other antihypertensive agents were included.
Laboratory Analysis
Blood samples were drawn after an overnight fasting. Samples were centrifuged at 3,000× g for 10 min, and stored at –70°C until analysis. SPA was measured after the 40‐fold dilution of serum with 2.5 mmol/l Mn2+ and 40 mmol/l trizma HCl buffer (pH 8.0) and pre‐incubated at 37°C for 2 hr. Then 1 ml of the reaction complex containing 30 mmol/l gly‐pro, 40 mmol/l trizma HCl buffer (pH 8.0), and 100 μl of pre‐incubation serum was incubated at 37°C for 30 min. The supernatant was used for the measurement of proline according to the method of Myara et al. (17), which is a modification of Chinard's method 18. The intra‐assay coefficient of variation was 3.8%. Urinary samples were drawn on the day of blood sampling and the day after the sampling. Microalbuminuria was measured by immuno‐turbidimetry method (Beckman Coulter System®) in early morning urine samples taken at two separate times.
Urinary creatinine was measured using colorimetric method (Abbott c1600®). Mean microalbuminuria to urinary creatinine ratio was used for determining ACR.
Statistical Analysis
All analyses were conducted using SPSS 11.5 (SPSS Inc., Chicago, IL). Continuous variables were expressed as mean ± SD and categorical variables as percentages. Categorical and continuous variables were compared between the two diabetic groups with or without microalbuminuria by χ2 and independent samples t‐test ,respectively. Comparison of categorical variables between the three groups was determined by χ2 test. Comparison of continuous variables between the three groups was performed using one‐way analysis of variance (ANOVA) and post hoc comparisons were performed using Scheffe procedure. Covariate analysis was performed to determine whether the effect of HDL‐C is present over the significance of the SPA difference between groups. Correlations between SPA and other clinical and laboratory parameters were assessed by Pearson's correlation coefficient. Linear regression analysis was performed to identify factors influencing SPA.
RESULTS
The demographic characteristics and hemodynamic parameters of the study groups are shown in Table 1. Age, sex distribution, weight, body mass index (BMI), systolic blood pressure (SBP), and diastolic blood pressure (DBP) of the three groups were similar. Antidiabetic therapy, number of patients having hypertension (not shown on table), duration of diabetes, fasting plasma glucose (FPG), glycosylated hemoglobin (HbA1c), and C‐peptide levels were similar between the two diabetic groups. FPG levels were significantly higher in both diabetic groups when compared to the control group (both P < 0.001). Serum triglyceride levels were significantly higher and HDL‐C levels were significantly lower in microalbuminuric group when compared with control group (both P < 0.05). Microalbuminuric group had significantly higher ACR than normoalbuminuric and control groups (both P < 0.001). The laboratory parameters are shown in Table 2.
Table 1.
Demographic and Clinical Features of the Study Population*
| Microalbuminuric group (n = 30) | Normoalbuminuric group (n = 30) | Control group (n = 28) | |
|---|---|---|---|
| Age (year) | 48.2 ± 6.4 | 46.3 ± 6.0 | 45.0 ± 6.4 |
| Sex (male/female) | 15/15 | 15/15 | 14/14 |
| Weight (kg) | 73.9 ± 8.2 | 74.6 ± 9.5 | 72.5 ± 10.5 |
| BMI (kg/m2) | 26.7 ± 2.3 | 26.7 ± 1.8 | 25.8 ± 1.9 |
| SBP (mmHg) | 119.3 ± 7.6 | 118.2 ± 5.5 | 118.1 ± 8.9 |
| DBP (mmHg) | 75.5 ± 4.6 | 76.0 ± 4.3 | 75.6 ± 6.9 |
| Antidiabetic therapy | |||
| Insulin | 9 | 3 | — |
| OAD | 21 | 27 | — |
| Duration of DM (year) | 6.6 ± 5.9 | 4.1 ± 3.4 | — |
BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure; DM, diabetes mellitus; OAD, oral antidiabetic drugs.
*All P > 0.05.
Table 2.
Comparison of Laboratory Variables of the Study Groups
| Microalbuminuric group (n = 30) | Normoalbuminuric group (n = 30) | Control group (n = 28) | |
|---|---|---|---|
| FPG (mmol/dl) | 11.43 ± 5.24a | 10.58 ± 4.15b | 4.97 ± 0.44 |
| HbA1c (%) | 9.3 ± 2.6 | 9.5 ± 2.7 | — |
| C‐peptide (nmol/l) | 0.96 ± 0.49 | 1.03 ± 0.63 | — |
| Urea (mmol/l) | 11.0 ± 3.0 | 9.8 ± 2.7 | 9.9 ± 2.2 |
| Creatinine (mmol/l) | 0.07 ± 0.02 | 0.07 ± 0.01 | 0.07 ± 0.01 |
| Albumin (μmol/l) | 667.0 ± 45.0 | 697.0 ± 45.0 | 667.0 ± 45.0 |
| Triglyceride (mmol/l) | 1.97 ± 0.95c | 1.70 ± 0.64 | 1.36 ± 0.68 |
| Total Cholesterol (mmol/l) | 4.52 ± 0.90 | 4.7 ± 0.93 | 4.82 ± 1.01 |
| HDL‐C (mmol/l) | 0.88 ± 0.17d | 0.97 ± 0.20 | 1.08 ± 0.26 |
| LDL‐C (mmol/l) | 2.72 ± 0.67 | 2.94 ± 0.82 | 3.10 ± 0.86 |
| ACR (μg/mg) | 91.8 ± 70.0a, e | 10.2 ± 4.5 | 8.8 ± 5.6 |
| SPA (U/l) | 668.0 ± 14.0a, f | 660.1 ± 11.4 | 654.1 ± 10.4 |
HbA1c, glycosylated hemoglobin; ACR, urine albumin‐to‐creatinine ratio; FPG, fasting plasma glucose; HDL‐C, high‐density lipoprotein cholesterol; LDL‐C, low‐density lipoprotein cholesterol; SPA, serum prolidase activity.
P < 0.001 Microalbuminuric group vs. control group.
P < 0.001 Normooalbuminuric group vs. control group.
P = 0.014 Microalbuminuric group vs. control group.
P = 0.030 Microalbuminuric group vs. control group.
P < 0.001 Microalbuminuric group vs. normoalbuminuric group.
P = 0.043 Microalbuminuric group vs. normoalbuminuric group.
SPA was significantly higher in microalbuminuric group when compared with normoalbuminuric and control groups (P < 0.05 and P < 0.001, respectively; Fig. 1). Significant difference in prolidase levels between groups existed when HDL‐C estimated values were analyzed with covariate analysis (P = 0.001, Partial Eta sq = 0.152). Estimated marginal means of the prolidase levels of groups were almost unchanged (for microalbuminuric group: 668.0 vs. 667.6 U/l, for normoalbuminuric group: 660.1 vs. 660.0 U/l and for control group: 654.1 vs. 654.5 U/l). In correlation analysis, SPA was negatively correlated with HDL‐C levels (r = –0.219, P < 0.05) and positively correlated with ACR (r = 0.394, P < 0.001; Fig. 2). There was no correlation between SPA and any other parameters. In linear regression analysis, ACR was the sole independent predictor of SPA (beta = 0.362, t = 3.391, P < 0.05).
Figure 1.
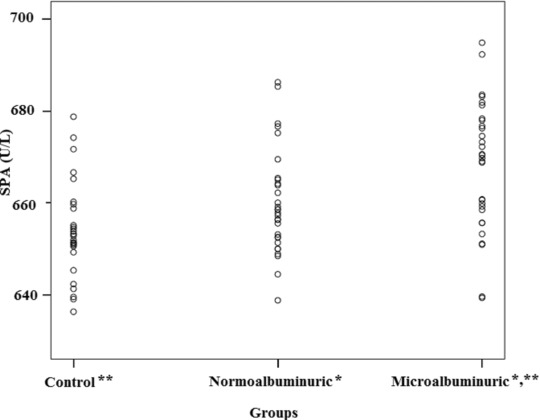
Serum prolidase activities of the study groups (SPA: Serum prolidase activity, *; P < 0.05 Microalbuminuric group vs. normoalbuminuric group, **; P < 0.001 Microalbuminuric group vs. control group).
Figure 2.
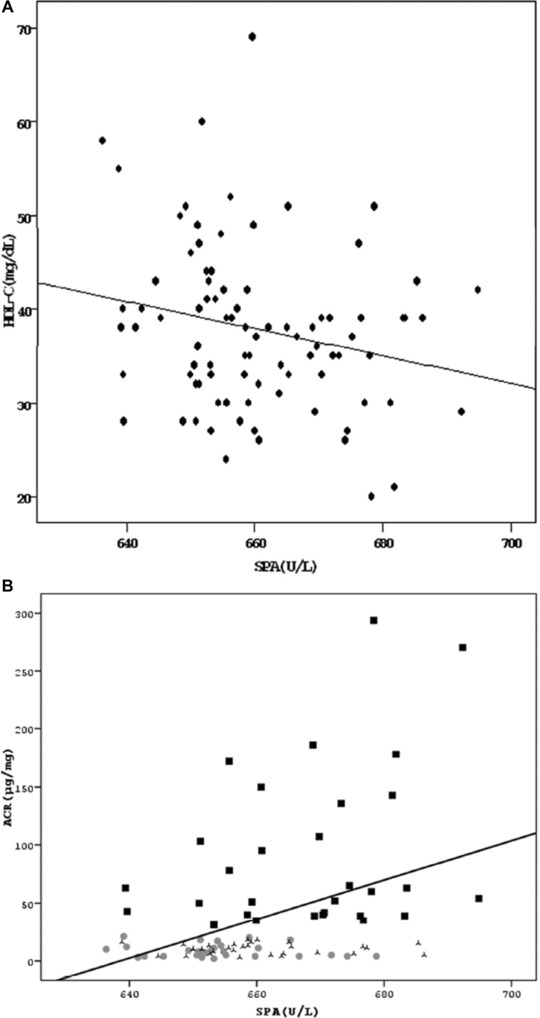
Correlations of serum prolidase activity with (A) HDL‐C and (B) ACR (ACR: Urinary albumin‐to‐creatinine ratio, HDL‐C: High density lipoprotein cholesterol, SPA: Serum prolidase activity).
DISCUSSION
In the current study, we observed that (a) SPA is significantly higher in type 2 DM patients having microalbuminuria compared to patients having normoalbuminuria and healthy controls; (b) SPA was significantly correlated with increased ACR; and (c) SPA was negatively correlated with HDL‐C levels.
Transcription of Type 4 collagen and the total amount of collagen have shown to be increased in DN 19. Microscopic alterations of glomerular basement membrane are early features of DN, which may result in microalbuminuria, followed by overt proteinuria, and eventual renal failure 20. An increase in prolidase enzyme activity correlates with increased rates of collagen turnover 21. Studies have shown that SPA is increased in coronary artery disease, nonalcoholic steatohepatitis, chronic liver disease, osteoarthritis, osteoporosis, Helicobacter pylori gastritis, epithelial ovarian and breast cancers, wound healing, and keloid formation, indicating increased collagen turnover 7, 8, 9, 10, 11, 12, 13, 14, 15, 16. Although SPA in early DN has not been evaluated before, prolidase has been shown to decrease in renal insufficiency possibly related with iminoacidopathy due to chronic uremia 22.
In type 2 diabetic population, SPA has been studied in only one study 11. In their study, Erbagci et al. showed that SPA was lower in diabetic patients than in controls and SPA did not differ between patients with or without nephropathy. In this study, which focused on diabetes‐related osteoporosis, the number of patients with nephropathy and the stage of DN were not clear. In our study, we found SPA of microalbuminuric group to be higher than the group with type 2 DM without microalbuminuria and the control. Microalbuminuria is a relatively early stage of nephropathy and has distinct histological appearance when compared with overt proteinuria. The exact number of patients having overt proteinuria in the former study has not been documented in the study and it limits us to render a conclusion about the role of SPA at different stages of DN. Because of their natural pathological consequences we can only assume that the distinct stages of DN may affect SPA in different ways. Since we focused on DN and had uniform patient groups with and without microalbuminuria, and found SPA elevated in microalbuminuric group when compared with other two study groups, we can conclude that early stages of DN can be predicted by elevated SPA. As the objective of our study is not designed to find the pathological causation between SPA and DN, we cannot speculate whether SPA is a casual factor in DN or not. If we have studied urinary and serum type 4 collagen and laminin sequential with measurements of SPA, the role of SPA in pathogenesis of DN could be more suggestive. However, we did not have opportunity to perform these measurements
In one study, SPA has been found to be significantly associated with the presence and severity of coronary artery disease, suggesting that elevated SPA might be an independent predictor of coronary atherosclerosis 7. Microalbuminuria is known to relate with the increased risk of coronary artery disease in type 2 DM 23, 24. Increased serum SPA in our patients with DN may be related with subclinical atherosclerosis. Lack of angiographic study limits us to deny the impact of subclinical atherosclerosis on high SPA levels that we have obtained in patients with microalbuminuria. Patients with microalbuminuria had significantly higher triglyceride and lower HDL‐C levels when compared with controls. These metabolical parameters are among well‐known cardiovascular risk factors in type 2 DM and the relationship between SPA and HDL‐C may be the result of subclinical atherosclerosis 25, 26, 27. In our study, there is a negative correlation between SPA and HDL‐C, which is concordant with the study in patients with coronary artery disease, but the clinical significance of this finding is not clear 7.
In the present study, we used two consecutive early morning urine samples to detect microalbuminuria. One potential limitation of the study is the lack of determination of microalbuminuria in 24‐hr urine sample. Although spot urinary microalbumin measurement is accepted as a reliable alternative method, 24‐hr urine collection is still the gold standard method in determining microalbuminuria 28.
In conclusion, SPA appears to be higher in type 2 DM patients having microalbuminuria than in patients with normoalbuminuria and healthy controls. This finding may highlight the role of prolidase as a distinct entity in the pathogenesis of DN, which has never been studied before. However, further clinical studies are needed to clarify the predictive and pathophysiological role and significance of SPA in the early phase and the progression of DN.
CONFLICT OF INTEREST
The authors report no conflicts of interest.
REFERENCES
- 1. Mauer SM, Steffes MW, Ellis EN, Sutherland DE, Brown DM, Goetz FC. Structural‐functional relationships in diabetic nephropathy. J Clin Invest 1984;74(4):1143–1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Soldatos G, Cooper ME. DN: Important pathophysiologic mechanisms. Diabetes Res Clin Pract 2008;82:75–79. [DOI] [PubMed] [Google Scholar]
- 3. Tsilibary EC. Microvascular basement membranes in diabetes mellitus. J Pathol 2003;200:537–546. [DOI] [PubMed] [Google Scholar]
- 4. Surazynski A, Miltyk W, Palka J, Phang JM. Prolidase‐dependent regulation of collagen biosynthesis. Amino Acids 2008;35:731–738. [DOI] [PubMed] [Google Scholar]
- 5. Hui KS, Lajtha A. Prolidase activity in brain: Comparison with other organs. J Neurochem 1978;30:321–327. [DOI] [PubMed] [Google Scholar]
- 6. Levidiotis V, Power DA. New insights into the molecular biology of the glomerular filtration barrier and associated disease. Nephrology (Carlton) 2005;10(2):157–166. [DOI] [PubMed] [Google Scholar]
- 7. Yildiz A, Demirbag R, Yilmaz R, et al. The association of serum prolidase activity with the presence and severity of coronary artery disease. Coron Artery Dis 2008;19:319–325. [DOI] [PubMed] [Google Scholar]
- 8. Horoz M, Aslan M, Bolukbas FF, et al. Serum prolidase enzyme activity and its relation to histopathological findings in patients with non‐alcoholic steatohepatitis. J Clin Lab Anal 2010;24:207–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Myara I, Myara A, Mangeot M, Fabre M, Charpentier C, Lemonnier A. Plasma prolidase activity: A possible index of collagen catabolism in chronic liver disease. Clin Chem 1984;30:211–215. [PubMed] [Google Scholar]
- 10. Altindag O, Erel O, Aksoy N, Selek S, Celik H, Karaoglanoglu M. Increased oxidativestress and its relation with collagen metabolism in knee osteoarthritis. Rheumatol Int 2007;27:339–344. [DOI] [PubMed] [Google Scholar]
- 11. Erbagci AB, Araz M, Erbagci A, Tarakçioğlu M, Namiduru ES. Serum prolidase activity as a marker of osteoporosis in type 2 diabetes mellitus. Clin Biochem 2002;35:263–268. [DOI] [PubMed] [Google Scholar]
- 12. Aslan M, Nazligul Y, Horoz M, et al. Serum prolidase activity and oxidative status in Helicobacter pylori infection. Clin Biochem 2007;40:37–40. [DOI] [PubMed] [Google Scholar]
- 13. Camuzcuoglu H, Arioz DT, Toy H, Kurt S, Celik H, Aksoy N. Assessment of preoperative serum prolidase activity in epithelial ovarian cancer. Eur J Obstet Gynecol Reprod Biol 2009;147:97–100. [DOI] [PubMed] [Google Scholar]
- 14. Cechowska‐Pasko M, Palka J,Wojtukiewicz MZ. Enhanced prolidase activity and decreased collagen content in breast cancer tissue. Int J Exp Pathol 2006;87:289–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Senboshi Y, Oono T, Arata J. Localization of prolidase gene expression in scar tissue using in situ hybridization. J Dermatol Sci 1996;12:163–171. [DOI] [PubMed] [Google Scholar]
- 16. Duong HS, Zhang QZ, Le AD, Kelly AP, Kamdar R, Messadi DV. Elevated prolidase activity in keloids: Correlation with type I collagen turnover. Br J Dermatol 2006;154:820–828. [DOI] [PubMed] [Google Scholar]
- 17. Myara I, Charpentier C, Lemonnier A. Optimal conditions for prolidase assay by proline colorimetric determination: Application to imminodipeptiduria. Clin Chim Acta 1982;125:193–205. [DOI] [PubMed] [Google Scholar]
- 18. Chinard FP. Photometric estimation of proline and ornithine. J Biol Chem 1952;199:91–95. [PubMed] [Google Scholar]
- 19. Adler SG, Feld S, Striker L, et al. Glomerular type IV collagen in patients with diabetic nephropathy with and without additional glomerular disease. Kidney Int 2000;57:2084–2092. [DOI] [PubMed] [Google Scholar]
- 20. Cooper ME. Pathogenesis, prevention, and treatment of diabetic nephropathy. Lancet 1998;352:213–219. [DOI] [PubMed] [Google Scholar]
- 21. Palka JA, Phang JM. Prolidase activity in fibroblasts is regulated by interaction of extracellular matrix with cell surface integrin receptors. J Cell Biochem 1997;67:166–175. [DOI] [PubMed] [Google Scholar]
- 22. Gejyo F, Kishore BK, Arakawa M. Prolidase and prolinase activities in the erythrocytes of patients with chronic uremia. Nephron 1983;35(1):58–61. [DOI] [PubMed] [Google Scholar]
- 23. Park HY, Schumock GT, Pickard AS, Akhras K. A structured review of the relationship between microalbuminuria and cardiovascular events in patients with diabetes and hypertension. Pharmacotherapy 2003;23:1611–1666. [DOI] [PubMed] [Google Scholar]
- 24. Drury PL, Ting R, Zannino D, et al. Estimated glomerular filtration rate and albuminuria are independent predictors of cardiovascular events and death in type 2 diabetes mellitus: The Fenofibrate Intervention and Event Lowering in Diabetes (FIELD) study. Diabetologia 2011;54(1):32–43. [DOI] [PubMed] [Google Scholar]
- 25. Gervaise N, Garrigue MA, Lasfargues G, Lecomte P. Triglycerides, apo C3 and Lp B:C3 and cardiovascular risk in type II diabetes. Diabetologia 2000;43:703–708. [DOI] [PubMed] [Google Scholar]
- 26. Laakso M, Lehto S, Penttilä I, Pyörälä K. Lipids and lipoproteins predicting coronary heart disease mortality and morbidity in patients with non‐insulin‐dependent diabetes. Circulation 1993;88:1421–1430. [DOI] [PubMed] [Google Scholar]
- 27. Turner RC, Millns H, Neil HA, et al. Risk factors for coronary artery disease in noninsulin dependent diabetes mellitus: United Kingdom Prospective Diabetes Study (UKPDS:23). BMJ 1998;316:823–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Witte EC, Lambers Heerspink HJ, de Zeeuw D, Bakker SJ, de Jong PE, Gansevoort R. First morning voids are more reliable than spot urine samples to assess microalbuminuria. J Am Soc Nephrol 2009;20:436–443. [DOI] [PMC free article] [PubMed] [Google Scholar]


