Abstract
Background
Psoriasis is a chronic and recurrent inflammatory skin disease. Previous studies have shown that bilirubin has anti‐inflammation and antioxidant effects. However, the various roles of bilirubin in psoriasis patients are still unclear.
Objective
To investigate the serum total bilirubin (TB) level in the individuals with psoriasis vulgaris and further evaluate the relationship between serum TB concentration and C‐reactive protein (CRP) to clarify the effect of bilirubin on inflammation.
Methods
A total of 214 patients with psoriasis vulgaris and 165 age‐ and gender‐matched healthy control subjects were recruited. The peripheral leukocyte count (white blood cell, WBC) and differential, serum biochemical and immunologic indexes including serum TB, immunoglobulin (Ig) G, IgA, IgM, complement C3 and C4, as well as serum CRP concentrations were measured.
Results
Results showed that the serum TB level decreased significantly and peripheral WBC, neutrophil, and serum CRP concentrations increased significantly in patients with psoriasis vulgaris. Meanwhile, the serum CRP was negatively correlated with serum TB levels but positively correlated with peripheral WBC and the Psoriasis Area and Severity Index (PASI). Logistic regression analysis showed that the serum TB was a protective factor for psoriasis vulgaris.
Conclusion
The present study suggests that lower serum TB is associated with the enhancement of the inflammatory response in psoriasis vulgaris. Therefore, lower serum TB has a prognostic significance for worsening psoriasis vulgaris. Bilirubin may play a crucial role in inflammation by contributing to the inhibition of the inflammatory response.
Keywords: psoriasis vulgaris; bilirubin, inflammation; CRP; logistic regression
INTRODUCTION
Psoriasis is an immune‐mediated chronic and recurrent inflammatory skin disease, affecting approximately 2% of the population in the world and impacting the patients’ quality of life 1. The C‐reactive protein (CRP) is an important inflammatory biomarker, produced and released by the liver under the stimulation of inflammatory mediators 2. Increased serum concentration of CRP reflects activation of systemic inflammation in the body 2, 3. The substantial inflammatory role played by CRP in the pathogenesis of psoriasis has been studied in several clinical investigations, and there is increasing evidence that the serum CRP level is significantly elevated in psoriasis patients and closely associated with the severity of psoriasis 3, 4.
The amount of circulating leukocytes in circulation plays a central role in inflammatory diseases. Increased peripheral leukocyte counts are correlated with the intensity of the inflammatory response and adverse outcomes 5. Similar to any inflammatory disease, psoriasis often induces an increase in blood leukocytes, especially neutrophils (NEUT). Several clinical studies reported increased levels of NEUT in the peripheral blood of these patients 2, 4. The activation of NEUT triggers a series of physiological and pathological responses including deregulation, enzyme release, and generation of reactive oxygen species (ROS). This has been shown to mediate the inflammatory process and greatly enhance the inflammatory response, and contribute to tissue damage 6. To counterbalance the injurious effects of the oxidants in inflammation, there are endogenous antioxidant systems in human body, which may promote the detoxification of ROS. Once the balance between the two systems is broken, enhanced tissue inflammation will occur and the psoriasis will worsen 7.
Oxidative stress is pathologically essential to process various inflammatory diseases 8, 9, 10. Bilirubin, a potent antioxidant under physiological conditions by inhibiting oxidation, has anti‐inflammatory effects 11, 12. Rapid regeneration of bilirubin by biliverdin reductase is enough to protect cells and tissues against the oxidants 13. The above‐mentioned mechanism may also help bilirubin to play a direct anti‐inflammatory role 14, 15. The bilirubin has been proved to act against atherosclerosis (AS) that is closely associated with chronic vascular wall inflammation 16. Several studies further revealed a relationship between bilirubin and AS diseases 17, 18. There is evidence that a decreased concentration of serum bilirubin is indicative of an elevated clinical risk of developing AS 19. The oxidative stress in inflammation is one of the main pathogenesis processes in psoriasis 20. As an important endogenous antioxidant, bilirubin can inhibit the inflammation in tissues 14, 15, 21. There are several studies trying to shed light on the change of serum bilirubin in psoriasis. However, the outcomes have been inconsistent 22, 23, 24, 25.
Although the antioxidant effects as well as the protective role against inflammation of bilirubin have been extensively explored in both retrospective and prospective studies 12, 13, 17, 18, 19, the change of serum bilirubin concentration and its relation to inflammation in psoriasis have not yet been elucidated. Our objective was to investigate the change of serum total bilirubin (TB) in psoriasis vulgaris patients to evaluate the anti‐inflammatory role of bilirubin in psoriasis.
MATERIALS AND METHODS
Study Subjects
This study had been approved by the Ethical Committee of Capital Medical University and Beijing Traditional Chinese Medicine (TCM) Hospital. A written informed consent was obtained from all participants prior to enrollment. The principle of the Helsinki Declaration for using human subjects was obeyed. A total of 214 patients with psoriasis vulgaris (55 females and 159 males) from the Department of Dermatology, TCM Hospital, were enrolled as the patient group. The diagnosis of psoriasis was based on clinical findings and laboratory examinations. Meanwhile, 165 healthy subjects (56 females and 109 males) were enrolled as controls. A questionnaire interview was conducted to collect individual information. Both patients and control subjects were then physically examined. Those with a past/present medical history of liver, renal, cardiovascular and cerebrovascular diseases, heart failure, peripheral arterial disease, hypertension, hypercholesterolemia, hypothyroidism, obstructive sleep apnea, diabetes, insulin resistance, chronic inflammatory disease, such as rheumatic arthritis, system lupus erythematous, inflammatory bowel disease, cutaneous lymphoma, nonmelanoma skin cancer, or any kinds of tumor, drinking and smoking were excluded from the study. The controls also had no history of any skin disease and presented normal hematological, biochemical, and immunological index values. In the patient group, the patients have no clinical finding of joint complaints and radiological evidence of psoriatic arthritis (PsA), the subjects of psoriasis coexisting PsA, and the individuals with dermatologic diseases other than psoriasis were also excluded. In addition, none of the psoriasis patients had received any systemic or specific nutritional supplement or medication, namely anti‐inflammatory drugs, antioxidants, local steroid medication, or any phototherapy treatment for at least 1 month prior to blood collection. Psoriasis severity was evaluated by the Psoriasis Area and Severity Index (PASI) presented at the time of blood collection 7. In order to diminish the subjectivity, the PASI of psoriasis patients was evaluated by the same group of dermatologist.
Blood Samples Collection
All blood samples were obtained by venipuncture and collected into vacuum blood collection tubes (INSEPACK®, Sekisui, Beijing). Peripheral whole blood samples were collected into tubes containing EDTA‐K+2. The peripheral leukocyte parameters were analyzed within 2 h after the blood sample collection. Serum samples were obtained by centrifugation of blood samples for 10 min at 3,500 rpm to obtain the cellular components. Subsequently, the serum samples were stored at −80°C until analysis.
Peripheral Blood WBC Parameter Analysis
The peripheral white blood cell (WBC) parameters including leukocyte count and differential count were determined with the LH780 hematology auto‐analyzer (Beckman Coulter Inc. Brea, CA, USA).
Serum Immunological Index Detection
The serum CRP, immunoglobulin (Ig) G, IgM, IgA and complement C3, C4 concentrations were measured with rate turbidimetry and nephelometry. The reagent kits were purchased from the Beckman Coulter, Inc. (Brea, CA, USA) and measured with the IMMAGE 800 Immunochemistry System (Beckman Coulter, Inc., Brea, CA, USA).
Serum ALT, CK, Cre, and TB Determination
The serum alanine aminotransferase (ALT), creatine kinase (CK), and creatinine (Cre) were measured with the enzyme kinetics assay. The ALT kit was purchased from the Beckman Coulter, Inc. (Brea, CA, USA); the CK kit was purchased from the Diasys Diagnostic Systems GmbH (Holzheim, Germany) and Cre kit was purchased from the Merit Choice Bioengineering Co., Ltd. (Beijing, China). The serum TB level was measured with the diazo colorimetric assay using a kit purchased from the Merit Choice Bioengineering Co., Ltd.. All the four indexes were measured on the Beckman Coulter AU5821 automatic analyzer (Beckman Coulter, Inc., Brea, CA, USA).
Statistical Analysis
The Statistical Package of Social Science (SPSS) 17.0 for Windows (SPSS Inc., Chicago, IL) was used for statistical analysis. The normality of data was tested by one sample Kolmogorov–Smirnov test. For the normal data, unpaired t‐test was used for comparison between the two groups, while Mann–Whitney U‐test was used for the data abnormally distributed. The χ2 test was used for comparing the dichotomous variables of the two groups, while Spearman correlation was performed in bivariate analyses. The influence of analyzed parameters including the age, gender, peripheral blood WBC parameter, serum TB, and immunological index on psoriasis was assessed by binary logistic regression analyses. For all tests, two‐tailed P values were reported and the results were considered statistically significant when P < 0.05.
RESULTS
General Information of Psoriasis Patients and Controls
General information on the subjects of this study was summarized in Table 1. The psoriasis vulgaris patients and control subjects appeared to adequately match with respect to age and gender. There was no significant difference in age and gender distributions between the psoriasis patient group and controls.
Table 1.
Subject Characteristics of Psoriasis Patients and Controls
| Psoriasis patients (n = 214) | Controls (n = 165) | |||||
|---|---|---|---|---|---|---|
| Mean (SD) | Media (IR) | Mean (SD) | Media (IR) | P‐value | ||
| Age | year | 41.02 (12.55) | 44.00 (20.30) | 42.22 (9.68) | 42.00 (14.00) | 0.116 |
| Gender | M/F (%) | 159/55 (74.30/25.70) | 109/56 (66.06/33.94) | 0.081 | ||
| PASI | – | 15.76 (7.35) | 15.8 (9.00) | – | – | – |
| Peripheral Blood WBC count and classification | ||||||
| WBC | (×109/l) | 6.94 (1.88) | 6.50 (2.31) | 6.17 (1.51) | 6.00 (2.06) | <0.001 |
| Neutrophil granulocyte | (%) | 61.53 (7.94) | 62.20 (10.00) | 58.03 (6.87) | 58.30 (9.60) | 0.002 |
| Neutrophil granulocyte | (×109/l) | 4.30 (1.39) | 4.15 (1.79) | 3.62 (1.17) | 3.53 (1.35) | <0.001 |
| Lymphocyte | (%) | 28.67 (8.83) | 27.40 (11.90) | 32.51 (6.65) | 32.20 (9.10) | 0.003 |
| Lymphocyte | (×109/l) | 1.95 (0.78) | 1.85 (0.87) | 1.98 (0.51) | 2.04 (0.61) | 0.075 |
| Mononuclear leukocyte | (%) | 5.97 (1.95) | 5.45 (2.30) | 6.73 (2.45) | 6.10 (2.20) | <0.001 |
| Mononuclear leukocyte | (×109/l) | 0.41 (0.18) | 0.37 (0.19) | 0.41 (0.15) | 0.39 (0.17) | 0.710 |
| Eosinophilic granulocyte | (%) | 4.07 (3.82) | 3.00 (2.70) | 2.00 (1.01) | 1.80 (0.90) | <0.001 |
| Eosinophilic granulocyte | (×109/l) | 0.31 (0.46) | 0.21 (0.20) | 0.12 (0.06) | 0.10 (0.06) | <0.001 |
| Serum biochemical and immunological index | ||||||
| Alanine aminotransferase | U/l | 23.81 (18.86) | 18.00 (16.00) | 22.51 (14.93) | 17.00 (15.00) | 0.594 |
| Creatine kinase | U/l | 75.95 (46.11) | 64.00 (38.00) | 78.40 (23.95) | 74.50 (30.00) | 0.721 |
| Creatinine | μmol/l | 71.83 (14.71) | 71.00 (23.00) | 68.04 (15.14) | 68.00 (26.00) | 0.095 |
| Total bilirubin | μmol/l | 11.69 (4.47) | 10.95 (5.20) | 13.64 (5.75) | 13.18 (7.80) | <0.001 |
| Immunoglobulin G | g/l | 12.21 (2.53) | 12.00 (3.20) | 11.33 (1.86) | 11.40 (2.40) | 0.002 |
| Immunoglobulin A | g/l | 2.60 (1.35) | 2.33 (1.41) | 2.26 (0.80) | 2.17 (0.99) | 0.064 |
| Immunoglobulin M | g/l | 0.89 (0.34) | 0.83 (0.43) | 1.02 (0.41) | 0.95 (0.54) | 0.001 |
| Complement C3 | g/l | 1.16 (0.24) | 1.13 (0.28) | 1.16 (0.25) | 1.12 (0.37) | 0.890 |
| Complement C4 | g/l | 0.28 (0.20) | 0.26 (0.09) | 0.25 (0.10) | 0.23 (0.07) | 0.020 |
| C‐reactive protein | mg/dl | 9.17 (14.98) | 3.37 (7.02) | 2.66 (2.47) | 2.11 (1.96) | <0.001 |
χ2 test, nonparametric test (Mann–Whitney test), or Student's t‐test were used, the SD is the standard deviation and IR is the interquartile range.
Peripheral Blood Leukocyte Count and Differential Count
The peripheral blood WBC (leukocyte) count and differential count are shown in Table 1. The peripheral blood leukocyte count of psoriasis patients was significantly higher than the controls. Meanwhile the differential count of peripheral blood leukocytes of psoriasis vulgaris patients showed that the absolute count and percentage of NEUT and eosinophils (EOS) were significantly higher than the controls (P < 0.001), while the percentage of lymphocytes (LYM) and monocytes (MONO) in psoriasis vulgaris patients were obviously lower than the controls (P < 0.001).
The Serum ALT, CK, Cre, and TB Levels
There was no significant difference in the serum ALT, CK, and Cre level between psoriasis patients and controls (P > 0.05; Table 1). The serum TB concentration of psoriasis patients was obviously lower than the controls (P < 0.05).
The CRP, IgG, IgM, IgA, and Complement C3, C4 Concentrations
Results of immunological marker levels between the psoriasis group and controls are shown in Table 1. The serum CRP, IgG, and complement C4 concentrations of psoriasis vulgaris patients were significantly higher than the controls (P < 0.05).
Correlations Among the Serum CRP, Serum TB levels, and Peripheral Blood WBC Parameters as well as Immunological Index in Psoriasis Patients
In psoriasis vulgaris patients, the peripheral blood WBC count was positively correlated with serum CRP concentration (Fig. 1; r = 0.297, P < 0.001) and the serum TB level was negatively correlated with the serum CRP concentration (Fig. 2; r = −0.145, P = 0.036). The correlations of serum CRP as well as serum TB concentrations with peripheral blood WBC count, differential count, and immunological indexes in psoriasis vulgaris patients are shown in Table 2. The serum CRP level was significantly positively correlated with the count and percentage of peripheral blood NEUT, MONO, and negatively correlated with LYM absolute count and percentage. The serum CRP level was also significantly positively correlated with the serum IgA, C3 and C4 concentrations. Meanwhile, the serum TB level was significantly correlated with the serum IgA concentrations.
Figure 1.
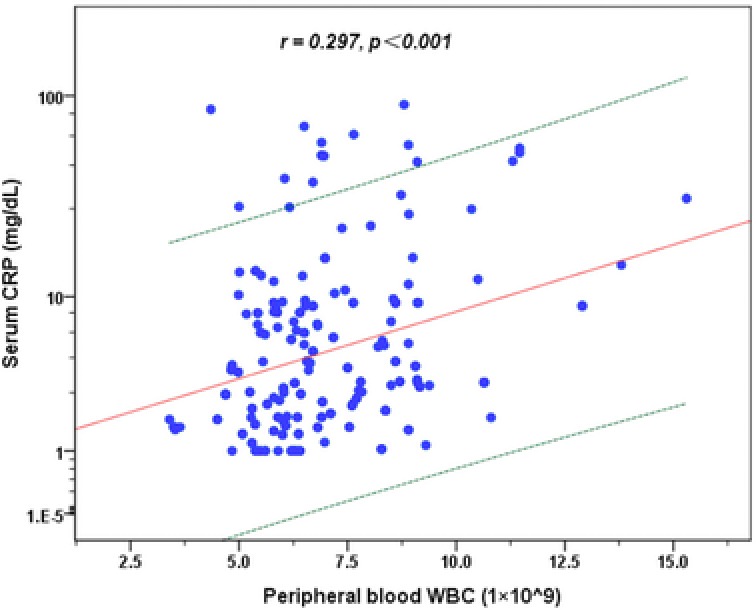
Correlation between serum CRP concentration and peripheral blood WBC count in psoriasis vulgaris patients. Regression prediction line and 95% individual confidence intervals (dotted line) are provided.
Figure 2.
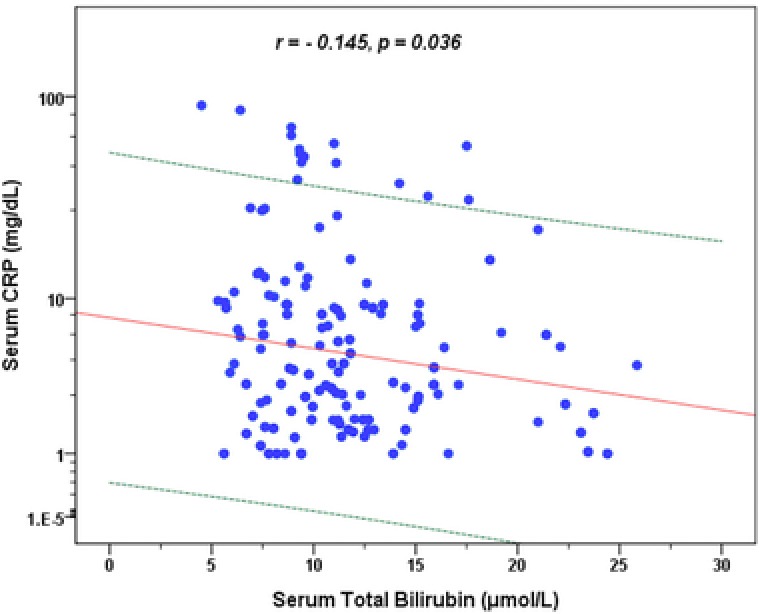
Correlation between serum CRP and TB concentration in psoriasis vulgaris patients. Regression prediction line and 95% individual confidence intervals (dotted line) are provided.
Table 2.
The Relationship Between Peripheral Blood WBC Classification as well as Immunological Index With Serum CRP and TB Concentrations in Psoriasis Patients
| Serum CRP | Serum TB | |||
|---|---|---|---|---|
| r | P‐value | r | P‐value | |
| Peripheral blood WBC count and classification | ||||
| Neutrophil granulocyte count | 0.431 | 0.000 | 0.067 | 0.331 |
| Neutrophil granulocyte (%) | 0.468 | 0.000 | −0.059 | 0.393 |
| Lymphocyte count | −0.209 | 0.002 | 0.059 | 0.393 |
| Lymphocyte (%) | −0.469 | 0.000 | 0.030 | 0.662 |
| Mononuclear leukocyte count | 0.375 | 0.000 | −0.008 | 0.910 |
| Mononuclear leukocyte (%) | 0.257 | 0.000 | −0.120 | 0.082 |
| Eosinophilic granulocyte count | 0.157 | 0.022 | 0.152 | 0.068 |
| Eosinophilic granulocyte (%) | 0.083 | 0.225 | 0.131 | 0.058 |
| Serum immunological index | ||||
| Immunoglobulin G | 0.066 | 0.337 | −0.051 | 0.461 |
| Immunoglobulin A | 0.233 | 0.001 | −0.229 | 0.001 |
| Immunoglobulin M | −0.087 | 0.209 | −0.152 | 0.058 |
| Complement C3 | 0.378 | 0.000 | −0.109 | 0.116 |
| Complement C4 | 0.449 | 0.000 | −0.128 | 0.066 |
The Spearman correlation was used. P < 0.05 is considered statistically significant.
Correlations Between the Serum CRP, Serum TB levels, and PASI in Psoriasis Vulgaris Patients
In the psoriasis patients, the serum CRP was significantly positively correlated with the PASI (r = 0.172, P = 0.012), whereas the serum TB was negatively correlated with the PASI but without significant (r = −0.125, P = 0.070).
Logistic Regression Analysis
The result of binary logistic regression analysis is shown in Table 3. The χ2 value of this logistic model was 208.796 (P < 0.001), −2 log‐likelihood was 286.114, and the overall predicted correct percentage of the model was 81.8 %. The results of logistic analyses showed that the serum TB was the protective factor (OR = 0.859, P < 0.001, 95 % CI of OR was 0.797–0.926) and the serum CRP was risk factor (OR = 1.259, P = 0.001, 95 % CI of OR was 1.104–1.436) for psoriasis vulgaris.
Table 3.
Logistic Regression Analysis of Psoriasis Patients and Controls
| Parameters | B | SE | Wald | OR | 95% CI for OR | P‐value |
|---|---|---|---|---|---|---|
| Age | 0.007 | 0.014 | 0.265 | 1.007 | 0.980–1.036 | 0.607 |
| Gender | 0.306 | 0.322 | 0.906 | 1.358 | 0.723–2.550 | 0.341 |
| WBC count | −1.084 | 0.607 | 3.192 | 0.338 | 0.103–1.111 | 0.074 |
| NEUT count by NEUT % | 0.021 | 0.010 | 4.277 | 1.021 | 1.001–1.041 | 0.039 |
| LYM count by LYM % | 0.033 | 0.018 | 3.248 | 1.033 | 0.997–1.071 | 0.071 |
| MONO count by MONO % | −0.345 | 0.129 | 7.123 | 0.708 | 0.549–0.912 | 0.008 |
| EOS count by EOS % | 2.972 | 0.507 | 34.339 | 19.530 | 7.228–52.770 | 0.000 |
| Serum TB | −0.152 | 0.038 | 15.749 | 0.859 | 0.797–0.926 | 0.000 |
| Serum IgG | 0.340 | 0.090 | 14.402 | 1.404 | 1.179–1.674 | 0.000 |
| Serum IgA | −0.029 | 0.203 | 0.020 | 0.972 | 0.652–1.447 | 0.887 |
| Serum IgM | −1.095 | 0.442 | 6.132 | 0.335 | 0.141–0.796 | 0.013 |
| Serum C3 | −1.813 | 0.839 | 4.675 | 0.163 | 0.032–0.844 | 0.031 |
| Serum C4 | 1.726 | 2.877 | 0.360 | 5.618 | 0.020–1,578.294 | 0.549 |
| Serum CRP | 0.231 | 0.067 | 11.823 | 1.259 | 1.104–1.436 | 0.001 |
| Constant | −0.965 | 1.546 | 0.390 | 0.381 | – | 0.532 |
P < 0.05 is considered statistically significant. B, estimated coefficient; SE, standard error; OR, odds ratio; CI, confidence interval.
DISCUSSION
During an inflammatory process, several inflammatory factors induce NEUT activation an in turn the release of activation products. This phenomenon seems to increase serum CRP and may contribute to the pathophysiology of psoriasis 2, 3. A recent study reported that elevated levels of serum CRP is a risk factor for chronic inflammatory diseases, such as cardiovascular diseases, and may predict a long‐term risk of cardiovascular events 26, 27, 28, 29, 30, 31. CRP itself may play an active role in inflammation by inhibiting NEUT chemotaxis and endothelial cell adhesion, reducing transmigration to the site of inflammation 26, 27.
It has been observed that psoriasis is associated with inflammatory conditions 30, 31. Earlier studies suggested that there is a strong linkage between the severity of psoriasis and enhancement in inflammatory response. The serum CRP level is closely associated with PASI in psoriasis 32, 33, and previously we have shown that there was a significant positive correlation between serum CRP levels and PASI scores in psoriasis patients. The present study reconfirmed the results. Therefore, the serum CRP could be used as an accurate and sensitive blood biomarker to evaluate and monitor the severity of psoriasis during diagnosis and treatment in clinic 34, 35.
In the case of inflammation, the NEUT seem to play a crucial role by contributing to the development of oxidative stress 2. The deterioration of psoriasis seems to be linked to an imbalance between enhanced inflammation and their inhibitors. The insufficiency of the antioxidant defense in psoriasis may lead to an enhanced and uncontrolled inflammatory process 7. Several conditions may trigger the enhancement of psoriasis, such as infections, skin traumas, and stress conditions. All of them could induce an additional inflammatory stimulus, which may disrupt the fragile balance between the inflammation products and counterbalancing of endogenous anti‐inflammatory factors 7. In this study, the peripheral WBC and NEUT were significantly increased in patients with psoriasis vulgaris, which suggest that the number of peripheral blood WBC and NEUT were closely related to the inflammatory condition in psoriasis vulgaris. Our data confirmed that the psoriasis patients were in a more severe inflammatory condition, as indicated by the significant increase in CRP levels in serum, blood total leucocytes, and NEUT compared to controls. Actually, we found that serum CRP was correlated positively and significantly with total leucocytes and NEUT in psoriasis vulgaris patients.
Several studies have shown an increased risk of cardiovascular disease in psoriasis patients 36, 37. Balta et al. reported that the psoriasis was significantly associated with subclinical AS even when the patient's PASI scores are lower 38, 39. Meanwhile, the epidemiological evidence suggested that lower TB was implicated in AS development 17, 18. Previous studies also demonstrated that lower serum TB was significantly related to oxidative and inflammatory conditions, while higher serum bilirubin could inhibit inflammation 40, serum bilirubin level was significantly negatively correlated with the artery intima media thickness (IMT) in nondiabetic and Type 2 diabetic subjects 41. These results further suggest that the bilirubin has significant anti‐inflammatory effect. Although the exact role of bilirubin on inflammation development in psoriasis remains unclear, given the remarkable anti‐inflammatory properties, bilirubin may inhibit oxidative stress and inflammation in psoriasis. There are only a few studies that have investigated the serum bilirubin level in psoriasis and are related with inflammation. However, the controversy exists regarding the change in serum bilirubin in psoriasis. Severin et al. and Coimbra et al. reported that the serum bilirubin level was slightly or significantly elevated in psoriasis patients 22, 25, whereas Balta et al. and Nemati et al. found that the serum bilirubin was lower or significantly decreased in psoriasis patients (23, 24). The result of those studies suggests that the serum bilirubin in psoriasis patients has been consistent and inconclusive. Furthermore, the relationship between the serum bilirubin and inflammation has also not been demonstrated clearly. Consequently, the clinical relevance of serum bilirubin with respect to inflammation in psoriasis is still debatable and should be investigated further.
We hypothesized that lower serum TB enhances chronic inflammation in psoriasis subjects, and the psoriasis individuals with a higher serum bilirubin level would be less likely to be vulnerable to the inflammatory mediators. In order to explore the possible relationship between chronic inflammation and bilirubin in psoriasis, we investigated the serum TB and CRP concentration in psoriasis vulgaris. Our results showed that the serum TB level of psoriasis vulgaris patients was obviously lower than the controls. It is noteworthy that we did identify a strong inverse correlation between serum TB and CRP levels in psoriasis vulgaris patients. The logistic analyses also showed that the serum TB was the protective factor, while the serum CRP was a risk factor for psoriasis vulgaris. These results further support the theory that bilirubin may suppress the inflammatory effects in psoriasis vulgaris.
The PASI score, the most common outcome measure in clinical practice of psoriasis, is a nonlinear scale that does not allow reliable assessment of subtle variations of erythema, induration, and desquamation 42. Meanwhile, the definitions of its constituent components require an estimation of the percentage body surface area, which is easily influenced by environmental factors and is difficult to measure accurately 43. Therefore, the PASI cannot reflect the severity of inflammation accurately in psoriasis. The present study also showed that the serum TB was insignificantly negatively related to the PASI in psoriasis patients.
Our study suggested that the serum TB may be a helpful biomarker in prediction and monitoring the inflammatory effects of psoriasis vulgaris patient in clinical practice. However, the present investigation has several limitations. First, we only observed the effects of serum TB on inflammatory condition in psoriasis vulgaris subjects and did not evaluate the effect of other antioxidants, such as vitamins B, C, E, folic acid, and glutathione, as well as selenium etc. Previous studies have shown that those substances also have anti‐oxidative or anti‐inflammatory effects, and have the potential to act synergistically, however further investigation is required. Second, all subjects in this study were Chinese, therefore the results may be different in other ethnic populations. Third, the number of psoriasis vulgaris subjects in this study was relatively small, further investigations with larger psoriasis vulgaris population are needed. Despite these limitations, our results still suggested that lower serum bilirubin level might enhance inflammation in psoriasis, which may in turn potentiate the tissue damage. The bilirubin may play an important role in the inflammatory process in psoriasis; lower concentrations of serum bilirubin may be a potential risk factor for psoriasis vulgaris patients.
CONCLUSION
The present study shows that lower serum TB is associated with the enhancement of inflammatory response in psoriasis vulgaris. Bilirubin appears to be a beneficial effect against inflammation and plays an important role in the inflammatory process in psoriasis vulgaris. The results of the present study might be helpful in understanding the role of bilirubin in psoriasis. Further detailed and comprehensive investigations are required to clarify the mechanism of this process.
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
Abbreviations
- AS
atherosclerosis
- CRP
C‐reactive protein
- EOS
eosinophils
- Ig
immunoglobulin
- LYM
lymphocytes
- MONO
monocytes
- NEUT
neutrophils
- PASI
Psoriasis Area and Severity Index
- ROS
reactive oxygen species
- TB
total bilirubin
- WBC
white blood cell or leukocyte
ACKNOWLEDGMENTS
We express our gratitude to other colleagues who were involved in this study but are not in the author list.
REFERENCES
- 1. Bhole VM, Choi HK, Burns LC, et al. Differences in body mass index among individuals with PsA, psoriasis, RA and the general population. Rheumatology (Oxford) 2012;51:552–556. [DOI] [PubMed] [Google Scholar]
- 2. Coimbra S, Oliveira H, Reis F, et al. C‐reactive protein and leucocyte activation in psoriasis vulgaris according to severity and therapy. J Eur Acad Dermatol Venereol 2010;24:789–796. [DOI] [PubMed] [Google Scholar]
- 3. Devaraj S, Kumaresan PR, Jialal I. C‐reactive protein induces release of both endothelial microparticles and circulating endothelial cells in vitro and in vivo: Further evidence of endothelial dysfunction. Clin Chem 2011;57:1757–1761. [DOI] [PubMed] [Google Scholar]
- 4. Di Napoli M, Papa F. Association between blood pressure and C‐reactive protein levels in acute ischemic stroke. Hypertension 2003;42:1117–1123. [DOI] [PubMed] [Google Scholar]
- 5. Wang J, Song J, Wu J, He C, Xu C, Liu Y. Leukocyte and leukocyte subset counts reveal compensatory mechanisms in coronary heart disease. Clin Chim Acta 2013;418:79–85. [DOI] [PubMed] [Google Scholar]
- 6. Enerbäck C. Soluble biomarkers in psoriasis. Eur J Dermatol 2011;21:844–850. [DOI] [PubMed] [Google Scholar]
- 7. Rocha‐Pereira P, Santos‐Silva A, Rebelo I, Figueiredo A, Quintanilha A, Teixeira F. The inflammatory response in mild and in severe psoriasis. Br J Dermatol 2004;150:917–928. [DOI] [PubMed] [Google Scholar]
- 8. Khansari N, Shakiba Y, Mahmoudi M. Chronic inflammation and oxidative stress as a major cause of age‐related diseases and cancer. Recent Pat Inflamm Allergy Drug Discov 2009;3:73–80. [DOI] [PubMed] [Google Scholar]
- 9. Bhattacharyya A, Chattopadhyay R, Mitra S, Crowe SE. Oxidative stress: An essential factor in the pathogenesis of gastrointestinal mucosal diseases. Physiol Rev 2014;94:329–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lugrin J, Rosenblatt‐Velin N, Parapanov R, Liaudet L. The role of oxidative stress during inflammatory processes. Biol Chem 2014;395:203–230. [DOI] [PubMed] [Google Scholar]
- 11. Chen YH, Chau LY, Chen JW, Lin SJ. Serum bilirubin and ferritin levels link heme oxygenase‐1 gene promoter polymorphism and susceptibility to coronary artery disease in diabetic patients. Diabetes Care 2008;31:1615–1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Paine A, Eiz‐Vesper B, Blasczyk R, Immenschuh S. Signaling to heme oxygenase‐1 and its anti‐inflammatory therapeutic potential. Biochem Pharmacol 2010;80:1895–1903. [DOI] [PubMed] [Google Scholar]
- 13. Zhang ZY, Bian LQ, Kim SJ, Zhou CC, Choi YH. Inverse relation of total serum bilirubin to coronary artery calcification score detected by multidetector computed tomography in males. Clin Cardiol 2012;35:301–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Vítek L. The role of bilirubin in diabetes, metabolic syndrome, and cardiovascular diseases. Front Pharmacol 2012;3:55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wegiel B, Otterbein LE. Go green: The anti‐inflammatory effects of biliverdin reductase. Front Pharmacol 2012;3:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gul M, Uyarel H, Ergelen M, et al. Prognostic value of total bilirubin in patients with ST‐segment elevation acute myocardial infarction undergoing primary coronary intervention. Am J Cardiol 2013;111:166–171. [DOI] [PubMed] [Google Scholar]
- 17. Mayer M. Association of serum bilirubin concentration with risk of coronary artery disease. Clin Chem 2000;46:1723–1727. [PubMed] [Google Scholar]
- 18. Ohnaka K, Kono S, Inoguchi T, et al. Inverse associations of serum bilirubin with high sensitivity C‐reactive protein, glycated hemoglobin, and prevalence of type 2 diabetes in middle‐aged and elderly Japanese men and women. Diabetes Res Clin Pract 2010;88:103–110. [DOI] [PubMed] [Google Scholar]
- 19. Yu K, Kim C, Sung E, Shin H, Lee H. Association of serum total bilirubin with serum high sensitivity C‐reactive protein in middle‐aged men. Korean J Fam Med 2011;32:327–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zhou Q, Mrowietz U, Rostami‐Yazdi M. Oxidative stress in the pathogenesis of psoriasis. Free Radic Biol Med 2009;47:891–905. [DOI] [PubMed] [Google Scholar]
- 21. Oh SW, Lee ES, Kim S, et al. Bilirubin attenuates the renal tubular injury by inhibition of oxidative stress and apoptosis. BMC Nephrol 2013;14:105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Severin E, Nave B, Ständer M, Ott R, Traupe H. Total antioxidative capacity is normal in sera from psoriasis patients despite elevated bilirubin, tocopherol and urate levels. Dermatology 1999;198:336–339. [DOI] [PubMed] [Google Scholar]
- 23. Balta S, Balta I, Mikhailidis DP, et al. Bilirubin levels and their association with carotid intima media thickness and high‐sensitivity C‐reactive protein in patients with psoriasis vulgaris. Am J Clin Dermatol 2014;15:137–142. [DOI] [PubMed] [Google Scholar]
- 24. Nemati H, Khodarahmi R, Sadeghi M, Ebrahimi A, Rezaei M, Vaisi‐Raygni A. Antioxidant status in patients with psoriasis. Cell Biochem Funct 2014;32:268–273. [DOI] [PubMed] [Google Scholar]
- 25. Coimbra S, Oliveira H, Reis F, et al. Erythroid disturbances before and after treatment of Portuguese psoriasis vulgaris patients: A cross‐sectional and longitudinal study. Am J Clin Dermatol 2012;13:37–47. [DOI] [PubMed] [Google Scholar]
- 26. Han TS, Sattar N, Williams K, Gonzalez‐Villalpando C, Lean ME, Haffner SM. Prospective study of C‐reactive protein in relation to the development of diabetes and metabolic syndrome in the Mexico City Diabetes Study. Diabetes Care 2002;25:2016–2021. [DOI] [PubMed] [Google Scholar]
- 27. Khan HA, Alhomida AS, Sobki SH, Moghairi AA, Koronki HE. Blood cell counts and their correlation with creatine kinase and C‐reactive protein in patients with acute myocardial infarction. Int J Clin Exp Med 2012;5:50–55. [PMC free article] [PubMed] [Google Scholar]
- 28. Yamada S, Gotoh T, Nakashima Y, et al. Distribution of serum C‐reactive protein and its association with atherosclerotic risk factors in a Japanese population: Jichi Medical School Cohort Study. Am J Epidemiol 2001;153:1183–1190. [DOI] [PubMed] [Google Scholar]
- 29. Yang JY, Nam JS, Choi HJ. Association between high‐sensitivity C‐reactive protein with arterial stiffness in healthy Korean adults. Clin Chim Acta 2012;413:1419–1423. [DOI] [PubMed] [Google Scholar]
- 30. Schön MP, Boehncke WH. Psoriasis. N Engl J Med 2005;352:1899–1912. [DOI] [PubMed] [Google Scholar]
- 31. Schön MP, Boehncke WH, Bröcker EB. Psoriasis: Clinical manifestations, pathogenesis and therapeutic perspectives. Discov Med 2005;5:253–258. [PubMed] [Google Scholar]
- 32. Suárez‐Fariñas M, Li K, Fuentes‐Duculan J, Hayden K, Brodmerkel C, Krueger JG. Expanding the psoriasis disease profile: Interrogation of the skin and serum of patients with moderate‐to‐severe psoriasis. J Invest Dermatol 2012;132:2552–2564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Tam LS, Tomlinson B, Chu TT, et al. Cardiovascular risk profile of patients with psoriatic arthritis compared to controls–the role of inflammation. Rheumatology (Oxford) 2008;47:718–723. [DOI] [PubMed] [Google Scholar]
- 34. Strober BE, Poulin Y, Teller C, Wang Y, Williams DA, Goldblum OM. Changes in C‐reactive protein in patients with moderate‐to‐severe psoriasis switched to adalimumab therapy after suboptimal response to etanercept, methotrexate or phototherapy. J Eur Acad Dermatol Venereol 2014;28:1701–1706. [DOI] [PubMed] [Google Scholar]
- 35. Beygi S, Lajevardi V, Abedini R. C‐reactive protein in psoriasis: A review of the literature. J Eur Acad Dermatol Venereol 2014;28:700–711. [DOI] [PubMed] [Google Scholar]
- 36. Xu T, Zhang YH. Association of psoriasis with stroke and myocardial infarction: Meta‐analysis of cohort studies. Br J Dermatol 2012;167:1345–1350. [DOI] [PubMed] [Google Scholar]
- 37. Maradit‐Kremers H, Icen M, Ernste FC, Dierkhising RA, McEvoy MT. Disease severity and therapy as predictors of cardiovascular risk in psoriasis: A population‐based cohort study. J Eur Acad Dermatol Venereol 2012;26:336–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Balta I, Balta S, Demirkol S, et al. Elevated serum levels of endocan in patients with psoriasis vulgaris: Correlations with cardiovascular risk and activity of disease. Br J Dermatol 2013;169:1066–1070. [DOI] [PubMed] [Google Scholar]
- 39. Balta I, Balta S, Demirkol S, et al. Aortic arterial stiffness is a moderate predictor of cardiovascular disease in patients with psoriasis vulgaris. Angiology 2014;65:74–78. [DOI] [PubMed] [Google Scholar]
- 40. Choi SH, Yun KE, Choi HJ. Relationships between serum total bilirubin levels and metabolic syndrome in Korean adults. Nutr Metab Cardiovasc Dis 2013;23:31–37. [DOI] [PubMed] [Google Scholar]
- 41. Dullaart RP, Kappelle PJ, de Vries R. Lower carotid intima media thickness is predicted by higher serum bilirubin in both non‐diabetic and Type 2 diabetic subjects. Clin Chim Acta 2012;414:161–165. [DOI] [PubMed] [Google Scholar]
- 42. Navarini AA, Poulin Y, Menter A, Gu Y, Teixeira HD. Analysis of body regions and components of PASI scores during adalimumab or methotrexate treatment for patients with moderate‐to‐severe psoriasis. J Drugs Dermatol 2014;13:554–562. [PubMed] [Google Scholar]
- 43. Chularojanamontri L, Griffiths CE, Chalmers RJ. The Simplified Psoriasis Index (SPI): A practical tool for assessing psoriasis. J Invest Dermatol 2013;133:1956–1962. [DOI] [PubMed] [Google Scholar]


