Abstract
Background
The reversion‐inducing‐cysteine‐rich protein with kazal motifs (RECK) gene is a transformation suppressor gene that can negatively regulate matrix metalloproteinases (MMPs) and inhibit tumor invasion, angiogenesis, and metastasis. So, the aim of this study was to analyze the effect of RECK gene rs 11788747 single nucleotide polymorphism (SNP) on hepatocellular carcinoma (HCC) susceptibility and its relation to various clinical and laboratory data of the patients.
Methods
This is a case–control study including 200 HCC patients and 200 healthy controls. RECK rs 11788747 genotyping was performed using polymerase chain reaction‐restriction fragment length polymorphism (PCR‐RFLP).
Results
RECK rs 11788747 A/G and G/G genotypes frequencies were significantly higher in HCC patients compared to the healthy controls. The HCC patients possessing at least one polymorphic G allele were significantly at a higher risk of developing lymph nodes involvement and distant metastasis.
Conclusion
This study revealed the role of RECK rs 11788747 SNP in HCC in Egyptian patients, which consequently might be used as a prognostic tool and could be added to its therapeutic strategies.
Keywords: RECK, rs 11788747, HCC, Egypt
INTRODUCTION
Hepatocellular carcinoma (HCC) is the fifth most common cause of cancer and the third leading cause of cancer‐related deaths worldwide 1. The development of HCC is a multistep and complex process. Multiple risk factors, including chronic hepatitis B virus (HBV) or HCV infection and liver cirrhosis, contribute to hepatocarcinogenesis 2. In addition, individual genetic predisposition may play a role in increasing the risk of HCC 3.
The reversion‐inducing‐cysteine‐rich protein with Kazal motifs (RECK) gene was first isolated as a tumor suppressor gene against Rat sarcoma (ras) oncogenes. This gene has been mapped on human chromosome 9p13–p12, consisting of 21 exons and 20 introns and with a length of 87kb 4. So far 13 single nucleotide polymorphisms (SNPs) have been identified; four of them are in the coding region of exons 1, 9, 13, and 15—which raises the possibility of disease involvement—where polymorphisms in the corresponding protein structure lead to abnormal function, and the remaining nine are in introns 5, 8, 10, 12, 15, and 17 5.
The RECK gene encodes a membrane‐anchored glycoprotein with three kazal‐type SPI‐like domains, where SPI is serine protease inhibitor. Given that matrix metalloproteinases (MMPs) are indeed proteases, these SPI‐like domains are likely to have a significant role in MMP inhibition 6.
MMPs can degrade most extracellular matrix (ECM) components and connective tissue proteins. Thus MMPs play a major role in pathological processes, such as tumor angiogenesis, invasion, and metastasis. RECK inhibits the activity of at least three MMP members, including MMP‐2, MMP‐9, and membrane type (MT‐MMPs) 7.
RECK downregulation has been confirmed in many human cancers and correlated with tumor metastasis or poor prognosis 4. However, to our knowledge, only one study conducted in Taiwan by Chung et al., 2012, has investigated the role of RECK gene variants in HCC development and clinical factors 8.
In this view point, we studied the frequencies of rs11788747 polymorphism at the RECK gene in patients with HCC, and compared them with those of healthy subjects in an attempt to assess the implication of the RECK gene rs11788747 polymorphism in susceptibility to HCC in a cohort of Egyptian patients and to study the association between this genetic polymorphism and the clinical and laboratory data of these patients.
METHODS
The present study included 200 HCC patients (172 males and 28 females, mean age 59.22 ± 7.993 years) diagnosed according to the guidelines formulated by the European Association for the Study of the Liver (EASL) for HCC diagnosis and management 9.
Patients were attending the outpatient clinic or the inpatient wards of the Department of Endemic Medicine, Faculty of Medicine, Cairo University. Two hundred ethnically matched healthy volunteers—age (mean 55.30 ± 8.313 years), sex (172 males and 28 females)—were included in the current study as a control group. Peripheral blood samples were obtained from all subjects after they were provided with a written informed consent. The study was approved by the hospital's ethical committee (according to the WMA Declaration of Helsinki).
All patients in the study were subjected to full history taking, careful clinical examination, laboratory investigations, abdominal ultrasonography (US), triphasic helical computed tomography (CT), and /or dynamic magnetic resonance imaging (MRI).
Genotyping
Total genomic DNA of patients and healthy controls was extracted from about 2 ml anticoagulated whole blood on EDTA using Qiagen extraction kit (catalog number 51104, USA). The genotyping of RECK gene (rs11788747) SNP was performed by using a polymerase chain reaction–restriction fragment length polymorphism (PCR‐RFLP) assay. For quality control, genotyping was repeated for random samples in each group to confirm our results.
The target gene was amplified by PCR using 5′‐ GTAGAAGAAGTGACTCATCC ‐3′ (sense) and 5′‐ ATCTCACTCCGAAGATAACC‐3′ (antisense) primers (spanning RECK exon 13 region containing rs11788747 site). PCR reactions were carried out in a 25 μl reaction mixture containing 5 μl extracted DNA, 12.5 μl of Dream Taq Green PCR Master Mix, 1 μl of each primer, and 5.5 μl of water, nuclease‐free. The PCR reaction tubes were then placed in the thermal cycler (GeneAmp PCR system 9700, Germany). The PCR amplifications cycles were performed as follow: initial denaturation at 94°C for 5 min followed by 35 cycles of denaturation at 94°C for 1 min, annealing at 60°C for 1 min, and extension at 72°C for 2 min . Final extension step was performed at 72°C for 20 min 8.
PCR products were then digested by RsaI, using the conditions recommended in the manufacturer's instructions (catalog number: FD1124; Fermentas, Lithuania) and DNA fragments were separated by electrophoresis in 2% agarose gel stained with ethidium bromide.
The homozygous wild genotype A/A does not contain a recognition site for the enzyme RsaI, so the 242 bp amplificon remains unaltered after incubation with RsaI. As for the homozygous polymorphic genotype G/G, it contains a recognition site for the FastDigest RsaI restriction enzyme, so the digestion product of the PCR amplificon having G/G genotype, using RsaI, yields two DNA fragments 140 and 102 bp in length.
In case of the heterozygous genotype A/G, incubation of the PCR amplification product with RsaI yields three DNA fragments 242, 140, and 102 bp in length (8, Fig. 1).
Figure 1.
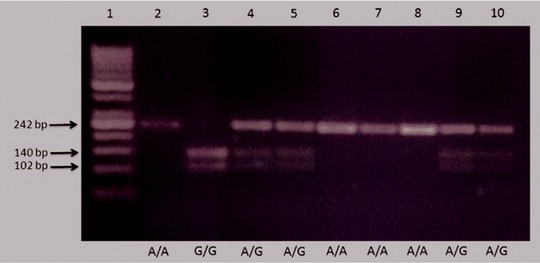
Gel electrophoresis showing different genotypes of RECK rs11788747 SNP:
Lane 1—50–500 bp ladder‐size PCR marker.
Lanes 2, 6, 7, 8—Homozygous wild genotype (A/A).
Lane 3—Homozygous mutant genotype (G/G).
Lanes 4, 5, 9, 10—Heterozygous genotype (A/G).
Statistical Methodology
Data were statistically described in terms of mean ± standard deviation (±SD), median and range, or frequencies (number of cases) and percentages when appropriate. Comparison of numerical variables between more than two groups was done using one‐way analysis of variance (ANOVA) test with posthoc multiple two‐group comparisons in normal data. For comparing categorical data, Chi‐square (χ2) test was performed. Exact test was used instead when the expected frequency is less than 5. Odds ratio (OR) and 95% confidence interval (CI) were calculated for the association of the polymorphic genotypes (A/G and G/G) and cases of hepatocellular carcinoma. All P‐values less than 0.05 were considered statistically significant. All statistical calculations were done using computer programs SPSS (Statistical Package for the Social Science; SPSS Inc., Chicago, IL) version 15 for Microsoft Windows.
RESULTS
Genotype frequencies of RECK gene (rs11788747) SNP in the case–control cohort of patients with HCC were shown in Table 1. These frequencies carry significant difference with regard to the susceptibility to HCC (OR = 3.807, 95% CI = 2.512–5.771).
Table 1.
Comparison Between the HCC Patients and the Healthy Controls With Regard to RECK Genotypes
| RECK genotype | HCC (N = 200) | Controls (N = 200) | OR (95% CI) a | P‐value |
|---|---|---|---|---|
| A/A | 76 (38%) | 140 (70%) | ||
| A/G | 100 (50%) | 60 (30%) | 3.807 | <0.001 |
| G/G | 24 (12%) | 0 (0%) | (2.512–5.771) |
P < 0.05 is statistically significant.
Odds Ratio (OR) and 95% confidence interval (CI) were calculated for association of the polymorphic genotypes (A/G and G/G) and cases of Hepatocellular Carcinoma (HCC).
The patients were classified according to RECK genotypes into three groups: A/A that included 76 patients, A/G that included 100 patients, and G/G that included 24 patients. The three groups were compared clinically regarding Child–Pugh class, lymph node metastasis and distant metastasis. We found statistically significant differences between the three groups with regard to all these points of comparison (Table 2).
Table 2.
Comparison Between RECK Genotypes With Regard to the Clinical Data of HCC patients
| Genotype groups | |||||
|---|---|---|---|---|---|
| Clinical data | A/A group (n = 76) | A/G group (n = 100) | G/G group (n = 24) | P‐value | |
| Child class | A | 40 (52.6%) | 56 (56%) | 4(16.7%) | <0.001 |
| B | 32 (42.1%) | 28 (28%) | 20 (83.3%) | ||
| C | 4 (5.3%) | 16 (16%) | 0 (0%) | ||
| Lymph node metastasis | Yes | 0 (0%) | 20 (20%) | 16 (66.7%) | <0.001 |
| No | 76 (100%) | 80 (80%) | 8 (33.3%) | ||
| Distant metastasis | Yes | 0 (0%) | 0 (0%) | 16 (66.7%) | <0.001 |
| No | 76 (100%) | 100 (100%) | 8 (33.3%) | ||
P < 0.05 is statistically significant.
Alpha fetoprotein (AFP), alanine transaminase (ALT), and aspartate transaminase (AST) are common clinical pathological markers of HCC. Our study analyzed the levels of these pathological markers associated with RECK genotypic frequencies. Statistically significant differences were detected between the three groups of HCC patients with regard to the ALT and the AFP with increased mean values in the group carrying the polymorphic genotype (GG). However, no statistical significant difference was elicited between the three groups regarding AST (Table 3).
Table 3.
Comparison Between RECK Genotypes With Regards to the Laboratory Data of HCC Patients
| Genotype groups | |||||
|---|---|---|---|---|---|
| Laboratory test | A/A group (n = 76) | A/G group (n = 100) | G/G group (n = 24) | P‐value | |
| ALT (U/L) | Mean ± SD | 42.53 ± 17.643 | 50.99 ± 21.531 | 56.17 ± 23.938 | 0.004 |
| AST (U/L) | Mean ± SD | 82.58 ± 56.016 | 88.69 ± 44.422 | 98.67 ± 27.496 | 0.337 |
| AFP (ng/ml) | Mean ± SD | 1,470.15 ± 3,392.179 | 1,436.40 ± 4,256.439 | 8,836.12 ± 14,178.189 | <0.001 |
P < 0.05 is statistically significant.
DISCUSSION
The RECK gene was initially isolated as a transformation suppressor gene encoding a membrane‐anchored glycoprotein and later found to suppress tumor invasion and metastasis by regulating MMPs 10. This study focused on the effects of SNP of RECK gene (rs 11788747) on HCC in Egyptian patients.
Analysis of the distribution of RECK genotypes among the HCC patients and controls showed higher frequency of A/G and G/G genotypes in HCC patients (50% and 12%, respectively) compared to the healthy controls (30% and 0%, respectively) and this difference was statistically significant. Thus, in our study the presence of at least one polymorphic G allele increases the susceptibility to HCC compared to the A/A wild‐type carriers.
In Chung et al.’s (2012) study in Taiwan, they showed that HCC patients carrying rs11788747 polymorphisms were not at a higher risk of developing HCC. Yet, the same study showed that individuals carrying the RECK promoter rs10814325 inheriting at least one polymorphic C allele had a 1.85‐fold higher risk of HCC compared to TT wild‐type carriers 8.
In our study 20% of HCC patients belonging to the A/G group and 66.7% of HCC patients belonging to the G/G group developed lymph nodal metastasis while none of the HCC patients belonging to the A/A group had any LN involvement. This difference was statistically significant.
This is in contrast to Chung et al.’s (2012) study, in which 4.8% of HCC belonging to the A/A group and a combined 5.8% of patients belonging to the A/G and the G/G groups had LN metastasis 8. No statistical significance was elicited regarding the presence of at least one polymorphic G allele and the risk of developing LN metastasis among the HCC patients recruited in that study.
These contradictory findings between our study and that conducted by Chung et al. (2012) may be the result of the different ethnic backgrounds of the patients 8. Thus, additional studies in different ethnic groups with larger sample sizes are needed to validate the different effects of the RECK polymorphisms on HCC.
Furthermore, in our study 66.7% HCC patients belonging to G/G group developed distant metastasis while none of the other HCC patients belonging to either A/A or A/G groups showed evidence of distant metastasis. This difference was statistically significant.
This finding was in parallel conjunction with that concluded by Chung et al. (2012). In this study, none of A/A patients had distant metastasis while a combined 11.5% of HCC patients belonging to the A/G and the G/G had evidence of distant metastasis and thus HCC patients carrying RECK rs11788747 polymorphisms had a higher risk of distant metastasis than wild‐type patients 8.
Previous research conducted by Takahashi et al. (1998) confirmed the relationships of RECK expression and Gene status with tumor metastasis. In their study, they artificially restored RECK expression in tumor cells, in which RECK was undetectable. This greatly suppressed their invasive and metastatic potentials 11.
Child–Pugh class A to C is used to assess the prognosis of chronic liver disease. The score employs five clinical measures of liver disease that are total bilirubin, serum albumin, prothrombin time, ascites, and hepatic encephalopathty 12. Our study showed that the presence of G/G genotype was associated with poorer prognosis in HCC patients.
Role of RECK has been demonstrated in several other malignancies. In pancreatic carcinoma, an inverse correlation between RECK expression and MMP‐2 activation in the tumor tissues, as well as their invasive potentials, was found. Thus, it is suggested that RECK plays an active role in suppressing malignant phenotypes of pancreatic cancer cells and can be used as a prognostic indicator in these patients 13.
The level of RECK mRNA expression was at lower levels in the majority of the colorectal cancer tissues examined as compared to the surrounding non tumor tissues. This low expression was associated with higher incidence of lymph node metastasis and shorter survival rates in these patients suggesting that RECK is a tumor suppressor gene; downregulation of which leads to poor prognosis 14.
In addition, our study compared the three different genotype groups with regard to the laboratory data: ALT, AST, and AFP. Statistical significant differences were elicited between the three groups with regard to these laboratory data except for the AST.
In the study of Chung et al. (2012), they analyzed the levels of sera AFP, ALT, and AST in association with RECK genotypic frequencies. No statistical significance was elicited from comparing these laboratory investigations among the different RECK genotypes except for ALT levels that were statistically different between the combined A/G and G/G groups and the wild‐type A/A group 8.
CONCLUSION
In conclusion, we found a higher frequency of A/G and G/G genotypes among HCC patients when compared to the control group, suggesting a possible role of RECK gene rs11788747 polymorphism in increasing the susceptibility of individuals carrying this polymorphism to HCC. More studies are needed to investigate the different RECK gene polymorphisms and their biological function in HCC patients. Furthermore, the feasibility and a cost–benefit analysis of applying RECK as a prognostic factor should be carefully assessed by more studies to come.
Finally, the possibility of applying RECK, a pharmaceutical mimetic, or drugs activating endogenous RECK expression as possible therapeutic or preventive agents for HCC should be explored.
REFERENCES
- 1. Shiraha H, Yamamoto K, Namba M. Human hepatocyte carcinogenesis. Int J Oncol 2013;42(4):1133–1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gomaa AI, Khan SA, Leen ELS, Waked I, Taylor‐Robinson SD. Diagnosis of hepatocellular carcinoma. World J Gastroenterol 2009;15(11):1301–1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Dragani TA. Risk of HCC: Genetic heterogeneity and complex genetics. J Hepatol 2010;52(2):252–257. [DOI] [PubMed] [Google Scholar]
- 4. Meng N, Li Y, Zhang H, Sun XF. Review: RECK, a novel matrix metalloproteinase regulator. Histol Histopathol 2008;23:1003–1010. [DOI] [PubMed] [Google Scholar]
- 5. Clark JCM, Thomas DM, Choong PFM, Dass CR. RECK—a newly discovered inhibitor of metastasis with prognostic significance in multiple forms of cancer. Cancer Metastasis Rev 2007;26(3):675–683. [DOI] [PubMed] [Google Scholar]
- 6. Noda M, Takahashi C. Recklessness as a hallmark of aggressive cancer. Cancer Sci 2007;98(11):1659–1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rosenthal EL, Matrisian, LM . Matrix metalloproteases in head and neck cancer. Head Neck 2006;28(7):639–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chung TT, Yeh CB, Li YC, et al. Effect of RECK gene polymorphisms on hepatocellular carcinoma susceptibility and clinicopathologic features. Plos One 2012;7(3):33517 http://www.plosone.org/article/info%3Adoi%2F10.1371%2Fjournal.pone.0033517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Llovet JM, Lencioni R, Di Bisceglie AM, et al. A. EASL‐EORTC clinical practice guidelines: Management of hepatocellular carcinoma. J Hepatol 2012;56(4):908–943. [DOI] [PubMed] [Google Scholar]
- 10. Oh J, Takahashi R, Kondo S, et al. The membrane‐anchored MMP inhibitor RECK is a key regulator of extracellular matrix integrity and angiogenesis. Cell 2001;107:789–800 [DOI] [PubMed] [Google Scholar]
- 11. Takahashi C, Sheng Z, Horan TP, et al. Regulation of matrix metalloproteinase‐9 and inhibition of tumor invasion by the membrane‐anchored glycoprotein RECK. Proc Natl Acad Sci USA 1998;95(22):13221–13226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Pugh RN, Murray‐Lyon IM, Dawson JL, Pietroni MC, Williams R. Transection of the oesophagus for bleeding oesophageal varices. Br J Surg 1973;60(8):646–649. [DOI] [PubMed] [Google Scholar]
- 13. Bloomston M, Shafii A, Zervos E, Rojiani A, Rosemurgy A. MMP‐2 and TIMP‐1 are derived from, not in response to, pancreatic cancer. J Surg Res 2002;102:35–38 [DOI] [PubMed] [Google Scholar]
- 14. Takeuchi T, Hisanaga M, Nagao M, et al. The membrane‐anchored matrix metalloproteinase (MMP) regulator RECK in combination with MMP‐9 serves as an informative prognostic indicator for colorectal cancer. Clin Cancer Res 2004;10:5572–5573. [DOI] [PubMed] [Google Scholar]


