Abstract
Background
Human epididymis protein 4 (HE4) is an available tumor biomarker for detecting ovarian cancer. However, it is unknown if serum HE4 could be a novel biomarker for diagnosis of lupus nephritis (LN) and chronic kidney disease (CKD) in patients with systemic lupus erythematosus (SLE).
Methods
This study enrolled 209 SLE patients, 75 patients with renal dysfunction without SLE and 32 healthy subjects. HE4 concentrations were analyzed by ELISA (enzyme‐linked immunosorbent assay; Fujirebio Diagnostics, Sweden). The receiver operating characteristic (ROC) curves were constructed to assess diagnostic accuracy of HE4 for LN or CKD in SLE.
Results
Serum HE4 level was significantly higher in SLE patients than that in healthy controls (P < 0.001), especially for those with LN or CKD. It was also higher in patients with renal dysfunction without SLE than healthy controls (P < 0.001), while there was no significant difference between these patients and those with SLE with CKD (P = 0.73). Multivariate analysis showed significant association between increased HE4 and LN or CKD after controlling for confounders. ROC curves showed the cutoff values were 150.1 pM (sensitivity, 76.8%; specificity, 91.1%) for the diagnosis of LN in SLE and 233.9 pM (sensitivity, 92.9%; specificity, 93.5%) for CKD in SLE.
Conclusions
Increased serum HE4 level is closely associated with the development of LN or CKD in SLE patients. Furthermore, it can be used as a novel and useful biomarker for diagnosis of LN or CKD.
Keywords: human epididymis protein 4, nephritis, chronic kidney disease, systemic lupus erythematosus
Introduction
Systemic lupus erythematosus (SLE) is a chronic autoimmune disease of unknown etiology, characterized by multiple organ involvements. Lupus nephritis (LN) is a common manifestation and one of the most serious organ involvements, affecting 50–70% of SLE patients 1. LN patients often have a low 5‐year survival 2, although the course of renal disease can be dramatically modified by early diagnosis and prompt treatment, which contributes to the improvement of long‐term survival 3. Therefore, it is of great importance to detect LN in SLE at an early stage.
The majority of SLE studies focused on renal failure or remission achievement in LN patients, whereas less attention was paid to the presence or absence of chronic kidney disease (CKD) in SLE patients 4, 5, 6, 7, 8. Actually, CKD is closely associated with mortality and cardiovascular disease as well as the development of end‐stage renal disease (ESRD) 9. Therefore, an accurate and prompt identification for CKD is of great significance to help clinicians improve clinical outcome and prevent the development of ESRD and cardiovascular disease as well as the death in SLE, especially for the patients with LN.
Human epididymis protein 4 (HE4), first identified and characterized as a human epididymis‐specific protein in 1991 10, is widely detected in respiratory tract, nasopharynx, salivary glands, kidney, and other organs 11, 12, 13, although several subsequent studies demonstrated the clinical value of HE4 alone or in combination with CA125 for diagnosing and predicting ovarian cancer 14, 15, 16, 17, 18, 19. Recently, significant increment of serum HE4 concentration has also been found in CKD, renal failure, and heart failure 20, 21, 22, 23. Nonetheless, the diagnostic efficacy of serum HE4 has not been explored in SLE, especially for those with LN or CKD. The aim of this study is to determine whether serum HE4 could be a novel and useful biomarker for diagnosis of LN and CKD in SLE patients.
Materials and Methods
Two hundred nine SLE patients (184 women and 25 men, mean ± SD, age 39 ± 16 years) were consecutively recruited from Changzheng Hospital between August 2008 and July 2013. All the patients fulfilled at least four of the 1997 revised American College of Rheumatology (ACR) classification criteria for SLE 24. Of those, 138 patients (120 women and 18 men, mean ± SD, age 38 ± 16 years) fulfilled ACR criteria for LN 25, and 70 patients (62 women and 8 men, mean ± SD, age 43 ± 16 years) had CKD according to the kidney disease: improving global outcome definition (estimated glomerular filtration rate [eGFR] < 60 ml/min/1.73 m2 of body surface area for 3 months or more) 26. Seventy‐five patients with renal dysfunction without SLE (mean ± SD, age 53 ± 17 years; female/male: 66/9), and 32 healthy subjects (mean ± SD, age 40 ± 8 years; female/male: 29/3) with no histories of SLE or other autoimmune or inflammatory diseases were randomly recruited. Of the controls, the subjects were excluded if they had abnormal renal function (eGFR<90 ml/min/1.73 m2), tumor, or gynecological diseases.
Some clinical and demographic characteristics and laboratory data of the patients were obtained by medical record review. The SLE disease activity index (SLEDAI) score 27 was determined for each patient at the day when blood was drawn for laboratory tests. According to the physical and radiological examination, no ovarian disorders were detected in the study cohorts. Creatinine, urea, and uric acid (UA) were measured by enzymatic assay (Cobas c701, Roche). eGFR was calculated by chronic kidney disease epidemiology collaboration (CKD‐EPI) equation, which is a newly developed and validated formula for eGFR. It is more accurate at normal or near‐normal eGFR (eGFR CKD‐EPI = 141 × min [Scr/κ, 1]α × max [Scr/κ, 1]−1.209 × 0.993Age × [1.018 if female] × [1.159 if black], where Scr is serum creatinine, κ is 0.7 for females and 0.9 for males, α is −0.329 for females and −0.411 for males, min indicates the minimum of Scr/κ or 1, and max indicates the maximum of Scr/κ or 1) 28, 29.
Serum samples were prepared immediately by centrifugation of peripheral venous blood and processed with cryopreservation (−80°C) for determination of HE4 level. Serum HE4 concentration was measured in duplicate by enzyme‐linked immunosorbent assay (ELISA; Fujirebio Diagnostics, Sweden), with a measuring range of 15–900 pM. The inter‐ and intra‐assay variation were <7% and <3%, respectively. The samples with HE4 concentrations over 900 pM were measured again after dilution with HE4 calibrator by 1/10 according to the instructions.
Statistical Analysis
Data analyses were performed using SPSS 17.0 statistical software. Since all the continuous variables but age showed a skewed distribution, median and range were used to describe the levels of these variables. Differences between groups were compared using Mann–Whitney test for continuous variables. Spearman's correlation coefficient (r s) was used to evaluate the correlation between HE4 and creatinine and eGFR. P‐value less than 0.05 was considered as statistically significant. Receiver operating characteristics (ROCs) curves were constructed to assess sensitivity, specificity, and areas under the curves (AUCs) with 95% confidence interval (CI). In accordance with a previous study, the optimum cutoff value for diagnosis was selected by maximizing the sum of sensitivity and specificity and minimizing the overall error (square root of the sum [1‐sensitivity]2 + [1‐specificity]2), and by minimizing the distance of cutoff value to the top‐left corner of the ROC curve 30.
Results
Comparison of HE4 Levels Between Various Subgroups
The median level of serum HE4 was 166.3 pM (range, 16.7–7084.9) in the SLE group, significantly higher than that in the control group (median, range: 40.6 pM, 34.5–61.1) (P < 0.001). Then SLE patients were divided into four groups, namely SLE without LN or CKD (n = 67), SLE only with LN (n = 72), SLE only with CKD (n = 4), and SLE with LN and CKD (n = 66). The median levels of serum HE4 were 64.3 pM (range: 16.7–425.7 pM), 161.4 pM (range: 42.8–1218.6 pM), 282.7 pM (range: 183.0–1399.5 pM), and 613.8 pM (range: 156.4–7084.9 pM) in SLE without LN or CKD, SLE only with LN, SLE only with CKD, and SLE with LN and CKD groups, respectively. All SLE subgroups showed significantly higher HE4 levels than the healthy control group. Compared with SLE without LN or CKD subgroup, others showed significantly increased HE4 levels. Especially SLE with LN and CKD subgroup showed significantly higher HE4 level than SLE only with LN subgroup. Serum HE4 level was also significantly higher in patients with renal dysfunction without SLE (median, range: 339.9 pM [47.3–2140]) than healthy controls (P < 0.001), while there was no significant difference between these patients and those with SLE only with CKD (P = 0.73; Fig. 1).
Figure 1.
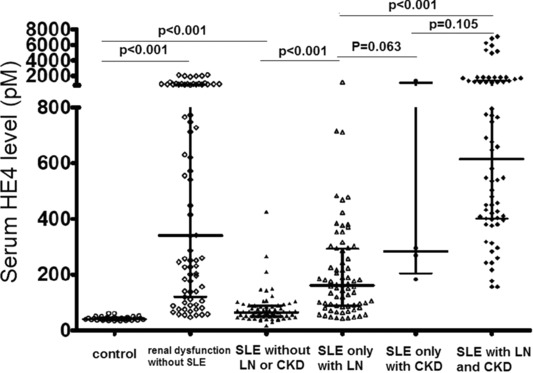
Serum HE4 levels in controls and various SLE subgroups.
Association of Various Variables With LN or CKD in SLE Patients
Compared with SLE patients without LN, those with LN were significantly more likely to have previously documented hypertension (P = 0.008), positive anti‐dsDNA antibody (P < 0.001), anemia (<0.001), leukopenia (P = 0.017), low C3 (P < 0.001), lower IgG (P = 0.001) and IgM (P = 0.018), higher SLEDAI score (P <0.001), and less likely to have anti‐SSA positive antibody (P = 0.039). However, other variables were not associated with LN (Table 1).
Table 1.
Clinical and Laboratory Characteristics of SLE Patients With LN or CKD Compared Without LN or CKD, Respectively
| SLE with LN (n = 138) | SLE without LN (n = 71) | P‐value | SLE with CKD (n = 70) | SLE without CKD (n = 139) | P‐value | |
|---|---|---|---|---|---|---|
| Age (mean ± SD, years) | 38 ± 16 | 40 ± 16 | 0.174 | 43 ± 16 | 37 ± 15 | 0.174 |
| Sex (Female/male) | 120/18 | 64/7 | 0.502 | 62/8 | 123/16 | 0.986 |
| Disease duration (months, range) | 12 (0.1–312) | 19 (0.3–276) | 0.149 | 24 (0.1–312) | 12 (0.1–276) | 0.156 |
| History of hypertension (%, n) | 25.4 (35) | 9.9 (7) | 0.008 | 34.3 (24) | 14.4 (20) | 0.001 |
| History of diabetes (%, n) | 0.7 (1) | 5.6 (4) | 0.085 | 0 (0) | 3.6 (5) | 0.260 |
| Current prednisolone (%, n) | 68.8 (95) | 67.6 (48) | 0.856 | 71.4 (50) | 67.6 (94) | 0.575 |
| Current cyclophosphamide (%, n) | 18.8 (26) | 18.3 (13) | 0.926 | 22.9 (16) | 16.5 (23) | 0.269 |
| Anti‐dsDNA antibody positive (%, n) | 52.9 (73) | 27.8 (19) | <0.001 | 55.7 (39) | 38.8 (54) | 0.021 |
| Anti‐SSA antibody positive (%, n) | 57.2 (79) | 71.8 (51) | 0.039 | 58.5 (41) | 64.0 (89) | 0.443 |
| Anti‐SSB antibody positive (%, n) | 20.3 (28) | 22.5 (16) | 0.706 | 15.7 (11) | 23.7 (33) | 0.179 |
| Anti‐U1RNP antibody positive (%, n) | 34.1 (47) | 39.4 (28) | 0.443 | 31.4 (22) | 38.1 (53) | 0.340 |
| Anti‐Smith antibody positive (%, n) | 25.4 (35) | 28.2 (20) | 0.663 | 24.3 (17) | 27.3 (38) | 0.636 |
| Anemia (%, n) | 56.5 (78) | 25.4 (18) | <0.001 | 77.1 (54) | 30.9 (43) | <0.001 |
| Leukopenia (%, n) | 40.6 (56) | 23.9 (17) | 0.017 | 37.1 (26) | 33.8 (47) | 0.634 |
| Thrombocytopenia (%, n) | 26.1 (36) | 22.5 (16) | 0.574 | 31.4 (22) | 21.6 (30) | 0.120 |
| Low C3 (%, n) | 92.0 (127) | 69.0 (49) | <0.001 | 88.6 (62) | 82.0 (114) | 0.220 |
| Low C4 (%, n) | 90.5 (125) | 84.5 (60) | 0.192 | 82.9 (58) | 91.4 (127) | 0.069 |
| IgG (g/l) | 11.0 (1.2‐47.2) | 14.9 (2.1–48.1) | 0.001 | 12.0 (2.07–48.10) | 5.4 (1.9–47.2) | 0.445 |
| IgM (g/l) | 0.88 (0.09–5.45) | 1.24 (0.15–9.70) | 0.018 | 0.87 (0.15 –5.45) | 1.06 (0.09–9.70) | 0.013 |
| IgA (g/l) | 2.31 (0.09–6.79) | 2.39 (0.55–6.42) | 0.341 | 2.33 (0.09–5.67) | 2.32 (0.55–6.79) | 0.341 |
| ESR (mm/hr) | 44 (3–150) | 38 (6–150) | 0.305 | 48 (6–150) | 38 (3–150) | 0.305 |
| HsCRP (mg/l) | 3.08 (0.2–283) | 4.17 (0.75–92.8) | 0.078 | 4.47 (0.22–72.48) | 4.17 (0.20–283) | 0.444 |
| SLEDAI score | 13 (4–31) | 7 (2–20) | <0.001 | 13 (4–31) | 9 (2–30) | <0.001 |
| LN (%, n) | / | / | / | 94.3 (66) | 51.8 (72) | <0.001 |
LN, lupus nephritis; SLE, systemic lupus erythematosus; CKD, chronic kidney disease; SD, standard deviation; SLEDAI, SLE disease activity index.
Next, by inclusion of these significant variables and HE4, multivariate logistic regression was used to examine whether or not HE4 was independently associated with LN in SLE patients. The results indicated that after controlling for above‐mentioned confounders, only HE4 and SLEDAI score remained significantly positive, but IgG inverse associations with LN (Table 2).
Table 2.
Multivariate Logistic Regression of the Relationship of Risk Factors With LN or CKD in SLE Patients
| Risk factors | OR (95% CI) | P‐value |
|---|---|---|
| LN | ||
| History of hypertension (yes, no) | 2.979 (0.873–10.167) | 0.081 |
| Leukopenia (yes, no) | 1.614 (0.617–4.223) | 0.330 |
| Anemia (yes, no) | 1.402 (0.497–3.956) | 0.523 |
| Low C3 (yes, no) | 2.577 (0.779–8.528) | 0.121 |
| IgG(g/l) | 0.916 (0.861–0.975) | 0.006 |
| IgM (g/l) | 0.865 (0.600–1.248) | 0.438 |
| Anti‐dsDNA antibody (yes, no) | 1.746 (0.694–4.394) | 0.236 |
| Anti‐SSA antibody (yes, no) | 0.639 (0.258–1.584) | 0.333 |
| SLEDAI score (per) | 1.313 (1.157–1.489) | <0.001 |
| HE4 (per pM) | 1.005 (1.001–1.008) | 0.005 |
| CKD | ||
| History of hypertension (yes, no) | 2.510 (0.859–7.338) | 0.093 |
| Anemia (yes, no) | 2.227 (0.840–5.899) | 0.107 |
| IgM (g/l) | 0.476 (0.215–1.051) | 0.066 |
| Anti‐dsDNA antibody (yes, no) | 2.387 (0.862–6.611) | 0.094 |
| SLEDAI score (per) | 0.980 (0.895–1.073) | 0.662 |
| LN (yes, no) | 2.019 (0.458–8.906) | 0.353 |
| HE4 (per pM) | 1.007 (1.004–1.010) | <0.001 |
LN, lupus nephritis; SLE, systemic lupus erythematosus; CKD, chronic kidney disease; SLEDAI, SLE disease activity index.
We also examined the association between various variables and CKD in SLE patients. We found that CKD patients were significantly more likely to have previously documented hypertension (P = 0.001), positive anti‐dsDNA antibody (P = 0.021), anemia (P < 0.001), LN (P < 0.001), higher SLEDAI score (P < 0.001), and lower IgM (P = 0.013). However, other variables were not associated with CKD (Table 1).
Also, multivariate logistic regression was used to examine whether or not HE4 was independently associated with CKD development in SLE patients by including these significant variables. The results suggested that after controlling for above‐mentioned confounders, only HE4 was significantly associated with CKD (Table 2).
Diagnostic Performance of HE4 for LN or CKD in SLE Patients
The median concentration of serum HE4 in SLE with LN patients (n = 138) was 317.1 pM (range: 42.8–7084.9), significantly higher than that in SLE without LN patients (median, range: 67.8 pM, 16.7–1399.5) (P < 0.001). ROC curves were used to investigate the diagnostic performance of serum HE4 for LN in 209 SLE patients, compared with that of serum creatinine, urea, and UA, which have been confirmed to be useful indicators for kidney diseases. Serum HE4 had significantly better diagnostic performance with 150.1 pM of optimum diagnostic cutoff value (AUC = 0.878, 95% CI: 0.829–0.926, sensitivity 76.8%, specificity 91.1%) for LN in SLE patients than did serum creatinine (AUC = 0.827, 95% CI: 0.771–0.883, sensitivity 74.6%, specificity 76.1%), urea (AUC = 0.818, 95% CI: 0.761–0.875, sensitivity 60.1%, specificity 94.4%), and UA (AUC = 0.803, 95% CI: 0.742–0.863, sensitivity 75.4%, specificity 77.5%), especially in SLE patients without CKD (AUC, 95% CI; HE4 vs. creatinine vs. urea vs. UA: 0.818, 0.747–0.889 vs. 0.716, 0.630–0.801 vs. 0.700, 0.614–0.787 vs. 0.724, 0.640–0.808) and in those with normal eGFR (AUC, 95% CI; HE4 vs. creatinine vs. urea vs. UA: 0.778, 0.689–0.867 vs. 0.681,0.578–0.784 vs. 0.649, 0.544–0.754 vs. 0.733, 0.638–0.828; Fig. 2 and Table 3).
Figure 2.
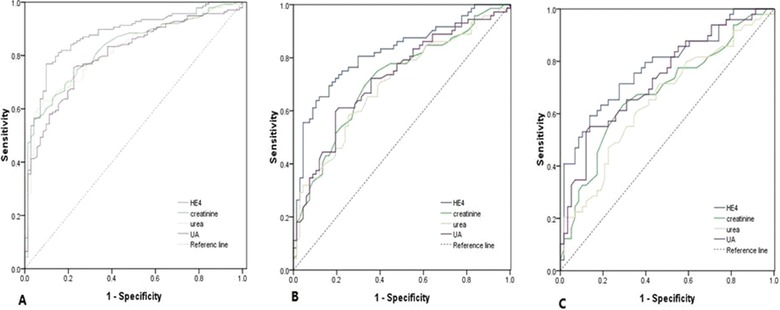
ROC curves of HE4, creatinine, urea, and UA for the diagnosis of LN in SLE patients. (A) LN versus SLE without LN, (B) LN without CKD versus SLE without CKD, (C) LN with normal eGFR versus SLE with normal eGFR.
Table 3.
Results for Measurement of Serum HE4, Creatinine, Urea, and UA in the Diagnosis of LN or CKD in SLE Patients
| AUC | 95% CI | P | Cutoff | Sen (%) | Spe (%) | PPV (%) | NPV (%) | PLR | NLR | Accuracy (%) | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| LN versus SLE without LN | |||||||||||
| HE4 | 0.878 ± 0.025 | 0.829 –0.926 | <0.001 | 150.1 | 76.8 | 91.1 | 94.6 | 67.0 | 8.629 | 0.255 | 81.8 |
| Creatinine | 0.827 ± 0.029 | 0.771 –0.883 | <0.001 | 65.5 | 74.6 | 76.1 | 85.8 | 60.7 | 3.121 | 0.334 | 75.1 |
| Urea | 0.818 ± 0.029 | 0.761 –0.875 | <0.001 | 7.75 | 60.1 | 94.4 | 95.4 | 54.9 | 10.732 | 0.423 | 71.8 |
| UA | 0.803 ± 0.031 | 0.742 –0.863 | <0.001 | 326.5 | 75.4 | 77.5 | 86.7 | 61.8 | 3.351 | 0.317 | 76.1 |
| LN without CKD versus SLE without CKD | |||||||||||
| HE4 | 0.818 ± 0.036 | 0.747–0.889 | <0.001 | 113.1 | 63.9 | 89.6 | 86.8 | 69.8 | 6.144 | 0.403 | 76.3 |
| Creatinine | 0.716 ± 0.043 | 0.630–0.801 | 0.016 | 58.5 | 69.4 | 67.2 | 69.4 | 67.2 | 2.116 | 0.455 | 68.3 |
| Urea | 0.700 ± 0.044 | 0.614–0.787 | <0.001 | 5.55 | 58.3 | 74.6 | 71.2 | 62.5 | 2.295 | 0.559 | 66.2 |
| UA | 0.724 ± 0.043 | 0.640–0.808 | <0.001 | 326.5 | 59.7 | 70.6 | 68.3 | 61.8 | 2.031 | 0.571 | 64.7 |
| LN with normal eGFR versus SLE with normal eGFR | |||||||||||
| HE4 | 0.778 ± 0.045 | 0.689–0.867 | <0.001 | 89.3 | 63.3 | 81.0 | 80.4 | 67.8 | 3.332 | 0.453 | 72.0 |
| Creatinine | 0.681 ± 0.053 | 0.578–0.784 | 0.001 | 57.5 | 63.3 | 70.7 | 72.6 | 64.8 | 2.160 | 0.519 | 67.3 |
| Urea | 0.649 ± 0.054 | 0.544–0.754 | 0.008 | 4.95 | 61.2 | 63.8 | 66.0 | 57.4 | 1.691 | 0.608 | 61.7 |
| UA | 0.733 ± 0.049 | 0.638–0.828 | <0.001 | 231.0 | 85.7 | 44.8 | 64.9 | 73.3 | 1.553 | 0.319 | 67.3 |
| CKD versus SLE without CKD | |||||||||||
| HE4 | 0.948 ± 0.013 | 0.922–0.975 | <0.001 | 233.9 | 92.9 | 93.5 | 89.0 | 96.3 | 14.292 | 0.076 | 93.3 |
| Urea | 0.920 ± 0.022 | 0.877–0.963 | <0.001 | 10.25 | 81.4 | 89.9 | 80.3 | 90.6 | 8.059 | 0.207 | 87.1 |
| UA | 0.857 ± 0.030 | 0.798–0.916 | <0.001 | 326.5 | 81.4 | 79.9 | 67.1 | 89.5 | 4.050 | 0.233 | 80.4 |
| CKD4‐5 versus SLE without CKD4‐5 | |||||||||||
| HE4 | 0.959 ± 0.013 | 0.934–0.984 | <0.001 | 390.3 | 97.1 | 84.5 | 55.7 | 99.3 | 6.265 | 0.034 | 86.6 |
| Urea | 0.944 ± 0.020 | 0.904–0.985 | <0.001 | 12.35 | 91.4 | 86.2 | 57.1 | 98.0 | 6.623 | 0.100 | 87.1 |
| UA | 0.847 ± 0.036 | 0.776–0.917 | <0.001 | 483.5 | 74.3 | 85.1 | 50.0 | 94.3 | 4.987 | 0.302 | 83.3 |
| CKD in LN versus LN without CKD | |||||||||||
| HE4 | 0.915 ± 0.023 | 0.870–0.960 | <0.001 | 380.14 | 81.8 | 88.9 | 87.1 | 84.2 | 7.369 | 0.205 | 85.5 |
| Urea | 0.907 ± 0.025 | 0.858–0.956 | <0.001 | 10.25 | 84.8 | 83.3 | 82.4 | 85.7 | 5.078 | 0.180 | 84.1 |
| UA | 0.813 ± 0.037 | 0.740–0.887 | <0.001 | 479.5 | 66.7 | 86.1 | 81.5 | 73.8 | 4.799 | 0.387 | 76.8 |
Note: The unit of Cutoff was picomolar, millimolar per liter, and micromolar per liter for HE4, urea, and UA, respectively.
AUC, area under curve; UA, uric acid; LN, lupus nephritis; SLE, systemic lupus erythematosus; CKD, chronic kidney disease; Sen, sensitivity; Spe, specificity; PPV, positive predictive value; NPV, negative predictive value; PLR, positive likehood ratio; NLR, negative likehood ratio.
Serum HE4 level in SLE with CKD patients (n = 70) was 564.2 pM (range: 156.4–7084.9), significantly higher than that was in SLE without CKD patients (median, range: 89.1 pM, 16.7–1218.6) (P < 0.001). Since serum creatinine was used to calculate eGFR, its diagnostic performance for CKD was not assessed by an ROC curve. However, correlations between HE4 and creatinine (r s = 0.638, P < 0.001) and eGFR (r s = −0.604, P < 0.001) were statistically significant in SLE with CKD patients (Fig. 3). ROC curves showed the optimum cutoff value of diagnosing CKD in SLE was 233.9 pM (AUC = 0.948, 95% CI: 0.922–0.975, sensitivity 92.9%, specificity 93.5%), 10.25 mmol/l (AUC = 0.920, 95% CI: 0.877–0.963, sensitivity 81.4%, specificity 89.9%), and 326.5 μmol/l (AUC = 0.857, 95% CI: 0.798–0.916, sensitivity 81.4%, specificity 79.9%) for HE4, urea, and UA, respectively. Moreover, in SLE patients with CKD4‐5 (eGFR < 30 ml/min/1.73 m2), the AUC was 0.959 (95% CI: 0.934–0.984) with sensitivity of 97.1% and specificity of 84.5%, 0.944 (95%CI: 0.904–0.985) with sensitivity 91.4% and specificity 86.2% and 0.847 (95% CI: 0.776–0.917) with sensitivity 74.3% and specificity 85.1%, respectively, for HE4, urea, and UA. Also, we investigated the diagnostic performance of HE4 for CKD in LN patients. Similarly, HE4 showed a great value for distinguishing CKD from LN patients (Fig. 4 and Table 3).
Figure 3.
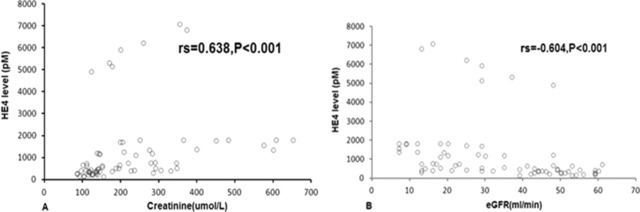
Scatterplots showing the correlation between HE4 and creatinine(A) and eGFR(B).
Figure 4.
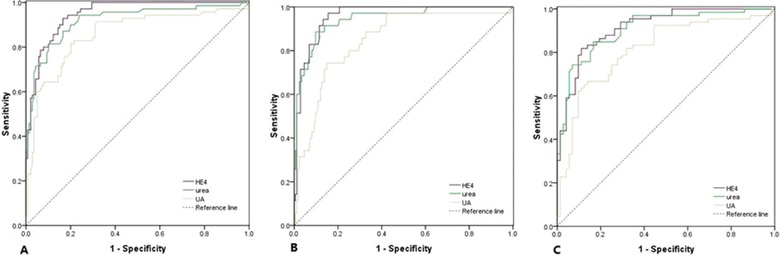
ROC curves of HE4, urea, and UA for the diagnosis of CKD in SLE patients. (A) CKD versus SLE without CKD, (B) CKD4‐5 versus SLE without CKD4‐5, (C) LN with CKD versus LN without CKD.
Discussion
This is, to our knowledge, the first study to investigate the association of serum HE4 level with LN or CKD in SLE patients. In this study, we found that increased serum HE4 is independently associated with the development of LN or CKD in SLE patients. Furthermore, HE4 could be used as a novel and valuable indicator for diagnosis of LN or CKD in SLE.
Although proteinuria, serum creatinine, C3, C4, anti‐dsDNA antibody, etc. has currently been used to screen and monitor LN development in SLE patients, their individual predictive value is modest 31. The diagnosis of LN needs to be confirmed by a renal biopsy. However, as an invasive procedure, repeated renal biopsies are not always accepted by patients. Moreover, the global kidney status cannot be reliably reflected by every renal biopsy. Accordingly, it is of great importance to identify a noninvasive biomarker that can early differentiate LN especially before obvious structural renal damage and loss of renal function from SLE for providing a window to treat early LN and obviate severe renal tissue injury. In current study, we found that serum HE4 level in LN patients is significantly higher than that in SLE patients who show even higher HE4 level than healthy controls do. Next, our multivariate analysis showed that HE4, IgG, and SLEDAI score are closely independently associated with LN development in SLE patients. As can be expected, serum IgG inversely correlates with LN, since an amount of IgG is consumed to form glomerular deposition of immune complexes. The association of SLEDAI score with LN is also expected as it is a standard parameter used for defining active LN. Interestingly, our data demonstrated no independent association of complement and anti‐dsDNA antibody with LN development. In fact, it has been widely reported that they are not specific and could not be used as optimal biomarkers for LN 32, 33, 34, 35, 36.
Next, our results showed a significant diagnostic performance of serum HE4 for LN. Furthermore, the performance is even better than that of serum creatinine, urea, and UA. Especially in SLE patients without CKD or with normal eGFR, the AUCs for HE4 still reached 0.818 and 0.778, respectively, although they were decreased, compared with those in total SLE patients. In contrast, the AUCs for serum creatinine, urea, and UA are below 0.75 in SLE patients without CKD or with normal eGFR. These results suggested that serum HE4 could be a promising, useful biomarker for early diagnosis of LN, especially before obvious structural renal damage and loss of renal function, in SLE patients.
Also, we explored, in this study, the association of serum HE4 with CKD in SLE patients. Our data demonstrated that HE4 is the only variable associated with CKD development after controlling for confounders. Interestingly, LN and SLEDAI score no longer remain independent associated with CKD. The results, comparable to those in two previous studies 8, 37, which found that the adjusted mean nonrenal SLEDAI or WHO classification for LN by biopsy is not a critical risk factor in predicting CKD development in SLE patients, mean that CKD development may be independent of LN and SLE activity. Prompt identification for CKD is necessary in SLE patients, with or without LN. We found, in current study, serum HE4 is a good biomarker for identifying CKD in SLE patients. It can also be well used to identify ESRD or pre‐ESRD (CKD4‐5).
Although HE4 has been approved by U.S. Food and Drug Administration for monitoring recurrence and progression of ovarian cancer, its clinical value has been frequently challenged by emerging studies that found significant increment of serum HE4 in CKD, renal failure, and heart failure 20, 21, 22, 23. Our study adds additional evidence that serum HE4 could be a sensitive and specific biomarker for diagnosis of LN and CKD in SLE patients. Despite little known about the reasons for close association between HE4 and LN or CKD development in SLE, the facts that HE4 might have immunomodulatory properties and that it can be expressed in kidney could provide some explanations 38, 39, since both LN and CKD are caused by systemic inflammation in SLE.
Although the precise mechanisms of HE4 elevation remain unknown, the renal dysfunction, which leads to the diminution of HE4 clearance, may be the main origin of HE4 elevation, because both the previous study 21 and our data indicated that the HE4 level is also significantly elevated in renal dysfunction by various risk factors rather than SLE. Furthermore, the elevated HE4 could contribute to CKD fibrosis, since a previous study reported that HE4 is a fibroblast‐derived mediator of fibrosis and administration of HE4‐neutralizing antibodies inhibited renal fibrosis 40. Therefore, HE4 elevation and renal dysfunction might be an interactive loop.
Some limitations should be addressed. First, our single‐center study included relatively small size of population, which might bias the relationship between HE4 and LN or CKD in SLE patients. Therefore, the results from our data should be interpreted with caution and be further confirmed in a larger size of studies. Second, this is a cross‐sectional study not designed to determine whether baseline or serial serum HE4 level can predict LN or CKD onset and progression. Also, this study cannot confirm a causative relationship between HE4 and LN or CKD. Future prospective studies are needed to establish the causation.
In summary, our study showed significantly increased serum HE4 level in SLE patients for the first time. In the clinical practice, use of different cutoff values of HE4 excellently discriminates between SLE patients with LN and CKD, confirming the important role of HE4 as a biomarker for the early diagnosis of LN and CKD in SLE.
Grant sponsor: The China National Natural Science Foundation Council (81001333, 81571591); Grant sponsor: Research Special Fund for Public Welfare Industry of Health; Grant number: 201202004.
Contributor Information
Lin Zhou, Email: lynnzhou36@163.com.
Yan Liang, Email: liangyan0829@163.com.
References
- 1. Seshan SV, Jennette JC. Renal disease in systemic lupus erythematosus with emphasis on classification of lupus glomerulonephritis advances and implications. Arch Pathol Lab Med 2009; 133:233–248. [DOI] [PubMed] [Google Scholar]
- 2. Korbet SM, Lewis EJ, Schwartz MM, et al. Factors predictive of outcome in severe lupus nephritis. Am J Kidney Dis 2000. 35: 904–14. [DOI] [PubMed] [Google Scholar]
- 3. Esdaile JM, Joseph L, Mackenzie T, et al. The benefit of early treatment with immunosuppressive agents in lupus nephritis. J Rheumatol 1994;21:2046–51. [PubMed] [Google Scholar]
- 4. Faurschou M, Starklint H, Halberg P, et al. Prognostic factors in lupus nephritis: Diagnostic and therapeutic delay increases the risk of terminal renal failure. J Rheumatol 2006;33:1563–1569. [PubMed] [Google Scholar]
- 5. Siso A, Ramos‐Casals M, Bove A, et al. Outcomes in biopsy‐proven lupus nephritis: evaluation of 190 white patients from a single center. Medicine 2010;89:300–307. [DOI] [PubMed] [Google Scholar]
- 6. Sun HO, Hu WX, Xie HL, et al. Long‐term outcome of Chinese patients with membranous lupus nephropathy. Lupus 2008;17:56–61. [DOI] [PubMed] [Google Scholar]
- 7. Reich HN, Gladman DD, Urowitz MB, et al. Persistent proteinuria and dyslipidemia increase the risk of progressive chronic kidney disease in lupus erythematosus. Kidney Int 2011; 79:914–920. [DOI] [PubMed] [Google Scholar]
- 8. Moon SJ, Kwok SK, Ju JH, et al. Predictors of chronic kidney disease in Korean patients with lupus nephritis. J Rheumatol 2011; 38:2588–2597. [DOI] [PubMed] [Google Scholar]
- 9. Ingelfinger JR. Acute kidney injury and chronic kidney disease as interconnected syndromes. N Engl J Med 2014;371:58–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kirchhoff C, Habben I, Ivell R, et al. A major human epididymisspecific cDNA encodes a protein with sequence homology to extracellular proteinase inhibitors. Biol Reprod 1991;45:350–357. [DOI] [PubMed] [Google Scholar]
- 11. Bingle L, Singleton V, Bingle CD. The putative ovarian tumour marker gene HE4 (WFDC2), is expressed in normal tissues and undergoes complex alternative splicing to yield multiple protein isoforms. Oncogene 2002;21:2768–2773. [DOI] [PubMed] [Google Scholar]
- 12. Bingle L, Cross SS, High AS, et al. WFDC2 (HE4): A potential role in the innate immunity of the oral cavity and respiratory tract and the development of adenocarcinomas of the lung. Respir Res 2006;7:61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Galgano MT, Hampton GM, Frierson HF Jr. Comprehensive analysis of HE4 expression in normal and malignant human tissues. Mod Pathol 2006;19:847–853. [DOI] [PubMed] [Google Scholar]
- 14. Moore RG, McMeekin DS, Brown AK, et al. A novel multiple marker bioassay utilizing HE4 and CA125 for the prediction of ovarian cancer in patients with a pelvic mass. Gynecol Oncol 2009;112:40–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Andersen MR, Goff BA, Lowe KA, et al. Use of a symptom index, CA125, and HE4 to predict ovarian cancer. Gynecol Oncol 2010;116:378–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Nolen B, Velikokhatnaya L, Marrangoni A, et al. Serum biomarker panels for the discrimination of benign from malignant cases in patients with an adnexal mass. Gynecol Oncol 2010;117:440–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Huhtinen K, Suvitie P, Hiissa J, et al. Serum HE4 concentration differentiates malignant ovarian tumours from ovarian endometriotic cysts. Br J Cancer 2009;100:1315–1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Anastasi E, Marchei GG, Viggiani V, et al. HE4: A new potential early biomarker for the recurrence of ovarian cancer. Tumour Biol 2010;31:113–119. [DOI] [PubMed] [Google Scholar]
- 19. Wang J, Gao J, Yao H, et al. Diagnostic accuracy of serum HE4, CA125 and ROMA in patients with ovarian cancer: a meta‐analysis. Tumour Biol 2014;35:6127–6138. [DOI] [PubMed] [Google Scholar]
- 20. Romeo V, Framarino Dei Malatesta M, Nudo F, et al. Is HE4 serum level a valid screening test in women candidates for kidney transplant? A case report and a review of literature.Clin Ter 2014;165:e162–e165. [DOI] [PubMed] [Google Scholar]
- 21. Jr Nagy B, Krasznai ZT, Balla H, et al. Elevated human epididymis protein 4 concentrations in chronic kidney disease. Ann Clin Biochem 2012;49:377–380. [DOI] [PubMed] [Google Scholar]
- 22. de Boer RA, Cao Q, Postmus D, et al. The WAP four‐disulfide core domain protein HE4: a novel biomarker for heart failure. JACC Heart Fail 2013;1:164–169. [DOI] [PubMed] [Google Scholar]
- 23. Escudero JM, Auge JM, Filella X, et al. Comparison of serum human epididymis protein 4 with cancer antigen 125 as a tumor marker in patients with malignant and nonmalignant diseases. Clin Chem 2011, 57:1534–1544. [DOI] [PubMed] [Google Scholar]
- 24. Hochberg MC. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum 1997; 40:1725. [DOI] [PubMed] [Google Scholar]
- 25. Renal Disease Subcommittee of the American College of Rheumatology Ad Hoc Committee on Systemic Lupus Erythematosus Response Criteria . The American College of Rheumatology response criteria for proliferative and membranous renal disease in systemic lupus erythematosus clinical trials. Arthritis Rheum 2006; 54: 421–432. [DOI] [PubMed] [Google Scholar]
- 26. Levey AS, Eckardt KU, Tsukamoto Y, et al. Definition and classification of chronic kidney disease: A position statement from Kidney Disease—Improving Global Outcomes (KDIGO). Kidney Int 2005;67: 2089–2100. [DOI] [PubMed] [Google Scholar]
- 27. Bombardier C, Gladman DD, Urowitz MB, Caron D, Chang CH. Derivation of the SLEDAI: a disease activity index for lupus patients. Arthritis Rheum 1992; 35: 630–640. [DOI] [PubMed] [Google Scholar]
- 28. Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med 2009, 150:604–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Matsushita K, Mahmoodi BK, Woodward M, et al. Comparison of risk prediction using the CKD‐EPI equation and the MDRD study equation for estimated glomerular filtration rate. JAMA 2012 307:1941–1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Shen Q, Fan J, Yang XR, et al. Serum DKK1 as a protein biomarker for the diagnosis of hepatocellular carcinoma: a large‐scale, multicentre study. Lancet Oncol 2012;13:817–826. [DOI] [PubMed] [Google Scholar]
- 31. Bertsias GK, Tektonidou M, Amoura Z, et al. Joint European League Against Rheumatism and European Renal Association‐European Dialysis and European Renal Association‐European Dialysis and Transplant Association (EULAR/ERA‐EDTA) recommendations for the management of adult and paediatric lupus nephritis. Ann Rheum Dis 2012;71:1771–1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Illei GG, Tackey E, Lapteva L, Lipsky PE, et al. Biomarkers in systemic lupus erythematosus: II. Markers of disease activity. Arthritis Rheum 2004:50:2048–2065. [DOI] [PubMed] [Google Scholar]
- 33. Illei GG, Lipsky PE. Biomarkers in systemic lupus erythematosus. Curr Rheumatol Rep 2004, 6:382–390. [DOI] [PubMed] [Google Scholar]
- 34. Nagy G, Brozik M, Varga L, et al. Usefulness of detection of complement activation products in evaluating SLE activity. Lupus 2000, 9:19–25. [DOI] [PubMed] [Google Scholar]
- 35. Schwartz N, Michaelson JS, Putterman C. Lipocalin‐2, TWEAK, and other cytokines as urinary biomarkers for lupus nephritis. Ann NY Acad Sci 2007, 1109:265–274. [DOI] [PubMed] [Google Scholar]
- 36. Schwartz N, Rubinstein T, Burkly LC, et al. Urinary TWEAK as a biomarker of lupus nephritis: A multicenter cohort study. Arthritis Res Ther 2009;11:R143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Reich HN, Gladman DD, Urowitz MB, et al. Persistent proteinuria and dyslipidemia increase the risk of progressive chronic kidney disease in lupus erythematosus. Kidney Int 2011;79:914–920. [DOI] [PubMed] [Google Scholar]
- 38. Bingle CD, Vyakarnam A. Novel innate immune functions of the whey acidic protein family. Trends Immunol 2008;29:444–453. [DOI] [PubMed] [Google Scholar]
- 39. Scott A, Weldon S, Taggart CC. SLPI and elafin: multifunctional antiproteases of the WFDC family. Biochem Soc Trans 2011;39:1437–1440. [DOI] [PubMed] [Google Scholar]
- 40. LeBleu VS, Teng Y, O'Connell J T, et al. Identification of human epididymis protein‐4 as a fibroblast‐derived mediator of fibrosis. Nat Med 2013;19:227–231. [DOI] [PMC free article] [PubMed] [Google Scholar]


