Abstract
Background
The metabolic syndrome (MetS) is a cluster of metabolic abnormalities including insulin resistance, dyslipidemia, high blood pressure, and abdominal adiposity. Obese patients develop leptin resistance, and an increased waist circumference (WC) due to deposition of abdominal fat. The aim of this study was to evaluate the association between circulating leptin levels and MetS among sample adult Mexican workers.
Method
A total of 204 workers aged 20–56 were evaluated. Anthropometric index, blood pressure, fasting plasma glucose, and lipid profile were measured by spectrophotometric methods. Fasting insulin and leptin were measured by inmunoenzimatic methods. Furthermore, homeostasis model assessment for insulin resistance (HOMA‐IR) was calculated.
Results
The prevalence of MetS according to the ATP‐III criteria was 33.8% and leptin concentrations were 2.5 times higher in women than men. Subjects with MetS had higher levels of leptin (26.7 ± 13.7) compared with those without MetS (20.1 ± 13.9; P <0.001). Leptin increased significantly while BMI increased as well (normal 14.0 ± 8.9, overweight 22.7 ± 11.7 and obese 31.4 ± 14.6) in addition to other variables such as WC, HDL‐C, insulin levels, and HOMA index. Each component of MetS was stratified by sex and submitted by linear regression with a 95% of accuracy. The 50% and 53% of the BMI is explained by the concentration of leptin in men and women, respectively (P < 0.001).
Conclusion
This study found that leptin was associated with the MetS, especially in obesity and insulin resistance, indicating a high risk for university workers to develop hypertension, DM2, and cardiovascular disease.
Keywords: leptin, obesity, metabolic syndrome
INTRODUCTION
The National Cholesterol Education Program: Adult Treatment Panel III 1 defines metabolic syndrome (MetS) as a cluster of metabolic abnormalities including one or any three of the following characteristics: abdominal adiposity, dyslipidemia, high blood pressure, fasting glucose, or insulin resistance. In Mexico, a prevalence of MetS of 36.8% in adults has been reported, which has rapidly increased in the past years, presenting a serious high risk of both type‐2 diabetes and cardiovascular disease (CVD) 2 patients. The etiology of the MetS still remains obscure, its various pathogenic mechanisms proposed are incompletely defined, and is under intense investigation 3. Recent research suggests that the adipocyte‐derived hormone leptin could be an important factor linking obesity, MetS, and CVD 4. Leptin plays a major role in energy regulation, control of appetite, and neuroendocrine regulation. It is secreted mainly by white adipose tissue, and the levels are positively correlated with the amount of body fat 5. Circulating leptin levels reflect, primarily, the amount of energy stored in fat, and, secondarily, the high changes in caloric intake 6. It has been reported that obese patients develop leptin resistance and increased waist circumference (WC) due to the deposition of abdominal fat 7. Circulating leptin levels were positively correlated with hiperlepidemias, diabetes mellitus, insulin resistance, and hypertension, whereby elevated leptin levels could be a risk factor for developing MetS in obese and non‐obese subjects. 8. Several studies have shown that in addition to the relationship between leptin and MetS components, there is also an ethnic component that plays an important role in the secretion of leptin 9, 10. Further studies have been conducted in different populations such as Korean, Caucasian, and Iranian 11, 12 where it has been demonstrated that leptin was positively correlated with MetS. In Mexico, there are a few reports about leptin and MetS. Mirza et al. 13 reported, in the border town of Texas‐Mexico, the correlation positive of the adiponectin/leptin index as a marker of MetS with high sensitivity and specificity. In this study, we aimed to evaluate the influence of obesity on the association between circulating leptin levels and MetS in a Mexican population from center of the country.
MATERIALS AND METHODS
Study Population
From October 2007 to April 2008, the workers have convened for participating in the study “Biochemical and molecular characterization of obesity in workers of Universidad Autónoma del Estado de Morelos.” All volunteers were informed in detail about the study and signed an informed consent. The following information was collected from all the volunteers: (1) Anthropometric data, (2) biochemical data, and (3) a lifestyle questionnaire about sociodemographic information, dietary habits, smoking, and alcohol consumption, as well as for determining their risk factors for diabetes, CVD, and hypertension. Subjects with missing information for the components of MetS such as WC, triglycerides (TG), HDL cholesterol, or blood pressure were excluded from the analysis. This study was approved by the ethics committee of the National Institute of Neurology and Neurosurgery (INNN) of the Ministry of Health and the labor union of the Universidad Autonoma del Estado de Morelos who reported no conflict of interest.
Anthropometry
WC was measured between the lower rib and the iliac crest, with the arms relaxed on the sides. This methodology was conducted during a clinical session, performed by a trained research personal. Briefly, the weight was measured while participants wore light clothing, using scales previously calibrated to an accuracy of ± 0.5 kg. Height was measured using a stadimeter with precision of 1 mm, measuring the participants without shoes, erect, and with the back against a flat surface. A calibrated sphygmomanometer Citizen TM digital oscillometric tensiometer (Citizen Watch Co., Ltd., Tokyo, Japan, model no. CH‐432B) was used to determine blood pressure at intervals of 5 min. The average of two measurements was recorded as systolic and diastolic blood pressures.
Biochemical Parameters
Participants were instructed to fast overnight for at least 10 h. Blood (5 ml) was obtained by venipuncture according to the standard protocol. The blood samples were centrifuged and the serum was analyzed. Fasting plasma glucose (FPG), total cholesterol (CHOD), and TG were determined by enzymatic colorimetric assay methods, using glucose oxidase test and cholesterol oxidase, p‐amino phenazone (Roche‐Hitachi 912 kit, Roche Diagnostic, Basel, Switzerland; intra‐ and interassay, coefficient of variation was 2.5% and 2.8%, respectively). High‐density lipoprotein cholesterol (HDL‐C) and low‐density cholesterol (LDL‐C) were measured using enzymatic direct methods: PEG cholesterol esterase and PEG cholesterol oxidase (HDL‐C plus third‐generation and LDL—second‐generation Roche‐Hitachi 912 kit, Roche Diagnostic).
Insulin levels were determined by an electrochemiluminicence immunoassay, using two monoclonal anti‐insulin chemiluminicent antibodies (Elecsys 2010 kit, Roche Diagnostic; Cat: 03184897). Serum leptin levels were measured by ELISA kit Human leptin (Millipore Corporation, MA, Cat EZHL‐80 SK). The sensitivity of the test was 0.5 ng/ ml. Homeostasis model assessment for insulin resistance (HOMA‐IR) was calculated as reported in Matthews et al. 14 using the following formula: Fasting insulin (mU/l) × Fasting glucose (mg/dl)/405.
Diagnosis of MetS
Diagnosis of MetS was based on the criteria described by the National Cholesterol Education Program: Adult Treatment Panel III (NCEP‐ATP III). We considered participants showing at least three of the following five factors:
Abdominal obesity: a WC ≥ 90 cm for men and ≥80 cm for women.
Triglyceride: a triglyceride level ≥150 mg/dl.
Low HDL cholesterol: HDL cholesterol <40 mg/dl for men and <50 mg/dl for women.
High blood pressure: a systolic blood pressure ≥130 mmHg or a diastolic blood pressure ≥85 mmHg.
Hyperglycemia: FPG levels ≥100 mg/dl International Diabetes Federation (IDF).
Statistical Analyses
With the information gathered from the questionnaires, anthropometric measurements, and laboratory results, a database has been organized. The following variables have been coded: BMI (normal, 18.5–24.9; overweight, 25.0–29.9; obese, ≥ 30 kg/m2), WC (normal ≤89 for men, ≤79 for women; central obesity ≥90 for men, ≥80 for women), waist–hip ratio (normal ≤0.89 for men, ≤0.84 for women; abnormal ≥0.90 for men, ≥0.85 for women), blood pressure (normal ≤129 mmHg systolic and ≤84 mmHg diastolic; high ≥130 mmHg systolic or ≥85 mmHg diastolic), glucose (normal ≤99; high ≥100 mg/dl), TG (recommended ≤149; borderline 150–199; risk ≥200), CHOD (recommended ≤199; borderline 200–239; risk ≥240), HDL (recommended ≥35; risk ≤34), LDL (recommended ≤129; borderline 130–159, high risk ≥160), insulin (recommended ≤20; risk ≥20.1), and HOMA (recommended ≤2.7; risk ≥2.71) conducted a descriptive analysis of the studied population where we obtained the proportion from each of the strata evaluated. Subsequently, we conducted a comparison of the mean leptin values by Student's t‐test and ANOVA test. Association between leptin and components of MetS was analyzed with a chi‐square test. Finally, we performed an analysis of Pearson correlation and linear regression stratified by sex. All statistical tests were performed with a confidence interval of 95% (α = 0.05). Statistical analysis was performed using SPSS 15.0.
RESULTS
This study involved 204 adults aged between 21 and 55, whose characteristics are described in Table 1. Of this population, 79.4% were women, 82.6% were nonsmokers, and 49.3% reported exercising. Based on the body mass index, it was observed that 38.2% of the population is obese and 19.1% was overweight. Central obesity was defined by the WC, and the 74.4% of the population was above the cutoff values and 74.9% of population was above the cutoff values for the waist‐to‐hip ratio (WHR). We observed high blood pressure in 34.3%, and the prevalence of MetS (according 2001, ATP III definition) in men and women was 33.8%. Comparing leptin concentration with different anthropometric and biochemical parameters (Table 2), we observed that women had 2.5 times higher leptin concentrations than men (25.3 ± 14.0 in women, 10.7 ± 7.0 in men). Subjects with MetS had higher levels of leptin (26.7 ± 13.7) compared with those without MetS (20.1 ± 13.9;(P < 0.001). In addition, leptin increases significantly with increases in BMI (normal 14.0 ± 8.9, overweight 22.7 + 11.7 and obese 31.4 + 14.6) as well as variables such as WC, insulin levels, and HOMA index. The other cardiovascular risk markers such as blood pressure, TG, and cholesterol showed a low association without statistical significance. Each component of MetS was stratified by sex and submitted by linear regression with a 95% of confidence (Fig. 1), and we observed that the augmentation of BMI in 50% for men and 53% for women was due the concentration of leptin (P < 0.001).
Table 1.
General Characteristic From a Sample of Mexican University Workers
| Variable | n | Percentage | |
|---|---|---|---|
| Sex | Men | 42 | 20.6 |
| Women | 162 | 79.4 | |
| Age group | 21–30 | 39 | 19.1 |
| 31–40 | 69 | 33.8 | |
| 41–50 | 67 | 32.8 | |
| 51+ | 29 | 14.2 | |
| Smoking status | Yes | 35 | 17.2 |
| No | 169 | 82.8 | |
| Exercise status | Yes | 100 | 49.0 |
| No | 104 | 51.0 | |
| BMI | Normal | 87 | 42.6 |
| Overweight | 39 | 19.1 | |
| Obese | 78 | 38.2 | |
| MetS | No | 135 | 66.2 |
| Yes | 69 | 33.8 | |
| Waist circumference | Normal | 53 | 26.0 |
| Obesity | 151 | 74.0 | |
| Waist‐to‐hip ratio | Normal | 52 | 25.5 |
| High | 152 | 74.5 | |
| Blood pressure | Normal | 134 | 65.7 |
| High | 70 | 34.3 | |
| Glucose | Normal | 189 | 92.6 |
| High | 15 | 7.4 | |
| Triglycerides | Normal | 134 | 65.7 |
| Borderline | 34 | 16.7 | |
| High risk | 36 | 17.6 | |
| Total cholesterol | Normal | 128 | 62.7 |
| Borderline | 62 | 30.4 | |
| High risk | 14 | 6.9 | |
| HDL‐C | Normal | 169 | 82.8 |
| High risk | 35 | 17.2 | |
| LDL‐C | Normal | 135 | 66.2 |
| Borderline | 55 | 27.0 | |
| High risk | 14 | 6.9 | |
| Insulin | Normal | 180 | 88.2 |
| High risk | 24 | 11.8 | |
| HOMA | Normal | 143 | 70.1 |
| High risk | 61 | 29.9 | |
Table 2.
Characteristics of Mexican University Workers According to Leptin Levels
| Variable | Media | SD | P | |
|---|---|---|---|---|
| Sex | Men | 10.7 | 7.0 | <0.001* |
| Women | 25.3 | 14.0 | ||
| Age group | 21–30 | 21.7 | 13.4 | 0.836 |
| 31–40 | 21.3 | 13.4 | ||
| 41–50 | 23.2 | 15.2 | ||
| 51+ | 23.5 | 14.9 | ||
| Smoking status | Yes | 24.3 | 17.1 | 0.469 |
| No | 22.1 | 13.5 | ||
| Exercise status | Yes | 21.7 | 14.3 | 0.505 |
| No | 23.1 | 14.2 | ||
| BMI | Normal | 14.0 | 8.9 | <0.001* |
| Overweight | 22.7 | 11.7 | ||
| Obese | 31.4 | 14.6 | ||
| MetS | No | 20.1 | 13.9 | <0.001* |
| Yes | 26.7 | 13.7 | ||
| Waist circumference | Normal | 13.2 | 9.1 | <0.001* |
| Obesity | 25.5 | 14.3 | ||
| Waist‐to‐hip ratio | Normal | 18.8 | 10.9 | 0.017* |
| High | 23.5 | 15.0 | ||
| Blood pressure | Normal | 20.6 | 12.7 | 0.029* |
| High | 25.5 | 16.3 | ||
| Glucose | Normal | 22.3 | 14.2 | 0.853 |
| High | 23.0 | 14.6 | ||
| Triglycerides | Normal | 21.8 | 14.3 | 0.043* |
| Borderline | 27.5 | 15.2 | ||
| High risk | 19.4 | 11.6 | ||
| Total cholesterol | Normal | 21.7 | 14.1 | 0.356 |
| Borderline | 24.2 | 14.8 | ||
| High risk | 19.2 | 12.1 | ||
| HDL‐C | Normal | 22.0 | 14.2 | 0.451 |
| High | 24.0 | 14.0 | ||
| LDL‐C | Normal | 22.5 | 14.3 | 0.668 |
| Borderline | 22.7 | 14.7 | ||
| High risk | 19.0 | 11.2 | ||
| Insulin | Normal | 20.9 | 13.7 | <0.001* |
| High | 32.9 | 13.2 | ||
| HOMA | Normal | 19.5 | 12.8 | <0.001* |
| High | 28.9 | 15.2 | ||
* p < 0.05 statistical significance.
Figure 1.
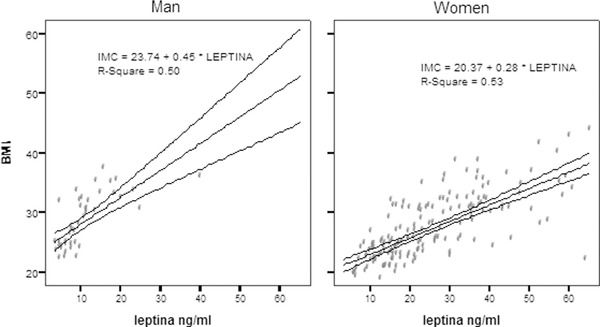
The 50% and 53% of the body mass index (BMI) is explained by the concentration of leptin in men and women, respectively. For each nanogram per milliliters of leptin increased, the BMI is increased by 0.45 for men and 0.28 for women.
DISCUSSION
In this study, we observed the main determinants of leptin levels were adiposity and sex. Women have higher leptin concentrations than men, and there is a direct correlation between the concentration of leptin and the body mass index. These data are consistent with those reported in other populations 8, 11.
Central obesity alone is considered a risk factor for cardiovascular disease and is defined as an accumulation of visceral fat. Several studies have shown the relationship between central adiposity hyperinsulinemia, hypertriglyceridemia, and hypertension to better predict the incidence of MetS independent of BMI 15. In this study, we found that excess of visceral fat is positively associated with lipid profile (Cholesterol, LDL, and TG) and inversely with HDL. Hypertriglyceridemia was found 34.3% of the population, 37.3% has hypercholesterolemia, and 17.2% has high HDL‐risk cholesterol. The prevalence of obesity and MetS was 38.2% and 33.8%, respectively. Patients with MetS have higher atherogenic risk, and they are more likely to develop type‐2 diabetes and heart disease. These results are similar to data reported in the last national health survey of Mexico 16
The insulin resistance and hyperleptinemia are characteristic of obesity and MetS, accumulation of fat in the abdominal region as TG causes metabolic disorders, particularly in insulin sensitivity. The best connection between obesity and DM2 is the resistance to insulin, which is known in both to precede abnormal glucose 17. Leptin acts in the insulin signaling hormones because both share the same signaling pathway. Leptin receptor and insulin receptors are expressed by brain neurons involved in energy intake 18. The results presented here show that leptin is strongly correlated with BMI and central obesity, as well as with risk factors for DM2 as insulin and HOMA index. We also observed the increase in plasma concentration of leptin is proportional to the degree of central adiposity, causing leptin resistance. Mayers et al. 19 conducted similar studies on population and suggested there is a transport mechanism of leptin from the blood to the CNS that is saturable, which causes leptin resistance. We do not know the exact mechanism that causes leptin resistance, what is known is that resistance to leptin stimulates hyperphagia, decreased energy expenditure, and stores excess of calories as visceral adipose tissue 7. In the clinic, leptin has been administered to decrease body weight and there has not been a significant reduction, supporting the idea that leptin is more an indicator of energy expenditure than a body weight controller 5.
CONCLUSIONS
Leptin is strongly associated with MetS and its components, especially with central obesity and insulin resistance, which are indicators of metabolic disorders such as dyslipidemia, DM2, and hypertension. This study has found that leptin was associated with the MetS, indicating a high risk of university workers to develop hypertension, DM2, and cardiovascular disease. We strongly recommend creating educational programs that include healthy food habits for the high‐risk population to reduce the chance of developing MetS and the pathology associated with the syndrome.
ACKNOWLEDGMENTS
The authors acknowledge the funding PROMEP—UAEMOR‐CA‐76, at the Faculty of Pharmacy of the University of the State of Morelos and all worker participants of this study.
Grant sponsor: PROMEP—UAEMOR‐CA‐76.
REFERENCES
- 1. NCEP_ATP III Expert panel on detection, evaluation and treatment of high blood cholesterol in adults . Executive summary of the third report of the National Cholesterol Education Program. Expert panel on detection, evaluation and treatment of high cholesterol. JAMA 2001;285(19):2486–2497. [DOI] [PubMed] [Google Scholar]
- 2. Rojas R, Aguilar‐Salinas CA, Jiménez‐Corona A, et al. Metabolic syndrome in Mexican adults: Results from the National Health and Nutrition Survey 2006. Salud Pública Méx 2010;1(52):S11–S18. [DOI] [PubMed] [Google Scholar]
- 3. Alberti KG, Zimmet P, Shaw J. The metabolic syndrome: A new worldwide definition. Lancet 2005;366:1059–1062. [DOI] [PubMed] [Google Scholar]
- 4. Patel SB, Reams GP, Spear RM, Freeman RH, Villarreal D. Leptin: Linking obesity, the metabolic syndrome, and cardiovascular disease. Curr Hypertens Rep 2008;10(2):131–137. [DOI] [PubMed] [Google Scholar]
- 5. Kelesidis T, Kelesidis I, Chou S, Mantzoros CS. Narrative review: The role of leptin in human physiology—Emerging clinical applications. Ann Intern Med 2010;152(2):93–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Williams DL, Schwartz MW. Neuroanatomy of body weight control: Lessons learned from leptin. J Clin Invest 2011;6(121):2152–2155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gade W, Schmit J, Collins M, Gade J. Beyond obesity: The diagnostic and patophysiology of metabolic syndrome. Clin Lab Sci 2010;1(23):51–61. [PubMed] [Google Scholar]
- 8. Esteghamati A, Noshad S, Khalilzadeh O, et al. Contribution of serum leptin to metabolic syndrome in obese and nonobese subjects. Arch Med Res 2011;3(42):244–251. [DOI] [PubMed] [Google Scholar]
- 9. Suyila Q, Cui H, Yang L, Zhao L, Zhang R, Su X. Serum leptin concentrations in Mongolian women. Obes Res Clin Pract 2013;7:75–80. [DOI] [PubMed] [Google Scholar]
- 10. Chateau‐Degat ML, Dewailly E, Poirier P, Gingras S, Egeland GM. Comparison of diagnostic criteria of the metabolic syndrome in 3 ethnic groups of Canada. Metabolism 2008;11(57):1526–1532. [DOI] [PubMed] [Google Scholar]
- 11. Yun JE, Kimm H, Jo J, Jee SH. Serum leptin is associated with metabolic syndrome in obese and nonobese Korean populations. Metabolism 2010;59:424–429. [DOI] [PubMed] [Google Scholar]
- 12. Perry HM 3rd, Morley JE, Horowitz M, Kaiser FE, Miller DK, Wittert G. Body composition and age in African‐American and Caucasian women: Relationship to plasma leptin levels. Metabolism 1997;12(46):1399–1405. [DOI] [PubMed] [Google Scholar]
- 13. Mirza S, Qu HQ, Li Q, et al. Adiponectin/leptin ratio and metabolic syndrome in a Mexican American population. Clin Invest Med 2011;5(34):E290–E297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment insulin resistant and beta‐cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985;28:412–419. [DOI] [PubMed] [Google Scholar]
- 15. Shamai L, Lurix E, Shen M, et al. Association of body mass index and lipid profiles: Evaluation of a broad spectrum of body mass index patients including the morbidly obese. Obes Surg 2011;21(1):42–47. [DOI] [PubMed] [Google Scholar]
- 16. Gutiérrez JP, Rivera‐Dommarco J, Shamah‐Levy T, et al. Encuesta Nacional de Salud y Nutrición 2012 Resultados Nacionales. Cuernavaca, México: Instituto Nacional de Salud Pública (MX), 2012. [Google Scholar]
- 17. Yaturu S. Obesity and type 2 diabetes. J Diabetes Mellitus 2011;1(4):79–95. [Google Scholar]
- 18. Singla P, Bardoloi A, Parkash AA. Metabolic effects of obesity: A review. World J Diabetes 2010;1(3):76–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Myers MG Jr, Leibel RL, Seeley RJ, Schwartz MW. Obesity and leptin resistance: Distinguishing cause from effect. Trends Endocrinol Metab 2010;21:643–651. [DOI] [PMC free article] [PubMed] [Google Scholar]


