Abstract
Background
The incidence and prevalence of urinary stone are increasing throughout the world. Compared to the past, recent demographics of patient with urolithiasis compositions are strikingly different. Furthermore, recent clinical studies implied that seasonal cyclicity might influence the distribution of stone composition.
Methods
We sought to determine the trends in pathogenesis of urolithiasis based on urinary stone analyses. Between 2002 and 2014, a total of 2,383 eligible urinary stone samples from different patients were collected in our center. Infrared spectroscopy was used for urinary calculi analysis. A logistic regression analysis was used to investigate the relationship between urinary calculi composition and calendar month (season), gender, and age in north China during the past 13 years.
Results
Calcium‐containing calculi were the most frequent with an overall incidence of 84.1%. Calcium phosphate (CaP) or magnesium ammonium phosphate (MAP) stones were more frequent in females, while monohydrate calcium oxalate (COM), dihydrate calcium oxalate (COD), or uric acid (UA) stones were more common in males. Older individuals were associated with an increased risk of UA stones and a decreased risk of COD, CaP, or cystine stones. Additionally, from 2002 to 2014, the frequency of COD and MAP stone increased, whereas the trend of CaP, UA and cystine stones decreased. However, calendar month (season) was not significantly associated with differences in composition.
Conclusion
This study provides the present distribution of urolithiasis compositions in China. From 2002 to 2014, age and gender were significantly associated with stone composition, whereas calendar month was not.
Keywords: age groups, China, seasons, sex, urolithiasis
Introduction
Worldwide epidemiological data have recently suggested that the incidence and prevalence of urolithiasis are increasing 1, 2, 3, 4. The underlying mechanisms are not entirely clear. To explore the possible etiology and pathophysiology of urinary calculi, physicians may investigate stone composition and abnormalities in urine biochemistry of each stone formed. However, in some cases, the prevalence of stone composition did not correlate with abnormalities in urine biochemistry, implicating the necessity to send for stone constitutional analysis in every different event 5, 6. Moreover, the incidence and composition of urinary lithiasis can be fairly affected by gender and age between countries 7, 8, 9. Besides, clinical observations imply a varying component of urinary calculus as
well as a dynamic of year‐ and season‐related incidences. For instance, the frequency of calcium‐containing stones and magnesium ammonium phosphate (MAP) stones increased and gradually decreased in the last four decades in Japan, respectively 2. Patients were more likely to suffer from calcium oxalate (CaOx) or uric acid (UA) stone during the summer months in the United States 10.
So far, little is known about stone composition analyses in north China where there are four definite seasonal variations. To our knowledge, given yearly and monthly (seasonal) variation of urinary calculi components, there are no published data of current stone analyses in China. In the present study, we examined the distribution of stone types with respect to the differences of calendar month (season), gender, and age in north China over a 13‐year period, from 2002 to 2014.
Material and Methods
Patient Selection
A retrospective review was performed for 2,813 Chinese patients with confirmed stone analysis in the Tianjin Institute of Urological Surgery between January 1, 2002 and December 31, 2014. All the stones were obtained either through spontaneous passage, shock wave lithotripsy (SWL), ureteroscopy (URS), percutaneous nephrolithotomy (PCNL), laparoscopic or open stone surgery. Stone specimens were excluded from the study in case when records for patient's age, gender, or other significant data (n = 318) were not available. Besides, only the first stone presented per person was included in this analysis to avoid overestimating the occurrence of a specific stone composition (n = 2,383).
Stone Analyses
All stones were analyzed in the Tianjin Institute of Urological Surgery using their standard operating procedure. Initially, dry stone specimens were weighed and inspected visually before crushing to reveal internal structure and characteristics. Samples were described by texture, color, and number of fragments submitted. A small part (1 mg) of stone specimen was mixed with potassium bromide (200 mg KBr), powdered, and then pressed into a small tablet that was analyzed by Shimadzu Fourier Transform Infrared Spectrophotometer 8300 (manufactured by Shimadzu Corporation, Japan).
Stone Classification
Stones were classified according to the fundamental component. For example, whewellite was grouped as monohydrate COM; wheddelite was grouped as dihydrate calcium oxalate (COD); while apatite, hydroxylapatite, whitlockite, dahllite, and brushite were grouped as calcium phosphate (CaP) stones. Rare stone compositions, such as xanthine, calcium carbonate, or ammonium urate, were categorized into a group termed “others.” Results for stones containing multiple compositions were reported by the percentage of specific component 11.
Statistical Analysis
A logistic regression analysis was used to assess presence of an individual stone composition using calendar year, calendar month, sex, and age groups (0–29, 30–39, 40–49, 50–59, 60–69, 70–79, and 80+ years) as covariates. Each type of urolithiasis component was analyzed as a dependent variable. The significance level was defined as statistically significant at P < 0.05, very significant at P < 0.01, and highly significant at P < 0.001. Every estimate was provided with odds ratios (OR) and 95% confidence interval (CI). In case OR > 1, the variable was an independent risk factor to the specific composition, when OR < 1 the variable was a protective factor. All statistical analyses were conducted using IBM SPSS, version 20.
Results
Stone Composition
A total of 2,383 patients met our eligibility criteria, and of these, 41.2% contained a single component, 49.2% contained two components, and 9.6% contained more than two components. Calcium‐containing calculi were predominant with an overall prevalence of 84.1%. COM was found in 58.7% of stones, CaP in 48.6%, COD in 21.2%, and UA in 11.8%. Nevertheless, MAP (3.5%), cystine (0.6%), and other rare stone components (0.4%) were less common (Fig. 1). These results, together with their 95% confidence limits, are presented in Table 1 using a logistic regression, the general associations between stone composition and gender, age, calendar year and calendar month. As a result of the small number of rare stone compositions in this population, significance could not be tested for the levels of “others.”
Figure 1.
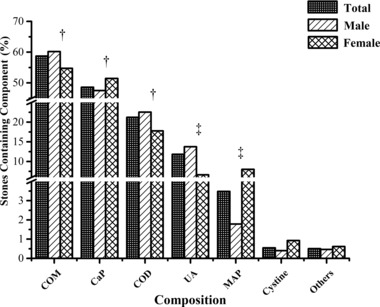
Overall distribution and the variations by gender of stone composition. ‡ P < 0.001; † P < 0.05.
Table 1.
The Essentials of Logistic Regression for Stone Components
| COM | COD | CaP | UA | MAP | Cystine | |
|---|---|---|---|---|---|---|
| Gender: | ||||||
| OR | 0.815 | 0.769 | 1.251 | 0.400 | 4.679 | 2.676 |
| 95% CI | (0.679–0.979) | (0.610–0.970) | (1.041–1.504) | (0.284–0.563) | (2.961–7.395) | (0.887–8.072) |
| P‐value | <0.05 | <0.05 | <0.05 | <0.001 | <0.001 | Not significant |
| Age | ||||||
| OR | 0.945 | 0.877 | 0.819 | 1.480 | 1.087 | 0.638 |
| 95% CI | (0.888–1.005) | (0.813–0.945) | (0.770–0.871) | (1.342–1.632) | (0.917–1.290) | (0.421–0.967) |
| P‐value | Not significant | <0.01 | <0.001 | <0.001 | Not significant | <0.05 |
| Year | ||||||
| OR | 0.996 | 1.031 | 0.955 | 0.960 | 1.083 | 0.854 |
| 95% CI | (0.974–1.018) | (1.003–1.060) | (0.934–0.977) | (0.928–0.992) | (1.011–1.160) | (0.744–0.981) |
| P‐value | Not significant | <0.05 | <0.001 | <0.05 | <0.05 | <0.05 |
| Month | ||||||
| OR | 1.024 | 1.004 | 0.984 | 1.011 | 0.951 | 1.057 |
| 95% CI | (0.999–1.050) | (0.975–1.034) | (0.960–1.009) | (0.973–1.050) | (0.890–1.016) | (0.893–1.251) |
| P‐value | Not significant | Not significant | Not significant | Not significant | Not significant | Not significant |
Effects of “others” were not significant.
Variations According to Gender
In general, 1,736 (72.8%) males and 647 (27.2%) females were included with a sex ratio of 2.7:1. Significant differences in stone composition were observed between males and females (Fig. 1). Women were associated with an increased risk of CaP (OR: 1.251; 95% CI: 1.041–1.504; P < 0.05) or MAP (OR: 4.679; 95% CI: 2.961–7.395; P < 0.001) stones, whereas men were more likely to submit COM (OR: 0.815; 95% CI: 0.679–0.979; P < 0.05), COD (OR: 0.769; 95% CI: 0.610–0.970; P < 0.05), or UA (OR: 0.400; 95% CI: 0.284–0.563; P < 0.001) stones (Table 1). Cysteine stones showed a slightly higher rate in females, however, the changes were not statistically significant. COM stones were most common in both genders, followed by CaP.
Variations According to Age
Patient's age at submission ranged from 1.2 to 92 years. The mean age was 49.0 ± 13.3 years. Significant differences in stone composition were observed among age groups (0–29, 30–39, 40–49, 50–59, 60–69, 70–79, and 80+ years; Fig. 2). Older individuals were associated with an increased risk of UA (OR: 1.480; 95% CI: 1.342–1.632; P < 0.001) stones, whereas the decreasing trends of COD (OR: 0.877; 95% CI: 0.813–0.945; P < 0.001), CaP (OR: 0.819; 95% CI: 0.770–0.871; P < 0.001), or cystine (OR: 0.638; 95% CI: 0.421–0.967; P < 0.05) stones were observed with aging (Table 1). It is of note that, there was a decreasing trend for COM stones and an increasing tendency for MAP stones over age of 70 years, but the general changes by age were not statistically significant. Although COM stones were most frequent in almost every age groups but in age group of ≥80 years; a striking proportional mix of stone type with regard to age groups was observed, with COM stones being relatively more common among the age group of 30–50 years, CaP stones being relatively more frequent among those in the age group of 0–29 years, COD stones being relatively more common among the age group of 30–40 years, and UA stones being relatively more common among those in 70+ years age group. Indeed, UA stones were most prevalent in individuals above 80 years of age (Fig. 2).
Figure 2.
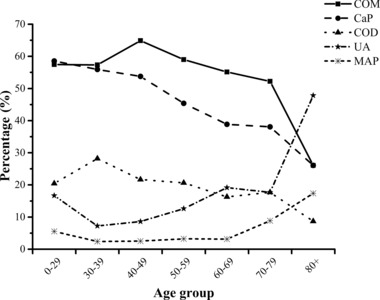
Association of age distribution with stone composition.
Distribution by Calendar Year
Overall, there were significant differences in stone composition in accordance with distribution by calendar year. From 2002 to 2014, a general tendency toward an increasing frequency of COD (OR: 1.031; 95% CI: 1.003–1.060; P < 0.05) and MAP (OR: 1.083; 95% CI: 1.011–1.160; P < 0.05) stones was observed, whereas the incidence of CaP (OR: 0.955; 95% CI: 0.934–0.977; P < 0.001), UA (OR: 0.960; 95% CI: 0.928–0.992; P < 0.05), and cystine (OR: 0.854; 95% CI: 0.744 to 0.981; P < 0.05) stones decreased (Table 1). Although there was an increasing trend for COM stones from 2002 to 2014, the general changes by years were not statistically significant. COM stones were most predominant according to calendar year, followed by CaP, COD, UA, and MAP (Fig. 3).
Figure 3.
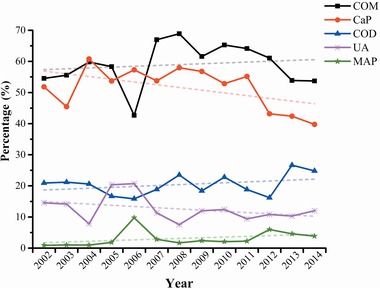
Association of calendar year with stone composition.
Distribution by Calendar Month
Given monthly variations (seasonal cyclicity) in climate and diet, we hypothesized that stone composition would be influenced by calendar month. Nevertheless, overall monthly patterns associated with stone types did not vary significantly (Table 1).
Discussion
This study evaluated the distribution of urinary calculi compositions in relation to the variations of calendar month (season), gender, and age in north China, including a 13‐year period. Our data indicate an increased percentage of CaP or MAP stones submitted from females, whereas males were more likely to submit COM, COD, or UA stones. UA stones were more common in older individuals, while COD, CaP, or cystine stones were more frequent in younger patients. Moreover, from 2002 to 2014, an increasing trend of COD and MAP stone was observed, whereas the incidence of CaP, UA, and cystine stones decreased. However, calendar month (season) was not associated with differences in composition.
On the basis of our data, calcium‐containing calculi (CaOx and/or CaP) were most common with an overall percentage of 84.1%, which was consistent with a historical series from other large referral laboratories 7, 10, 12. Moreover, we observed that COM was found in 58.7% of stones, CaP in 48.6%, COD in 21.2%, UA in 11.8%, MAP in 3.5%, and cystine in 0.6%. These findings were similar with a recent American series in which 83.4% of the stones were composed of calcium in the form of CaOx or CaP, whereas UA, MAP, and cystine stones comprised another 8%, 3%, and 0.35%, respectively 10. Interestingly, although almost identical results were reported in the proportions of calcium‐containing calculi (CaOx and/or CaP), MAP, and cystine stones, our data were considerably different from previous studies in France and south China that found UA calculi comprised about 5.6–6.9% of urinary stones 13, 14, 15. One possible explanation for this discrepancy may be that different dietary habits and metabolism of distinct subpopulations are strongly correlated with the formation of UA stones.
It is well documented that urolithiasis occurs more commonly in men than women. Early epidemiologic studies showed the ratio of urinary calculi in men is 2.2–3.4 times that of women 16. In general, our data included 1,736 (72.8%) males and 647 (27.2%) females with a sex ratio of 2.7:1. However, the reported male‐to‐female rate of stone disease was highly changed among different nations in recent studies 10, 14, 15, 17. In addition, our data indicated there were significant differences in stone composition between males and females. We found that although COM stones were most common in both genders, followed by CaP, men were associated with an increased risk of COM, COD, and UA stones, whereas women were more likely to submit CaP and MAP stones. Similar results were reported by Lieske et al., who noted that more females than males were possible to submit CaP (25.0% vs. 9.6%) or MAP stones (4.6% vs. 1.8%), while males compared to females had significantly more CaOx (74.1% vs. 58.1%) or UA stones (10.3% vs. 5.5%) 10. An increased prevalence of CaOx (COM and/or COD) stones submitted from males may be in part because of excessive high‐protein diet tendencies in males, which resulted in increase in urinary oxalate excretion and CaOx product 18. Similarly, meat‐containing diet tendencies in males also increased urinary risk factors for UA stone formation and consequently the risk of UA crystallization 19. However, the most likely explanations for the prevalence of CaP stones in women were the increased risk of urinary tract infection in females, which could raise urinary pH and favor CaP supersaturations 20. Obviously, these could be the possible reasons that women were particularly more likely than men to have MAP stones.
As in previous studies 6, 17, the present study found that average age of patients submitting stones for analysis was around 49.0 years. Different distributions in stone composition were observed among age groups. First, our data presented that the prevalence of calcium‐containing calculi (CaOx and/or CaP) was significantly reduced in older individuals. Such a decline in the proportion of calcium‐containing stones with age may be related to the decrease in the degree of hypercalciuria with age 21. Similar findings were supported by Costa‐Bauza et al. and Daudon et al., who attributed these trends to the decrease in urinary phosphorus and calcium concentration with age 9, 15. As it was found by several larger cohorts 7, 9, 10, 22, the data presented here demonstrated a clear increase of UA stones with age, especially above 70 years in both sexes. Although Costa‐Bauza et al. and Lieske et al. had recently reported that after the age of 50 years UA stone composition began to increase strikingly in both sexes 9, 10, it has been widely accepted that there is a clear increase of UA stones with age in both genders. Such trends could be explained by an increasing prevalence of metabolic syndrome with aging in both genders 23. This metabolic syndrome could result in increasing risk of acidic urine and UA nephrolithiasis in older patients 24. Unfortunately, causes and mechanisms of the decreased prevalence of cystine stones remain unknown, but may account for differences in the rate of underlying genetic predisposition to such stones.
Although our study demonstrated that COM stones were predominant in all age groups, there were significant differences in relation to varying proportions of stone composition among age groups (0–29, 30–39, 40–49, 50–59, 60–69, 70–79, and 80+ years). COM stones were relatively more common among those 30–50 years of age regardless of sex, which was similar to other studies 10, 17. Also, our data suggest that CaP calculi were relatively more common among those 20–29 years younger individuals, which were in agreement with previous studies 9, 10, 22. In addition, a more significant trend that adolescent girls (10‐19 years old) and young adult women (20‐29 years old) were more susceptible to CaP stones as opposed to men were showed by another study 10. As was also found by other authors 7, 9, 10, 11, UA stones tended to become more common among those 70+ years of age. It could be related with metabolic syndrome that appeared to play central role in UA stone formation by decreasing urinary ammonium and pH 24. The prevalence of metabolic syndrome increased almost five times from 20 to 29 years age group to 80 to 89 years group in male, and almost six times for female in the corresponding age groups 25. These tendencies were consistently in favor of a high percentage of UA stones among those 70+ years of age.
From 2002 to 2014, our data showed that CaOx‐containing stones were observed with an increasing frequency, whereas the incidence of UA and cystine stones decreased slightly. These findings were consistent with recent largest series of urolithiasis compositions analysis 7. Alternatively, a study from Veterans Administration medical center across the United States found an increasing proportion of CaP in a given individual by calendar year, which showed a different trend from ours. In addition, MAP stones showed a decreasing trend in the last few years in the developed countries 2, 4, 26, whereas we noted that MAP stones presented a slightly increasing trend between 2002 and 2014. However, another current study in China did not show a decreasing trend with years as many previous published studies 13. We can only hypothesize to explain these conflicting results. These findings might result from patients in China with a substandard treatment of urinary tract infection and extensively antibiotic resistance 27, which might have some influence on the different distribution of MAP between countries. Further epidemiological studies must evaluate this hypothesis.
Striking and definitive seasonal variations in the monthly urinary calculi attack rates for all age and gender populations had been found, however, few researchers had focused on the associations between calendar month (season) and stone composition 28, 29. A recent study in the United States showed that, as to the types of urinary stones, the warmer summer weather increases the risk to pass certain types of stones (CaOx and UA) but not others, as insensible water loss in warm weather will increase urinary supersaturations for both CaOx and UA 10. Nevertheless, we did not find any significant differences in overall monthly patterns associated with stone types. The results may imply factors leading to stone formation and affecting urolithiasis compositions are relatively uniform across populations and countries.
This study inevitably has certain potential shortcomings. Our data cannot reflect real incidences due to stones submitted for analysis only from a single reference laboratory. We certainly missed stones that were not collected after intervention or spontaneous passage. Furthermore, our data may lead to a bias toward stones requiring medical interventions, whereas stones that do not may be underestimated. Finally, detailed data on patient characteristics were not available other than calendar year, calendar month, gender, and age. Despite these limitations, this study has its value as this is the one of very few studies given the effect of calendar month (season), gender, and age on urolithiasis compositions in recent years.
Conclusions
Our study has used a logistic regression analysis to investigate the variations of urinary calculi composition based on calendar month (season), gender, and age in north China over a 13‐year period. Given gender and age distribution, these data present significant differences in the composition of urinary calculi. Additionally, an increasing trend of COD and MAP stone, as well as a decreasing incidence of CaP, UA, and cystine stones was observed from 2002 to 2014. Further investigations are required to elucidate the mechanisms behind these differences. Moreover, development of effective preventive and treatment strategies for urolithiasis relies on a better understanding of stone composition in the future.
Ethical approval: This article does not contain any studies with human participants or animals performed by any of the authors.
Conflict of Interest
All the authors declare that they have no conflict of interest.
Acknowledgments
This work was supported by grants from National Natural Science Foundation of China (grant numbers: 81172451, 81402124, and 81472416), Chinese Academy of Science and Technology of strategic leading science and technology (grant numbers: XDB14040401 and XDB14010300), the Application Base and Frontier Technology Project of Tianjin (grant number: 14JCQNJC10800), Science and Technology Program of Tianjin (grant number: 11ZCGYSY02300), and Tianjin City High School Science & Technology Fund Planning Project (grant number: 20130124). We thank laboratory members for great assistance with experiments and reagents.
Grant sponsor: National Natural Science Foundation of China; Grant numbers: 81172451, 81402124, and 81472416; Grant sponsor: Chinese Academy of Science and Technology; Grant numbers: XDB14040401 and XDB14010300; Grant sponsor: Application Base and Frontier Technology Project of Tianjin; Grant number: 14JCQNJC10800; Grant sponsor: Science and Technology Program of Tianjin; Grant number: 11ZCGYSY02300; Grant sponsor: Tianjin City High School Science & Technology Fund Planning Project; Grant number: 20130124.
References
- 1. Turney BW, Reynard JM, Noble JG, Keoghane SR. Trends in urological stone disease. BJU Int 2012;109(7):1082–1087. [DOI] [PubMed] [Google Scholar]
- 2. Yasui T, Iguchi M, Suzuki S, Kohri K. Prevalence and epidemiological characteristics of urolithiasis in Japan: National trends between 1965 and 2005. Urology 2008;71(2):209–213. [DOI] [PubMed] [Google Scholar]
- 3. Pearle MS, Calhoun EA, Curhan GC. Urologic diseases in America project: Urolithiasis. J Urol 2005;173(3):848–857. [DOI] [PubMed] [Google Scholar]
- 4. Hesse A, Brandle E, Wilbert D, Kohrmann KU, Alken P. Study on the prevalence and incidence of urolithiasis in Germany comparing the years 1979 vs. 2000. Eur Urol 2003;44(6):709–713. [DOI] [PubMed] [Google Scholar]
- 5. Cloutier J, Villa L, Traxer O, Daudon M. Kidney stone analysis: “Give me your stone, I will tell you who you are!”. World J Urol 2015;33(2):157–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wu W, Yang D, Tiselius HG, et al. The characteristics of the stone and urine composition in Chinese stone formers: Primary report of a single‐center results. Urology 2014;83(4):732–737. [DOI] [PubMed] [Google Scholar]
- 7. Knoll T, Schubert AB, Fahlenkamp D, Leusmann DB, Wendt‐Nordahl G, Schubert G. Urolithiasis through the ages: Data on more than 200,000 urinary stone analyses. J Urol 2011;185(4):1304–1311. [DOI] [PubMed] [Google Scholar]
- 8. Strope SA, Wolf JJ, Hollenbeck BK. Changes in gender distribution of urinary stone disease. Urology 2010;75(3):543–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Costa‐Bauza A, Ramis M, Montesinos V, et al. Type of renal calculi: Variation with age and sex. World J Urol 2007;25(4):415–421. [DOI] [PubMed] [Google Scholar]
- 10. Lieske JC, Rule AD, Krambeck AE, et al. Stone composition as a function of age and sex. Clin J Am Soc Nephrol 2014;9(12):2141–2146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gabrielsen JS, Laciak RJ, Frank EL, et al. Pediatric urinary stone composition in the United States. J Urol 2012;187(6):2182–2187. [DOI] [PubMed] [Google Scholar]
- 12. Herring LC. Observations on the analysis of ten thousand urinary calculi. J Urol 1962;88:545–562. [DOI] [PubMed] [Google Scholar]
- 13. Wu W, Yang B, Ou L, et al. Urinary stone analysis on 12,846 patients: A report from a single center in China. Urolithiasis 2014;42(1):39–43. [DOI] [PubMed] [Google Scholar]
- 14. Sun X, Shen L, Cong X, Zhu H, He L, Lu J. Infrared spectroscopic analysis of 5,248 urinary stones from Chinese patients presenting with the first stone episode. Urol Res 2011;39(5):339–343. [DOI] [PubMed] [Google Scholar]
- 15. Daudon M, Dore JC, Jungers P, Lacour B. Changes in stone composition according to age and gender of patients: A multivariate epidemiological approach. Urol Res 2004;32(3):241–247. [DOI] [PubMed] [Google Scholar]
- 16. Johnson CM, Wilson DM, O'Fallon WM, Malek RS, Kurland LT. Renal stone epidemiology: A 25‐year study in Rochester, Minnesota. Kidney Int 1979;16(5):624–631. [DOI] [PubMed] [Google Scholar]
- 17. Lieske JC, Pena DLVL, Slezak JM, et al. Renal stone epidemiology in Rochester, Minnesota: An update. Kidney Int 2006;69(4):760–764. [DOI] [PubMed] [Google Scholar]
- 18. Borghi L, Schianchi T, Meschi T, et al. Comparison of two diets for the prevention of recurrent stones in idiopathic hypercalciuria. N Engl J Med 2002;346(2):77–84. [DOI] [PubMed] [Google Scholar]
- 19. Siener R, Hesse A. The effect of a vegetarian and different omnivorous diets on urinary risk factors for uric acid stone formation. Eur J Nutr 2003;42(6):332–337. [DOI] [PubMed] [Google Scholar]
- 20. Parks JH, Coe FL, Strauss AL. Calcium nephrolithiasis and medullary sponge kidney in women. N Engl J Med 1982;306(18):1088–1091. [DOI] [PubMed] [Google Scholar]
- 21. Goldfarb DS, Parks JH, Coe FL. Renal stone disease in older adults. Clin Geriatr Med 1998;14(2):367–381. [PubMed] [Google Scholar]
- 22. Krambeck AE, Lieske JC, Li X, Bergstralh EJ, Melton LR, Rule AD. Effect of age on the clinical presentation of incident symptomatic urolithiasis in the general population. J Urol 2013;189(1):158–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ford ES, Giles WH, Dietz WH. Prevalence of the metabolic syndrome among US adults: Findings from the third National Health and Nutrition Examination Survey. J Am Med Assoc 2002;287(3):356–359. [DOI] [PubMed] [Google Scholar]
- 24. Abate N, Chandalia M, Cabo‐Chan AJ, Moe OW, Sakhaee K. The metabolic syndrome and uric acid nephrolithiasis: Novel features of renal manifestation of insulin resistance. Kidney Int 2004;65(2):386–392. [DOI] [PubMed] [Google Scholar]
- 25. Hildrum B, Mykletun A, Hole T, Midthjell K, Dahl AA. Age‐specific prevalence of the metabolic syndrome defined by the International Diabetes Federation and the National Cholesterol Education Program: The Norwegian HUNT 2 study. BMC Public Health 2007;7. doi: 10.1186/1471-2458-7-220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lee MC, Bariol SV. Changes in upper urinary tract stone composition in Australia over the past 30 years. BJU Int 2013;112(Suppl 2):65–68. [DOI] [PubMed] [Google Scholar]
- 27. Zhao L, Chen X, Zhu X, et al. Prevalence of virulence factors and antimicrobial resistance of uropathogenic Escherichia coli in Jiangsu province (China). Urology 2009;74(3):702–707. [DOI] [PubMed] [Google Scholar]
- 28. Lo SS, Johnston R, Al SA, Metcalf PA, Rice ML, Masters JG. Seasonal variation in the acute presentation of urinary calculi over 8 years in Auckland, New Zealand. BJU Int 2010;106(1):96–101. [DOI] [PubMed] [Google Scholar]
- 29. Chen YK, Lin HC, Chen CS, Yeh SD. Seasonal variations in urinary calculi attacks and their association with climate: A population based study. J Urol 2008;179(2):564–569. [DOI] [PubMed] [Google Scholar]


