Abstract
Background
This study was designed to determine the diagnostic role of urinary kidney injury molecule (KIM)‐1 levels in renal damage in patients with type 2 diabetes mellitus according to the urinary albumin/creatinine ratio.
Methods
Patients with type 2 diabetes mellitus admitted to different polyclinics in our hospital enrolled in the study and were subdivided into three groups according to albumin/creatinine ratio – normalbuminuric (n: 20); microalbuminuric (n: 20); albuminuric (n: 18) – and compared with the control group. Urine albumin was analyzed using the immunoturbidimetric method (Architect C16000, Abbott Diagnostics). uKIM‐1 was determined using a commercially available enzyme‐linked immunosorbent assay test kit (USCN Life Science, Hankou, Wuhan, China). One‐sample Kolmogorov–Smirnov test, Spearman correlation and Kruskal–Wallis non‐parametric tests were performed. Post hoc comparisons were made using Bonferroni‐corrected Mann–Whitney U tests.
Results
The differences between the controls and normalbuminuric, microalbuminuric and albuminuric groups were highly significant for KIM‐1. Positive correlation was found between KIM‐1 and urine microalbumin–urine microalbumin/creatinine (r = 0.479 P < 0.001; r = 0. 400, P < 0.001; respectively).
Conclusion
In our study, KIM‐1 levels were significantly different suggesting that urinary KIM‐1 levels may be an early marker in patients with diabetic nephropathy. J. Clin. Lab. Anal. 00:1–6, 2016.
Keywords: diabetic nephropathy, KIM‐1 protein, microalbuminuria
Introduction
Diabetes is a chronic disorder of glucose metabolism associated with microvascular (retinopathy, nephropathy, neuropathy) and macrovascular complications. Type 2 diabetes accounts for 85–90% of all diabetes and seems to be a global pandemic problem due to reasons such as increasing levels of obesity, arising from energy‐rich diets, and sedentary lifestyles 1, 2.
Diabetic nephropathy (DN) is the one of the major complications of diabetes mellitus (DM) 3, 4. Early diagnosis of DN is necessary in that this may reduce the progression of end‐stage renal disease (ESRD) 5. Urinary albumin level is important in DN and albumin in the urine between 30 and 300 mg/day or urinary albumin/creatinine ratio between 30 and 300 mg/g in spot urine are defined as microalbuminuria (subclinical albuminuria). Urinary albumin below these values is termed as normal urinary albumin excretion and urinary albumin above these values is termed macroalbuminuria or clinical proteinuria 6, 7, 8. In addition, microalbuminuria may also occur in hematuria, acute systemic illness/fever, severe physical exercise, heart failure, cardiovascular diseases, obesity, insulin resistance, hyperglycemia, rheumatoid arthritis in non‐diabetic normotensive patients and in elderly people 9, 10, 11.
Early diagnosis, prognosis and treatment of DN is important, hence new, sensitive and specific markers are needed 12. Kidney injury molecule‐1 (KIM‐1) is a cell membrane glycoprotein that is expressed after ischemia or toxicity from proximal tubules of the kidney 13.
There are several characteristic features of KIM‐1 that can be used as an ideal marker of kidney injury like the absence of KIM‐1 expression in the normal kidney and ex vivo room temperature stability 14.
The aim of this study was to evaluate the diagnostic role of urinary KIM‐1 levels in renal damage in patients with type 2 DM according to the urinary albumin/creatinine ratio.
Materials and Methods
Study Group
This prospective and controlled study was conducted in the Biochemistry Laboratory of Izmir Ataturk Training and Research Hospital and was ethically approved by a local ethics committee (date: 24 December 2009, number 79). The patients with type 2 DM admitted to different polyclinics in our hospital between 12 April 2010 and 5 June 2010 were enrolled in the study. All participants were informed about the study and gave consent to participate in it. Information about history of diabetes and its treatment, other health problems, use of medications was reported. All volunteers were women. Patients with serious comorbidities such as nondiabetic kidney disease, chronic liver disease, type I DM, ages <30 and >65 were excluded.
Patients with systolic and diastolic blood pressure equal to or above 140/90 mmHg respectively were considered as hypertensive.
On the basis of albumin/creatinine ratio, patients were categorized into three groups – normalbuminuric (n: 20); microalbuminuric (n: 20); albuminuric (n: 18). The classification of patient and control groups is shown in Table 1. Twenty healthy controls were matched for age and sex. Both patient and control groups included 78 female subjects.
Table 1.
Classification of patient and healthy control groups according to albumin/creatinine ratio
| Group number | Groups (n: 78) | Albumin/creatinine ratio in spot urine (mg/g) |
|---|---|---|
| Group I | Control group (n: 20) | <30 |
| Group II | Normalbuminuric group (n: 20) | <30 |
| Group III | Microalbuminuric group (n: 20) | 30–299 |
| Group IV | Albuminuric group (n: 18) | ≥300 |
Sample Collection
Blood and urine samples were obtained from the patient and control groups.
Blood
Serum fasting glucose, total cholesterol, triglyceride, blood urea nitrogen, creatinine, total protein, albumin (Alb) levels were analyzed with enzymatic colorimetric method, and aspartate aminotransferase and alanine aminotransferase with kinetic enzymatic method (Architect C16000; Abbott Diagnostics, USA). HbA1C levels were analyzed with HPLC method (Adams HA‐8160; Arkray, Inc., Japan).
Urine
Creatinine and albumin were examined from the first morning urine. (midstream) and the rest of the urine voided directly into a sterile container. Aliquot urine was centrifuged for 3 min at 3,000 × g and stored at −80°C until further (KIM‐1) analysis.
Urine albumin was analyzed using the immunoturbidimetric method (Architect C16000; Abbott Diagnostics).
KIM‐1 Enzyme‐linked Immunosorbent Assay
uKIM‐1 was determined using a commercially available enzyme‐linked immunosorbent assay test kit (USCN Life Science, Hankou, Wuhan, China). Biotinylated polyclonal antibody specific for KIM‐1 was used in the urine samples. Horseradish peroxidase‐conjugated avidin was added, followed by a color‐forming peroxidase substrate containing tetramethylbenzidine. The color was then measured at 450 nm by a microtiter plate reader and the concentration of KIM‐1 in the samples was then determined by comparing the O.D. of the samples to the standard curve. Lower detection limit of KIM‐1 was 0.042 ng/ml.
Statistic
SPSS (Version 15.0; SPSS Inc, Chicago, IL) statistical software package was used for analysis. One‐sample Kolmogorov–Smirnov test, Spearman correlation and Kruskal–Wallis non‐parametric tests were performed. Post hoc comparisons were made using Bonferroni‐corrected Mann–Whitney U tests and P < 0.0083 was considered statistically significant.
Results
Patients with type 2 DM (n: 58) and healthy controls (n: 20) were enrolled in the study.
Clinical characteristics, medications and blood markers of the study group are shown in Table 2. Patients in the albuminuric group had longer diabetes duration and lower MDRDeGFR compared with other groups. Individuals in the control group were slightly younger and had low HbA1c and fasting glucose in comparison with the other three patient groups.
Table 2.
Clinical characteristics, medications, and blood markers of the study group
| Clinical characteristics | Group I (n = 20) | Group II (n = 20) | Group III (n = 20) | Group IV (n = 18) | P‐valuea |
|---|---|---|---|---|---|
| Age (years) | 48.40 ± 5.384 | 52.10 ± 7.504 | 58.10 ± 5.973 | 56 ± 5.499 | <0.001 |
| BMI (kg/m2) | 27.21 ± 5.60 | 31.22 ± 5.57 | 33.16 ± 5.21 | 30.53 ± 5.75 | 0.012 |
| WHR | 0.85 ± 0.08 | 0.90 ± 0.04 | 0.90 ± 0.04 | 0.88 ± 0.04 | 0.054 |
| SBP (mmHg) | 115.75 ± 13.40 | 131.00 ± 15.77 | 142.00 ± 17.19 | 147.78 ± 17.16 | <0.001 |
| DBP (mmHg) | 73.25 ± 9.35 | 83.25 ± 8.62 | 90 ± 8.11 | 88.61 ± 7.62 | <0.001 |
| MDRDeGFR (ml/dak/l.73 m2) | 116.45 ± 9.51 | 97.66 ± 35.50 | 90.07 ± 27.43 | 54.58 ± 42.27 | <0.001 |
| Duration of DM (years) | 0 | 6.05 | 9.65 | 10.27 | <0.001 |
| Medications | |||||
| OAD (n) | 0 | 12 | 19 | 14 | |
| Ins (n) | 0 | 8 | 4 | 11 | |
| AH (n) | 0 | 12 | 18 | 18 | |
| AL (n) | 0 | 3 | 9 | 10 | |
| Blood markers | |||||
| F.Glucose (mg/dl) | 96.15 ± 6.52 | 154.35 ± 67.04 | 214.80 ± 103.34 | 171.22 ± 79.75 | <0.001 |
| HbA1c (%) | 4.095 ± 0.53 | 7.72 ± 1.73 | 8.30 ± 1.86 | 7.62 ± 1.11 | <0.001 |
| T.Cholesterol (mg/dl) | 208.40 ± 37.30 | 209.65 ± 73.47 | 190.05 ± 35.16 | 246.0 ± 68.34 | 0.030 |
| Triglyceride (mg/l) | 113.80 ± 59.64 | 175.70 ± 81.34 | 229.70 ± 191.62 | 343.67 ± 435.14 | 0.001 |
| BUN (mg/dl) | 10.50 ± 3.56 | 15.50 ± 11.48 | 15.80 ± 6.61 | 32.05 ± 17.46 | <0.001 |
| Creatinine (mg/dl) | 0.58 ± 0.04 | 0.83 ± 0.52 | 0.78 ± 0.30 | 1.77 ± 1.12 | <0.001 |
| Protein (g/dl) | 6.98 ± 0.47 | 6.3 ± 0.58 | 6.84 ± 0.50 | 6.61 ± 0.80 | 0.008 |
| Albumin (g/dl) | 4.28 ± 0.16 | 4.23 ± 0.28 | 4.37 ± 0.26 | 4.03 ± 0.69 | 0.379 |
| AST (U/l) | 17.05 ± 2.41 | 16.30 ± 5.82 | 17.65 ± 10.41 | 16.22 ± 4.94 | 0.532 |
| ALT (U/l) | 20.15 ± 6.65 | 17.15 ± 9.11 | 19.60 ± 14.0 | 16.33 ± 6.65 | 0.168 |
| Na (mmol/l) | 137.65 ± 1.95 | 136.15 ± 4.15 | 138.05 ± 3.22 | 138.50 ± 4.17 | 0.082 |
| K (mmol/l) | 4.25 ± 0.35 | 4.34 ± 0.33 | 4.61 ± 0.40 | 4.77 ± 055 | 0.001 |
| Cl (mmol/l) | 106.25 ± 1.94 | 103.05 ± 2.46 | 103.80 ± 2.09 | 107.06 ± 4.22 | <0.001 |
Modification of diet in renal disease (MDRD) eGFR (ml/dak/l.73 m2) = 186.3 × serum creatinine−1.154 × age (year)−0.203 × 0.742 (for women) formula was used.
BMI (kg/m2), body mass index; WHR, waist‐to‐hip ratio; SBP (mmHg), systolic blood pressure; DBP (mmHg), diastolic blood pressure; OAD, oral antidiabetic; I, insulin; AHT, antihypertensive; AL, antilipid; BUN, blood urea nitrogen; AST, aspartate aminotransferase; ALT, alanine aminotransferase.
Kruskal–Wallis test.
Minimum–maximum, mean ± SD, median and P values of the urine parameters for all groups are given in Table 3.
Table 3.
Urine parameter values for patient and control groups
| Parameter | Group I (n: 20) | Group II (n: 20) | Group III (n: 20) | Group IV (n: 18) | P a |
|---|---|---|---|---|---|
| Urine creatinine (mg/dl) | 0.001 | ||||
| Mean ± SD | 143.03 ± 63.07 | 154.40 ± 71.78 | 128.23 ± 69.45 | 82.77 ± 41.36 | |
| Median | 115.71 | 132.03 | 96.49 | 67.90 | |
| Minimum–maximum | 63.81–288.91 | 46.58–288.51 | 51.27–286.75 | 25.39–197.87 | |
| Urine microalbumin (mg/l) | <0.001 | ||||
| Mean ± SD | 1.33 ± 1.40 | 1.94 ± 1.41 | 12.09 ± 8.87 | 309.67 ± 430.64 | |
| Median | 0.8 | 1.7 | 9.85 | 164.50 | |
| Minimum–maximum | 0.5–5.7 | 0.5–5.7 | 2.1–32.0 | 25.6–1 928 | |
| UrineMalb/Cre (mg/g) | <0.001 | ||||
| Mean ± SD | 8.68 ± 5.73 | 12.78 ± 7.01 | 110.74 ± 89.60 | 4,992.87 ± 7,806.95 | |
| Median | 6.66 | 10.47 | 63.14 | 2,361.65 | |
| Minimum–maximum | 3.04–23.33 | 2.24–29.07 | 30.53–271.93 | 347.09–34,851.77 | |
| KIM‐1 (ng/ml) | 0.035 | ||||
| Mean ± SD | 0.70 ± 0.52 | 1.07 ± 1.21 | 0.98 ± 0.66 | 1.77 ± 1.45 | |
| Median | 0.62 | 0.46 | 0.71 | 1.46 | |
| Minimum–maximum | 0.156–2.024 | 0.156–4.129 | 0.268–2.692 | 0.189–5.337 | |
UrineMalb/Cre, urine microalbumin/creatinine.
Kruskal–Wallis test.
Urinary concentrations of creatinine and microalbumin and marker of tubular injury KIM‐1 were significantly different among the four groups. Concentrations were lowest in the control group and highest in the albuminuric group. The differences between the controls and normalbuminuric, microalbuminuric and albuminuric groups were highly significant for KIM‐1.
Kidney injury molecule‐1 results evaluated with Bonferroni‐corrected Mann–Whitney U test showed that there was a significant difference only between the control group and the albuminuric patient group (P < 0.006). Regarding urine microalbumin and urine microalbumin/creatinine, there were significant differences among all groups (except between the control group and the normoalbuminuric group; P < 0.001).
Except the control group, patients with and without systolic and diastolic blood pressure equal to or above 140/90 mmHg were distributed into two groups and their KIM‐1, urine microalbumin, urine microalbumin/creatinine values were compared with Bonferroni‐corrected Mann–Whitney U test. There was a significant difference between the control group compared to groups with blood pressure <140/90 mmHg and the group with blood pressure ≥140/90 mmHg regarding urine microalbumin and urine microalbumin/creatinine (P < 0.001 for both groups). There was no significant difference between the control group and KIM‐1 levels (P = 0.199 and 0.066, respectively). The comparison of the two groups (group with blood pressure <140/90 mmHg and the group with blood pressure ≥140/90 mmHg) for urine microalbumin, urine microalbumin/creatinine and KIM‐1 levels with Bonferroni‐corrected Mann–Whitney U test revealed no significant differences (P = 0.147, 0.077, and 0.779, respectively).
Positive correlation was found between KIM‐1 and urine microalbumin‐urine microalbumin/creatinine with Spearman's correlation test (r = 0.479, P < 0.001, r = 0. 400, P < 0.001; respectively).
The urine KIM‐1 levels are shown in Figure 1.
Figure 1.
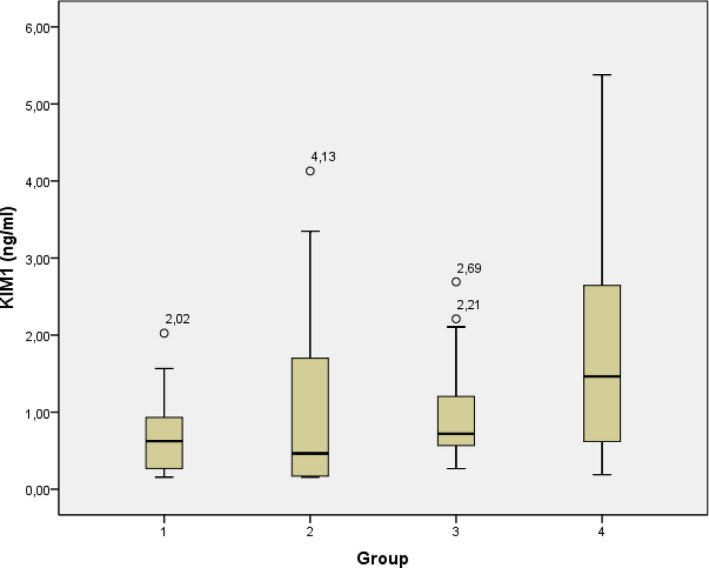
Kidney injury molecule‐1 levels in urine (ng/ml).
Discussion
Diabetes mellitus is the common cause of end‐stage renal failure that causes medical, social and economic problems and nephropathy develops in 20–30% of patients with type 1 or 2 diabetes 15. DN remains the most common cause for ESRD as the burden of diabetes increases worldwide in which the earliest sign of DN is the development of microalbuminuria but it is not specific to type 2 diabetes 16, 17. Microalbuminuria may also occur in hematuria, acute systemic illness or fever, intense physical exercise, heart failure, hyperglycemia, and elderly patients 10, 18. There is a strong relationship between microalbuminuria and vascular disorders in hypertensive patients so that it can be recommended as a marker of vascular and/or endothelial damage 11. The usefulness of the biomarkers is low for the early prediction of chronic kidney disease progression and one of the promising urinary proteins is KIM‐1 19. KIM‐1 is a type‐1 transmembrane protein that is not normally present and expressed on the proximal tubule apical membrane with injury, and it is a hallmark of virtually all proteinuric, toxic, and ischemic kidney diseases 20, 21.
In this study, we studied the KIM‐1 levels in patients with type 2 diabetes in order to evaluate the possible diagnostic role of KIM‐1 in DN. The patients were classified according to the urinary albumin/creatinine ratio. KIM‐1 was reported as a useful biomarker for renal proximal tubule injury, upregulated in renal disease with renal fibrosis and inflammation indicating that it can be used as a noninvasive biomarker in renal disease 13, 22.
von Eynatten et al. 23 evaluated renal functions related to chronic ischemic damage in patients with DN. uKIM‐1 was not significantly associated with kidney function and the authors indicate that L‐FABP could be a novel biomarker for chronic intrarenal ischemia. von Eynatten and his colleagues conducted this study in patients with DN but the groups were not created according to the level of albuminuria. In our study, KIM‐1 was positively correlated with urinary microalbumin and urine microalbumin/creatinine. In our study, there was a significant difference between the control group and the albuminuric group (P < 0.006). However, as the patient number of the study was limited and there was not such a difference among other groups, we believe that this study should be designed with a greater patient number. On the other hand, in another study, KIM‐1 levels were evaluated in diabetic patients and were not significantly associated with albuminuria and eGFR. The investigators reported that the reason of this absence of difference is the relation of KIM‐1 molecule with proximal tubule damage 24. Interestingly, we did not observe any difference in the control group compared to the normoalbuminuric group and the albuminuric group. Therefore, one should keep in mind that the significant difference between the control group and the albuminuric group in our study may be misleading. After grouping the patients with and without systolic and diastolic blood pressure equal to or above 140/90 mmHg, the comparison of KIM‐1 and urine microalbumin/creatinine levels displayed no significant difference, which may be a proof that the high blood pressure has no effect on the results.
Kidney injury molecule‐1 level was associated with the regression of microalbuminuria in type 1 DM and with or without albuminuria elevated urine‐KIM‐1 was evaluated as a marker indicating tubular damage at an early stage of type 1 DM 25, 26. The higher patient numbers in the study conducted by Vaidya et al. enabled them to demonstrate the role of KIM‐1 on type 1 DM more objectively. However, the fact that the groups in our study consisted of patients with type 2 DM and the patient number was small restricted us from demonstrating the role of KIM‐1 as a marker in kidney damage.
In a study of 117 patients with type 2 DM, de Carvalho et al. 27 reported that urinary KIM‐1 levels were increased in patients with moderate albuminuria and it may be a marker for early diabetic renal damage. Contrarily, in our study we found an increase in the albuminuric group. Therefore, further studies are needed to confirm the potential of urinary KIM‐1 levels as a marker of early kidney damage.
In this study, urinary KIM‐1 levels correlated with urinary microalbumin/creatinine (r = 0.400, P < 0.001), but correlation coefficients were low and this may be a result of the low number of patients. Urinary KIM‐1 levels were higher especially in the albuminuric group; this may be due to severe kidney damage and as this difference was not observed in other groups, the severity of kidney damage, and the lack of a correlation between the proximal tubule and the KIM‐1 reflecting the damage may be due to the limited number of patients.
Kidney injury molecule‐1 is not secreted from normal kidney and its epithelial cell secretion returns to normal when cell repair occurs; patients with normoalbuminuria and microalbuminuria are under treatment and this may cause low‐level detection. Such studies need to be repeated in patients with newly diagnosed type 2 diabetes, so that the role of KIM‐1 levels and the correlation with microalbumin/creatinine can be demonstrated more clearly.
Conclusion
The diagnostic value of KIM‐1 can be evaluated more clearly in newly diagnosed or untreated patients with type 2 DM. As different pathophysiological mechanisms underlie tubulointerstitial disease, new markers are needed in determining the early damage caused by DN. This preliminary study underscores that urinary KIM‐1 levels may be an early marker in patients with DN.
Acknowledgments
This study was supported by “Acar Medikal Bilg. Sis. San.ve Tic.Ltd.Şti. and ““Meditera İthalat İhracat Ltd. Şti.”.
References
- 1. Forouhi NT, Wareham NJ. Epidemiology of diabetes. Diabetes Basic Facts 2006;34:57–60. [Google Scholar]
- 2. Zimmet P. The burden of type 2 diabetes: Are we doing enough? Diabetes Metab 2003;29:9–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Jawa A, Kcomt J, Fonseca VA. Diabetic nephropathy and retinopathy. Med Clin North Am 2004;88:1001–1036. [DOI] [PubMed] [Google Scholar]
- 4. Fraser DJ, Phillips AO. Diabetic nephropathy. Medicine 2007;35:503–506. [Google Scholar]
- 5. Arora S. Renal function in diabetic nephropathy. World J Diabetes 2010;15:48–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mogensen CE, Chachati A, Christensen CK, et al. Microalbuminuria: An early marker of renal involvement in diabetes Uremia Invest 1985. –86;9:85–95. [DOI] [PubMed] [Google Scholar]
- 7. Zelmanovitz T, Gerchman F, Balthazar PSA, Thomazelli CSF, Matos DJ, Canani HL. Diabetic nephropathy. Diabetol Metab Syndr 2009;21:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sarafidis PA, Bakris GL. Microalbuminuria and chronic kidney disease as risk factors for cardiovascular disease. Nephrol Dial Transplant 2006;21:2366–2374. [DOI] [PubMed] [Google Scholar]
- 9. Hurst RT, Lee RW. Increased incidence of coronary atherosclerosis in type 2 diabetes mellitus: Mechanisms and management. Ann Intern Med 2003;139:824–834. [DOI] [PubMed] [Google Scholar]
- 10. Atkins RC, Zimmet P. Diabetic kidney disease: Act now or pay later. Blood Purif 2010;29:317–320. [DOI] [PubMed] [Google Scholar]
- 11. Satchell SC, Tooke JE. What is the mechanism of microalbuminuria in diabetes: A role for the glomerular endothelium? Diabetologia 2008;51:714–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chen H, Zheng Z, Li R, et al. Urinary pigment epithelium‐derived factor as a marker of diabetic nephropathy. Am J Nephrol 2010;32:47–56. [DOI] [PubMed] [Google Scholar]
- 13. Han WK, Bailly V, Abichandani R, et al. Kidney injury molecule‐1 (KIM‐1): A novel biomarker for human renal proximal tubule injury. Kidney Int 2002;62:237–244. [DOI] [PubMed] [Google Scholar]
- 14. Bonventre JV. Kidney injury molecule‐1 (KIM‐1): A urinary biomarker and much more. Nephrol Dial Transplant 2009;24:3265–3268. [DOI] [PubMed] [Google Scholar]
- 15. American Diabetes Association . Nephropathy in diabetes. Diabetes Care 2004;27:79–83. [Google Scholar]
- 16. Choudhury D, Tuncel M, Levi M. Diabetic nephropathy – a multifaceted target of new therapies. Discov Med 2010;10:406–415. [PubMed] [Google Scholar]
- 17. Shields J, Maxwell AP. Managing diabetic nephropathy. Clin Med 2010;10:500–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zelmanovitz T, Gerchman F, Balthazar PSA, Thomazelli CSF, Matos DJ, Canani HL. Diabetic nephropathy. Diabetol Metab Syndr 2009;1:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Devarajan P. The use of targeted biomarkers for chronic kidney disease. Adv Chronic Kidney Dis 2010;17:469–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bonventre JV. Kidney injury molecule‐1 (KIM‐1): A specific and sensitive biomarker of kidney injury. Scand J Clin Lab Invest Suppl 2008;241:78–83. [DOI] [PubMed] [Google Scholar]
- 21. Waanders F, van Timmeren MM, Stegeman CA, Bakker SJ, van Goor H. Kidney injury molecule‐1 in renal disease. J Pathol 2010;220:7–16. [DOI] [PubMed] [Google Scholar]
- 22. van Timmeren MM, van den Heuvel MC, Bailly V, Bakker SJ, van Goor H, Stegeman CA. Tubular kidney injury molecule‐1 (KIM‐1) in human renal disease. J Pathol 2007;212:209–217. [DOI] [PubMed] [Google Scholar]
- 23. von Eynatten M, Baumann M, Heemann U, et al. Urinary L‐FABP and anaemia: Distinct roles of urinary markers in type 2 diabetes. Eur J Clin Invest 2010;40:95–102. [DOI] [PubMed] [Google Scholar]
- 24. Nauta FL, Boertien WE, Bakker SJ, et al. Glomerular and tubular damage markers are elevated in patients with diabetes. Diabetes Care 2011;34:975–981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Vaidya VS, Niewczas MA, Ficociello LH, et al. Regression of microalbuminuria in type 1 diabetes is associated with lower levels of urinary tubular injury biomarkers, kidney injury molecule‐1, and N‐acetyl‐β‐D‐glucosaminidase. Kidney Int 2011;79:464–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Nielsen SE, Schjoedt KJ, Astrup AS, et al. Neutrophil gelatinase‐associated lipocalin (NGAL) and kidney injury molecule 1 (KIM1) in patients with diabetic nephropathy: A cross‐sectional study and the effects of lisinopril. Diabet Med 2010;27:1144–1150. [DOI] [PubMed] [Google Scholar]
- 27. de Carvalho JA, Tatsch E, Hausen BS, et al. Urinary kidney injury molecule‐1 and neutrophil gelatinase‐associated lipocalin as indicators of tubular damage in normoalbuminuric patients with type 2 diabetes. Clin Biochem 2016;49:232–236. [DOI] [PubMed] [Google Scholar]


