Abstract
Background
Tumor marker measurements are becoming essential for prognosis and follow‐up of patients in oncology. In this context, we aimed to compare a new analyzer, Lumipulse® G1200 (Fujirebio group, distributed in Europe by the Innogenetics group) with Kryptor® (Thermo Fisher Scientific B.R.A.H.M.S, Asnières, France) and Modular® Elecsys E170 (Roche Diagnostics, Meylan, France) for the measurement of seven tumor markers: PSA, AFP, CEA, CA 15‐3, CA 125, CA 19‐9, and Cyfra 21‐1.
Methods
A total of 471 serum samples from patients with elevated tumor markers and 100 serum from healthy patients were analyzed with Lumipulse® G1200 and either Kryptor® (for AFP) or Modular® (for the six other markers).
Results
The good precision of Lumipulse® G1200 assays was confirmed with CVs < 2.5% and < 5.0%, obtained, respectively, for within‐run imprecision and intermediate imprecision (except for Cyfra 21‐1: CV < 13%). For all markers, Lumipulse results were well correlated with Modular or Kryptor results (r ≥ 0.94). Concordance of results interpretation was > 95% and tumor marker kinetics were all similar.
Conclusion
We confirmed the analytical performances of Lumipulse® tumor marker assays except for the CYFRA 21‐1 assay for which performances were poor in this study. We noticed a few discrepancies for the CEA assay. Besides, values obtained for CA 19‐9 were higher with Lumipulse leading to a bias (slope = 1.5). But for the four other tumor markers assays (PSA, AFP, CA 125, CA 15‐3), the results were directly transferable between Lumipulse and Kryptor or Modular, thus facilitating an eventual substitution of one system by another.
Keywords: imprecision, evaluation, comparability, kinetic, luminescent measurements, prostate‐specific antigen, alpha‐fetoproteins, mucin‐1, CA‐125 antigen, CA‐19‐9 antigen
INTRODUCTION
As the incidence of cancers increased in the last century, tumor marker measurements became increasingly important for the evaluation of prognosis, patient follow‐up under treatment and early detection of relapses.
This study focused on seven tumor markers assessed in daily routine. First, alpha‐fetoprotein (AFP) is a glycoprotein of 70 kDa, produced physiologically by the yolk sac and the liver during fetal development and abnormally produced by malignant hepatocytes or germ‐cell tumor 1, 2, 3. The second one, the prostate specific antigen (PSA) produced by prostate epithelial cells, is elevated in various prostatic disorders such as benign prostate hyperplasia or prostate cancer 4, 5. Carcinoembryonic antigen (CEA) is a glycophosphatidyl‐inositol (GPI)‐anchored, intercellular adhesion molecule normally produced only during fetal development. CEA is upregulated in various types of cancer like lung or colorectal cancer 6, 7, in which it inhibits cell differentiation and anoikis 8, 9, thus increasing tumorogenecity and metastasis potential. Mucin‐1 is a transmembrane dimeric protein expressed on normal secretory cells, implicated in the formation of gels and chemical barriers. In malignant conditions, such as breast cancer 10, 11, mucin‐1 is increased, abnormally glycosylated and detected by CA 15‐3 assays. CA 125 is a glycoprotein secreted by normal endometrium and present in the epithelia of many organs such as ovaries, colon, lung, kidney, pancreas, and gall bladder. It increases in ovarian cancer 12, 13, as well as in benign ovarian conditions and serous inflammation. CA 19‐9 is a modified sialylated Lewis blood group antigen, increased in pancreatic cancer and other gastrointestinal conditions like jaundice or cirrhosis 14, 15, 16. Last, Cyfra 21‐1 is a soluble fragment of cytokeratin 19, an intermediate filament protein important for epithelial cell stability. Cyfra 21‐1 concentration is increased in many types of cancer, especially nonsmall‐cell lung carcinoma 17, 18, 19. So, these circulating tumor markers became important in clinical and biological practice.
But, it is well known that tumor marker concentrations in a given sample measured by different analyzers vary according to assay methods, antibodies used, and reagent specificities. Hence, it is of great importance that results given by different analytical systems are exact, precise, and above all comparable. Especially when a changeover of system is taking place in a laboratory, it is important to know if the new tumor marker assays are comparable to the existing ones for accurate result interpretation.
The Lumipulse® G1200 (Fujirebio group, distributed in Europe by the Innogenetics group) is a fully automated chemiluminescent enzyme immunoassay analyzer. It is launched by Fujirebio, well known for its expertise in oncology, and is widely used in Japan since 2008. It has been recently introduced in Europe via its affiliated companies of the Innogenetics group (in France, Innogenetics SARL, a Fujirebio company, Les Ulis, France) with a large assay menu including Fujirebio markers in various fields like endrocrinology and oncology. Unlike other systems, both the analyzer and the monoclonal antibodies (mAb) are provided by the same manufacturer, Fujirebio, and should guarantee good performances.
Tumor marker measurement systems differ in many aspects but often have in common the mAb used, manufactured by Fujirebio Diagnostics 20. Thus, a relative agreement between the different methods is expected. Actually, while some systems give equivalent results 21, 22, 23, some show no transferability 24, 25, 26, 27, 28.
The aim of this study was to evaluate Lumipulse G1200 performances for the measurement of AFP, PSA, CEA, CA 15‐3, CA 125, CA 19‐9, and Cyfra 21‐1. Results transferability with our routine analyzers, Kryptor and Modular Elecsys was carried out using individual results and kinetics of tumor markers established during patient follow‐up. Moreover, the practicability of the system under routine laboratory conditions was also evaluated.
MATERIALS AND METHODS
Instruments
Lumipulse® G1200 is a fully automated chemiluminescence‐based enzyme immunoanalyzer (CLEIA). It is a midsized analyzer, with a unique mono‐test cartridge concept and continuous sample loading. All measurements are performed in 25 min, allowing a highthroughput of 120 tests per hour.
All assays relay on two mAb, one labeled with alkaline phosphatase (ALP) and the other one coated on iron beads. Chemiluminescence is produced after AMPPD (3‐(2′‐Spiroadamantane)‐4‐methoxy‐4‐(3′′−phosphoryloxy)phenyl−1,2−dioxetane) hydrolysis by ALP into an unstable product which stabilizes by emitting light, measured at 477 nm.
Kryptor® (Thermo Fisher Scientific B.R.A.H.M.S, Asnières, France) is an automated analyzer with a patented detection system, Time Resolved Amplified Cryptate Emission (TRACE). TRACE technology is based on a nonradiative energy transfer between a donor (cage‐like structure with an europium ion (cryptate)) and an acceptor (XL665, an algual alophycocianin) 29, both coupled to a mAb specific to each assay.
Modular® Elecsys E170 (Roche Diagnostics, Meylan, France) is an electrochemiluminescence‐based immunoanalyzer. The Elecsys AFP assay for Modular analyzer uses a mAb labeled with biotin and another mAb coupled with Ruthenium. In the presence of the antigen (AFP), immunocomplexes are immobilized onto the surface of the electrode with magnetic beads labeled with streptavidin. Application of an electric voltage to the electrode then induces chemiluminescence detected by a spectrophotometer.
Samples and controls
According to the Clinical and Laboratory Standard Institute (CLSI) guidelines 30, document EP9‐A2, samples were selected to cover a clinically meaningful range of concentrations. A first series of samples represented high values of tumor marker concentrations. They were screened from the Pitié‐Salpêtrière laboratory database. Samples collected between April 2011 and January 2012 with at least one tumor marker above reference system cut‐off value were selected. They had been first analyzed as part of routine activity with the reference systems, and then stored at −20°C. Before analysis on the Lumipulse G1200, they were thawed at room temperature, homogenized, and centrifuged.
A second series of samples represented normal tumor marker values and were obtained from sera of blood donors collected at the “Etablissement Français du Sang, Paris” and immediately analyzed with both systems. The clinical status of patients with elevated marker concentration was not documented but the healthy donors were considered to be free of any tumoral disease because they were screened with a questionnaire.
For the quality controls (QC), three levels of Seronorm™ Immunoassay QC (Alere) were used. The low level was near the cut‐off value of both systems, while the medium and high levels explored the pathological concentrations. During the study, those controls were stored at −20°C; then thawed and kept at 4°C for a week. Due to the absence of Cyfra 21‐1 in this control, two levels of Kryptor Cyfra 21‐1 QC were also used. Those controls were stored at −20°C, then thawed and kept at 4°C for a day.
All assays were performed according to manufacturer's recommendations.
Imprecision evaluation
Measurements were performed twice a day during 10 days for the intermediate imprecision evaluation and ten times in a single run for within‐run imprecision.
Method and kinetic comparisons
Method comparison was conducted according to CLSI guidelines 30 using 551 results obtained from 360 patient samples. All outliers values were controlled with both systems. Passing‐Bablock regression and Bland‐Altman diagrams were plotted using MedCalc 12®, with a percent y scale for Bland‐Altman diagrams, rather than an absolute scale, because the standard deviation increased with the concentration. Cut‐off values comparison was performed using manufacturer's reference values to discriminate positive results from negative results and then calculate positive and negative concordance.
Kinetic patterns were established from serial measurements of these markers during chemotherapy. Curves were obtained by plotting logarithm of tumor marker concentrations in function of the time with either Kryptor or Modular and Lumipulse results. The pairs of kinetics obtained were compared on their shapes, half‐lives, doubling‐times, and nadir values.
RESULTS
Only 471 out of 577 samples initially selected were retrieved and analyzed to obtain a total of 630 tumor marker results. Results ten times superior to Lumipulse assays linearity limits were excluded, leaving 551 tumor marker results from 360 patient samples for the method comparison.
Analytical performances
The analytical performances of the seven tumor marker assays are summarized in Table 1. Within‐run imprecision and intermediate imprecision CVs were very good for AFP, PSA, CEA, and the three CA markers, with CVs below 2.5% and 5.0%, respectively, for all three levels of controls. Imprecision was slightly better for medium and high concentrations with intermediate imprecision CVs below 3.5%. These performances were comparable to those obtained by Cho in a previous study 31.
Table 1.
Precision Performances of Lumipulse G1200 in Measuring Seven Tumor Markers
| Coefficient of variation (%) | ||||
|---|---|---|---|---|
| Number of measurements | Mean concentration | Within‐run Imprecision | Intermediate fidelity | |
| CA 15‐3 | ||||
| Low | 24 | 32.44 | 1.24 | 3.63 |
| Medium | 22 | 66.73 | 0.87 | 2.56 |
| High | 19 | 88.28 | 1.11 | 2.79 |
| CA 125 | ||||
| Low | 22 | 31.55 | 1.61 | 4.90 |
| Medium | 21 | 55.88 | 1.20 | 2.96 |
| High | 20 | 98.69 | 1.20 | 3.08 |
| CA 19‐9 | ||||
| Low | 21 | 31.69 | 2.07 | 3.41 |
| Medium | 21 | 149.00 | 1.01 | 2.68 |
| High | 18 | 241.42 | 0.90 | 2.75 |
| CEA | ||||
| Low | 22 | 3.86 | 2.41 | 3.72 |
| Medium | 18 | 14.10 | 0.89 | 3.09 |
| High | 21 | 44.66 | 1.14 | 2.94 |
| AFP | ||||
| Low | 22 | 7.98 | 1.59 | 2.98 |
| Medium | 22 | 122.15 | 1.40 | 2.87 |
| High | 22 | 249.72 | 1.46 | 2.43 |
| PSA | ||||
| Low | 24 | 3.94 | 1.29 | 2.28 |
| Medium | 21 | 10.65 | 0.90 | 2.05 |
| High | 22 | 15.73 | 1.22 | 1.85 |
| Cyfra21‐1 | ||||
| Low | 10 | 1.67 | 4.80 | 12.95 |
| High | 9 | 11.04 | 8.45 | 8.44 |
CA, Carbohydrate antigen; CEA, Carcinoembryonic antigen; AFP, Alpha‐foetoprotein; PSA, Prostate Specific Antigen. Units are kU/L for CA 15‐3, CA 125 and CA 19‐9 and ng/ml for PSA, AFP, CEA, and Cyfra 21‐1. Seronorm Immunoassay QC was used for all markers except for Cyfra 21‐1 (Kryptor QC).
Cyfra 21‐1 assay seemed less precise than the others, with within‐run imprecision CV below 8.5% and intermediate imprecision CV below 13%. But its imprecision was assessed with only ten measurements and with a different control material (Cyfra 21‐1 Kryptor QC) and should be verified with more measurements.
Method comparison
The method comparison consisted in a Passing‐Bablock regression analysis 32 and a Bland‐Altman difference plot 33, using concentrations within and above linearity limits of both systems (Fig. 1).
Figure 1.
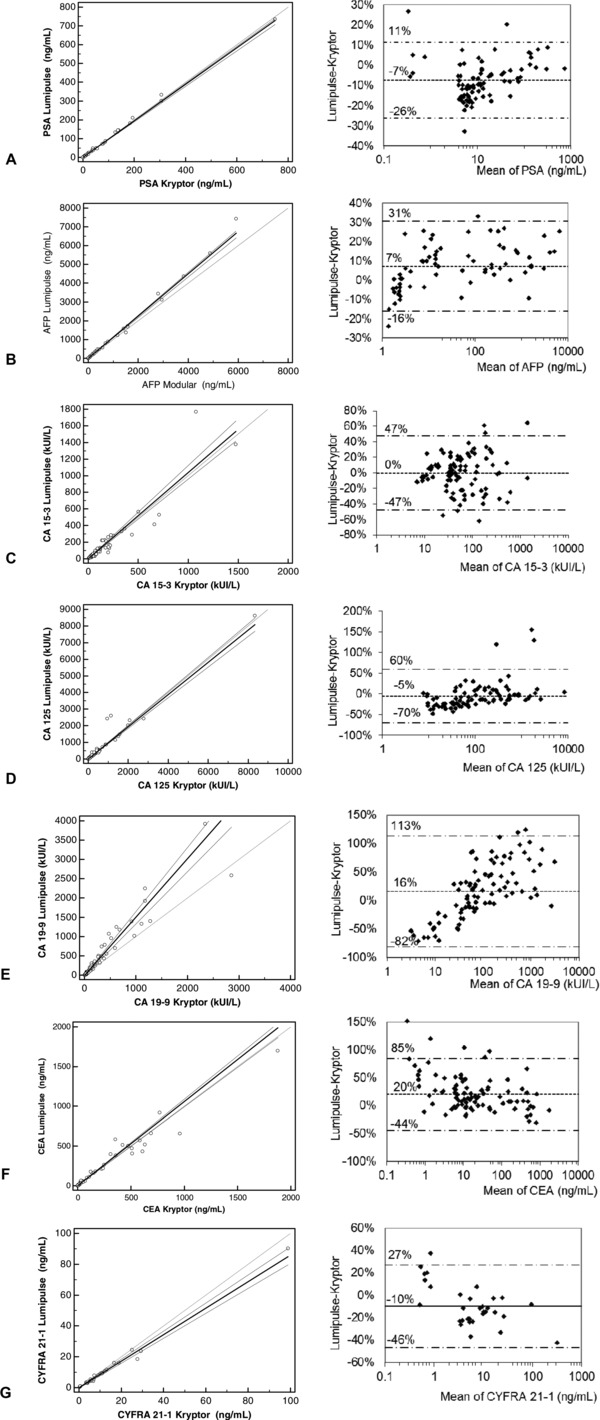
Method comparison for PSA (A), AFP (B), CA 15‐3 (C), CA 125 (D), CA 19‐9 (E), CEA (F) and Cyfra 21‐1 (G). Left column: Passing‐Bablock regression plots with the regression line (solid line), the confidence interval for the regression line (dashed lines) and the identity line (x = y, dotted line). Right column : Bland‐Altman plots with mean of differences and 95% confidence intervals.
As summarized in Table 2, Passing‐Bablock regression parameters exhibited a good correlation between analyzers for PSA, AFP, CEA, CA 125, CA 15‐3, and Cyfra 21‐1 (r ≥ 0.94) with slopes ranging from 0.86 to 1.13 and intercepts ranging from −4.9 to 0.4. For CA 19‐9, results were higher with Lumipulse than with Kryptor (slope = 1.5), indicating the presence of a proportional bias between Lumipulse and Kryptor results.
Table 2.
Passing‐Bablock Regression Parameters for LUMIPULSE Assays
| Marker | Reference system | N | Slope (95% CI) | Intercept | r |
|---|---|---|---|---|---|
| CA 15‐3 | Kryptor | 96 | 1.04 (0.96–1.12) | −0.83 | 0.938 |
| CA 125 | Kryptor | 85 | 0.96 (0.91–1.00) | −4.68 | 0.998 |
| CA 19‐9 | Kryptor | 92 | 1.52 (1.36–1.66) | −13.84 | 0.948 |
| CEA | Kryptor | 96 | 1.06 (0.99–1.09) | 0.43 | 0.980 |
| PSA | Kryptor | 83 | 0.97 (0.94–0.98) | −0.59 | 0.997 |
| Cyfra 21‐1 | Kryptor | 31 | 0.86 (0.81–0.91) | 0.09 | 0.996 |
| AFP | Modular | 68 | 1.13 (1.08–1.15) | −0.33 | 0.998 |
N, number of samples; r, correlation coefficient; CI, confidence interval.
Linear regression results were similar when including only values within the linearity range of both systems (data not shown) and confirmed results obtained by Cho 31.
The Bland‐Altman plots for PSA, AFP, CA 15‐3, CA 125, and Cyfra 21‐1 (Fig. 1A, B, C, D, and G) showed very good means of differences (−10% to 7%) and 95% limits of agreement (−60% to +50%), thus confirming the possible result transferability between Lumipulse and analyzers used in this study (Kryptor and Modular). For CA 19‐9 (Fig. 1E), the mean of differences was 16% with a large 95% confidence interval (−82% to +113%). Furthermore, differences between assays increased with the analyte concentration, confirming the presence of a significant proportional bias and a poor result transferability between Lumipulse and Kryptor CA 19‐9 results. This result contrasts with the fact that both assays use the same monoclonal antibody 26 (Centocor 1116‐NS‐19‐9) but is concordant with previous studies which demonstrated that Kryptor tends to underestimate CA 19‐9 values 26 compared to chemiluminescence‐based analyzers.
CEA Lumipulse and Kryptor assays were well correlated (r ≥ 0.980), yet some discrepancies were noted on the Passing‐Bablock and Bland‐Altman plots (Fig. 1 F) with a mean difference of +20% and large 95% limits of agreement (−45 to +85%), suggesting a poor result transferability between Lumipulse and Kryptor CEA results.
One patient, suffering from colic adenocarcinoma, had discordant Lumipulse and Kryptor results. At different sampling times, one CA 125 and two CA 19‐9 Lumipulse results were two to three times higher than Kryptor's but no analytical explanation was found. Consequently, those results were considered as outliers and excluded from the analysis.
Cut‐off values comparison
We compared all tumor marker concentrations to the respective cut‐off value recommended by each manufacturer. For all tumor markers, positive and negative concordances were ≥95% (except for CA 19‐9, 93%), meaning that for 95% of patients, the clinical interpretation of a tumor marker measurement did not differ whether the analysis was performed with Lumipulse or Kryptor (or Modular for AFP). For CA 15‐3, CA 125, and AFP, discordances concerned samples with values above Kryptor cut‐off and below Lumipulse cut‐off. For CA19‐9, the bias was responsible for some of the discrepancies observed (mostly values above Lumipulse cut‐off and below Kryptor cut‐off). For PSA, CEA, and CYFRA 21‐1, discordances were observed in less than 1% of samples, thus interpretation results did not differ between Lumipulse and Kryptor. All these discrepancies affected only border‐line values, limiting false clinical interpretations.
Patient kinetics comparison
Tumor marker measurement plays a critical role in the monitoring of patients with cancer. For this purpose, several studies 34, 35, 36, 37 have suggested that a kinetic approach is more appropriate than individual tumor marker measurements because it allows the calculation of parameters such as half‐life, doubling time, and the representation of the exponential nature of tumor growth.
Thus, the follow‐up of patients with cancer often includes tumor markers kinetics, performed by plotting log tumor marker concentration in function of time and calculating various kinetic parameters (half‐life, doubling‐time, and nadir), which are powerful indicators of therapeutic efficiency.
A total of 43 kinetics (≥ 4 kinetics for each tumor marker, except only one kinetic for CYFRA 21‐1) were analyzed, all of them had similar profiles, doubling times, half‐lives, and nadir, whether the analyzer was Lumipulse or Kryptor (Modular for AFP). Four of them are illustrated in Figure 2. The first case (Fig. 2A), is a CA 15‐3 kinetic plotted with Kryptor vs. Lumipulse results. With both systems, similar doubling‐times (67 vs. 64 days), half‐lives (18 vs. 15 days) and nadir (23 vs. 25 kUI/l at the same time) were calculated, thus leading to similar clinico‐biological interpretations. All the three other kinetics (Fig. 2B, C, and D) had similar profiles and parameters, suggesting that CA 125 and AFP Lumipulse kinetics lead to the same clinico‐biological interpretations as the reference systems kinetics. Finally, all 39 other Lumipulse kinetics (≥1 for each tumor marker) had profiles similar to Kryptor or Modular kinetics. To conclude, for all seven tumor markers, Lumipulse kinetics lead to the same clinico‐biological interpretations as the reference systems, whether the objective was the evaluation of response to treatment or the early detection of relapse.
Figure 2.
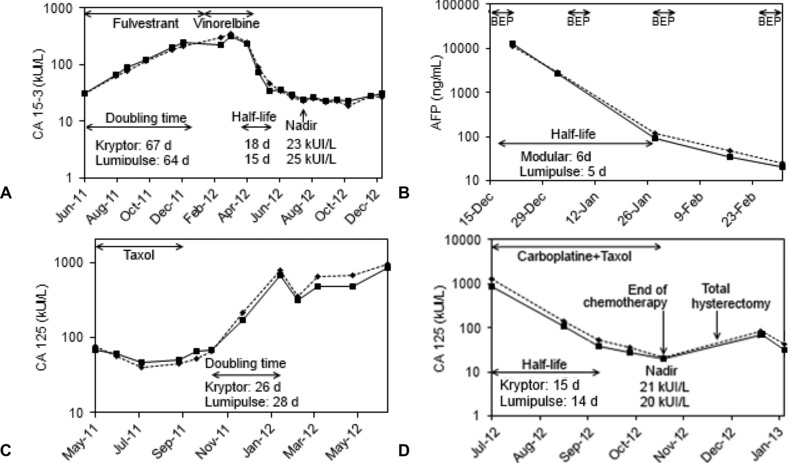
Lumipulse results (plain line) and Modular results (dot line, graph C) or Kryptor (dot line, graph A, B, and D), d = days. (A) Ms. P, metastatic breast adenocarcinoma; (B) Mr. E, nonseminomatous mediastinal germinal tumor, under treatment with Bleomycin, Etoposide, and Cisplatine (BEP); (C) Ms. J, ovarian adenocarcinoma; (D) Ms B, ovarian adenocarcinoma with peritoneal carcinosis.
One patient out of 43 had discordant Lumipulse and Kryptor kinetics. He was suffering from gastric adenocarcinoma, treated by cetuximab and monitored by CA 19‐9 kinetic (Data not shown). A quenching effect was suspected of interfering with CA 19‐9 Kryptor measurement, lowering dramatically the Kryptor's results. This interference was drastically reduced after sufficient sample dilution. Cetuximab or tumoral metabolites were suspected as quenching agent. It seemed that Lumipulse was not affected by this interference.
DISCUSSION
In this study, we confirmed the analytical performances of Lumipulse G1200, especially its good precision with a within‐run imprecision CV < 2.5% and an intermediate imprecision CV < 5.0% for PSA, CEA, AFP, CA 15‐3, CA 125, and CA 19‐9 assays. Cyfra 21‐1 assay imprecision was not that good, with CV < 9% for within‐run imprecision and CV < 13% for intermediate fidelity, possibly due to the use of a different control and the small number of measurements.
Method comparison between Lumipulse and Kryptor (or Modular for AFP) exhibited good correlations for all assays (r ≥ 0.94) and even result transferability between systems (mean of differences ± 10% and limits of agreement ± 60%) for PSA, CA 15‐3, CA 125, and Cyfra 21‐1 assays. On the contrary, comparison of CA 19‐9 and CEA Lumipulse vs. Kryptor assays revealed a significant bias (mean of differences = 15–20%) and large limits of agreement (−80% to +110%), suggesting a poor equivalence between systems. Cut‐off values comparison confirmed that Lumipulse and Kryptor (or Modular) discriminate the same samples as normal or pathological for all tumor markers except for CA 19‐9. Finally, analysis of 43 patient tumor markers kinetics and comparison of their parameters demonstrated that patient follow‐up by tumor markers kinetics with either Lumipulse or Kryptor (or Modular for AFP) leads to similar interpretations. Those results suggest that both analyzers are interchangeable for patient follow‐up, except for CA 19‐9 and CEA due to a positive bias between Lumipulse and Kryptor assays.
During this study, we also evaluated Lumipulse practicability, as a new analyzer introduced in a biochemistry hospital laboratory. Only a short training session (2–5 h) is required to perform measurements and daily maintenances. Its user‐friendly interface and its simple reagents replacement makes it easy‐to‐use. Additionally, its quick daily maintenance (10 min) and its constant assay duration (25 min) minimize working time spent on the analyzer.
REFERENCES
- 1. Gish RG. Hepatocellular carcinoma: Overcoming challenges in disease management. Clin Gastroenterol Hepatol 2006;4:252–261. [DOI] [PubMed] [Google Scholar]
- 2. Toyoda H, Kumada T, Tada T, Niinomi T, Ito T, Kaneoka Y, et al. Prognostic significance of a combination of pre‐ and post‐treatment tumor markers for hepatocellular carcinoma curatively treated with hepatectomy. J Hepatol;2012;57:1251–1257. [DOI] [PubMed] [Google Scholar]
- 3. Yuen MF, Lai CL. Serological markers of liver cancer. Best Pract Res Clin Gastroenterol 2005;19:91–99. [DOI] [PubMed] [Google Scholar]
- 4. Filella X, Alcover J, Molina R, Rodríguez A, Carretero P, Ballesta AM. Clinical evaluation of free PSA/Total PSA (Prostate‐specific Antigen) ratio in the diagnosis of prostate cancer. Eur J Cancer 1997;33:1226–1229. [DOI] [PubMed] [Google Scholar]
- 5. Freedland SJ, Hotaling JM, Fitzsimons NJ, Presti Jr JC, Kane CJ, Terris MK, et al. PSA in the New Millennium: A Powerful Predictor of Prostate Cancer Prognosis and Radical Prostatectomy Outcomes—Results from the SEARCH Database. EurUrol 2008;53:758–766. [DOI] [PubMed] [Google Scholar]
- 6. Grunnet M, Sorensen JB. Carcinoembryonic antigen (CEA) as tumor marker in lung cancer. Lung Cancer 2012;76:138–143. [DOI] [PubMed] [Google Scholar]
- 7. Goldstein MJ, Mitchell EP. Carcinoembryonic antigen in the staging and follow‐up of patients with colorectal cancer. Cancer Invest 2005;23:338–351. [DOI] [PubMed] [Google Scholar]
- 8. Eidelman FJ, Fuks A, DeMarte L, Taheri M, Stanners CP. Human carcinoembryonic antigen, an intercellular adhesion molecule, blocks fusion and differentiation of rat myoblasts. J Cell Biol 1993;123:467–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ordonez C, Screaton RA, Ilantzis C, Stanners CP. Human carcinoembryonic antigen functions as a general inhibitor of anoikis. Cancer Res 2000;60:3419–3424. [PubMed] [Google Scholar]
- 10. Brouckaert O, Laenen A, Wildiers H, Floris G, Moerman P, Van Limbergen E, et al. The prognostic role of preoperative and (early) postoperatively change in CA15.3 serum levels in a single hospital cohort of primary operable breast cancers. Breast J 2013;22:254–262. [DOI] [PubMed] [Google Scholar]
- 11. Duffy MJ, Evoy D, McDermott EW. CA 15‐3: Uses and limitation as a biomarker for breast cancer. Clinica Chimica Acta 2010;411:1869–1874. [DOI] [PubMed] [Google Scholar]
- 12. Xu J‐L, Commins J, Partridge E, Riley TL, Prorok PC, Johnson CC, et al. Longitudinal evaluation of CA‐125 velocity and prediction of ovarian cancer. Gynecol Oncol 2012;125:70–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mano A, Falcão A, Godinho I, Santos J, Leitão F, Oliveira C, et al. CA‐125 AUC as a new prognostic factor for patients with ovarian cancer. Gynecol Oncol 2005;97:529–534. [DOI] [PubMed] [Google Scholar]
- 14. Kang CM, Kim JY, Choi GH, Kim KS, Choi JS, Lee WJ, et al. The Use of Adjusted Preoperative CA 19‐9 to Predict the Recurrence of Resectable Pancreatic Cancer. J Surg Res 2007;140:31–35. [DOI] [PubMed] [Google Scholar]
- 15. Koom WS, Seong J, Kim YB, Pyun HO, Song SY. CA 19‐9 as a predictor for response and survival in advanced pancreatic cancer patients treated with chemoradiotherapy. Int J Radiat Oncol Biol Phys 2009;73:1148–1154. [DOI] [PubMed] [Google Scholar]
- 16. Micke O, Bruns F, Kurowski R, Horst E, deVries AF, Hausler JW, et al. Predictive value of carbohydrate antigen 19‐9 in pancreatic cancer treated with radiochemotherapy. Int J Radiat Oncol Biol Phys 2003;57:90–97. [DOI] [PubMed] [Google Scholar]
- 17. Hamzaoui A, Thomas P, Castelnau O, Roux N, Roux F, Kleisbauer JP. Usefulness of longitudinal evaluation of Cyfra 21‐1 variations in advanced lung cancer monitoring. Lung Cancer 1997;16:191–202. [DOI] [PubMed] [Google Scholar]
- 18. Hanagiri T, Sugaya M, Takenaka M, Oka S, Baba T, Shigematsu Y, et al. Preoperative CYFRA 21‐1 and CEA as prognostic factors in patients with stage I non‐small cell lung cancer. Lung Cancer 2011;74:112–117. [DOI] [PubMed] [Google Scholar]
- 19. Pujol J‐L, Boher J‐M, Grenier J, Quantin X. Cyfra 21‐1, neuron specific enolase and prognosis of non‐small cell lung cancer: prospective study in 621 patients. Lung Cancer 2001;31:221–231. [DOI] [PubMed] [Google Scholar]
- 20. Pichon MF, Brun GL, Hacene K, Basuyau JP, Riedinger JM, Eche N, et al. Comparison of fifteen immunoassays for the measurement of serum MUC‐1/CA 15‐3 in breast cancer patients. Clin Chem Lab Med 2009;47:985–992. doi: 10.1515/CCLM.2009.213. [DOI] [PubMed] [Google Scholar]
- 21. Glikmanas G, Plouvier E, Thuillier F. Évaluation du dosage de l'ACE, du PSA et de l'AFP sur l'Elecsys™ BoehringerMannheim. Transférabilité des résultats entre l'ES 300™ et l'Elecsys™. Immuno‐analyse et Biologie Spécialisée 1997;12:138–142. [Google Scholar]
- 22. Yan G, Ju H, Liang Z, Zhang T. Technical and clinical comparison of two fully automated methods for the immunoassay of CA 125 in serum. J Immunol Methods 1999;225:1–8. [DOI] [PubMed] [Google Scholar]
- 23. Moutereau S, Rymer JC. Kryptor—Évaluation finale en situation de routine. Immuno‐analyse et biologie Spécialisée 1998;13:307–316. [Google Scholar]
- 24. Stern P, Friedecky B, Bartos V, Bezdickova D, Vavrova J, Uhrova J, et al. Comparison of different immunoassays for CA 19‐9. Clin Chem Lab Med 2001;39:1278–1282. [DOI] [PubMed] [Google Scholar]
- 25. Choi J, Park Y, Kim J‐H, Kim H‐S. Evaluation of automated serum des‐gamma‐carboxyprothrombin (DCP) assays for detecting hepatocellular carcinoma. Clin Biochem 2011;44:1464–1468. [DOI] [PubMed] [Google Scholar]
- 26. Passerini R, Riggio D, Salvatici M, Zorzino L, Radice D, Sandri MT. Interchangeability of measurements of CA 19‐9 in serum with four frequently used assays: an update. Clin Chem Lab Med 2007;45:100–104. [DOI] [PubMed] [Google Scholar]
- 27. Park Y, Park J, Kim HS. Evaluation of the UniCel DxI 800 immunoassay analyzer in measuring five tumor markers. Yonsei Med J 2012;53:557–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zur B, Holdenrieder S, Albers E, Walgenbach‐Brunagel G, Stoffel‐Wagner B. Method comparison for CA 15‐3, CA 19‐9, and CA 125 determination using the new LOCI technique of Dimension Vista 1500 and Immulite 2000 XPI. J Immunoassay Immunochem 2012;33:435–445. doi: 10.1080/15321819.2012.666221. [DOI] [PubMed] [Google Scholar]
- 29. Mathis G. Rare earth cryptates and homogeneous fluoroimmunoassays with human sera. Clin Chem 1993;39:1953–1959. [PubMed] [Google Scholar]
- 30. Clinical and Laboratory Standards Institute (CLSI) Method Comparison and Bias estimation using patient samples; Approved guidelines‐Second Edition 2002; Document EP9‐A2.
- 31. Cho J‐H, Lee C‐M, Park CM, Moon H‐W, Hur M, Yun Y‐M, et al. Evaluation of the Performance of Lumipulse G1200 for Tumor Marker Assays. Lab Med Online 2012;2:131–138. [Google Scholar]
- 32. Passing H, Bablok. A new biometrical procedure for testing the equality of measurements from two different analytical methods. Application of linear regression procedures for method comparison studies in clinical chemistry, Part I. J Clin Chem Clin Biochem 1983;21:709–720. [DOI] [PubMed] [Google Scholar]
- 33. Dewitte K, Fierens C, Stockl D, Thienpont LM. Application of the Bland‐Altman plot for interpretation of method‐comparison studies: a critical investigation of its practice. Clin Chem 2002;48:799–801. [PubMed] [Google Scholar]
- 34. Bidart JM, Thuillier F, Augereau C, Chalas J, Daver A, Jacob N, et al. Kinetics of serum tumor marker concentrations and usefulness in clinical monitoring. Clin Chem 1999;45:1695–1707. [PubMed] [Google Scholar]
- 35. Cheung KL, Graves CRL, Robertson JFR. Tumour marker measurements in the diagnosis and monitoring of breast cancer. Cancer Treat Rev 2000;26:91–102. [DOI] [PubMed] [Google Scholar]
- 36. Hess V, Glimelius B, Grawe P, Dietrich D, Bodoky G, Ruhstaller T, et al. CA 19‐9 tumour‐marker response to chemotherapy in patients with advanced pancreatic cancer enrolled in a randomised controlled trial. Lancet Oncol 2008;9:132–138. [DOI] [PubMed] [Google Scholar]
- 37. Sölétormos G, Nielsen D, Schiøler V, Mouridsen H, Dombernowsky P. Monitoring different stages of breast cancer using tumour markers CA 15‐3, CEA and TPA. Eur J Cancer 2004;40:481–486. [DOI] [PubMed] [Google Scholar]


