Abstract
Background
Fecal calprotectin is a noninvasive marker for bowel diseases and it is high valuable to follow disease activity in Crohn's disease (CD) and ulcerative colitis (UC). In this study, we evaluated the diagnostic performance of the recently introduced immunochromatographic assay CalFast in comparison to the well‐known ELISA tests for calprotectin assay to obtain a rapid diagnosis of bowel inflammation in pediatric patients.
Methods
CalFast was tested in parallel to the classic ELISA tests CalPrest and PhiCal (gold standards for the calprotectin determination) on 148 fecal samples from pediatric subjects including 104 healthy subjects, 29 with CD, and 15 with UC.
Results
In this study, the sensitivity and specificity of CalFast, CalPrest, and PhiCal were 86.4%, 88.6%, and 93.2% and 86.6%, 74%, and 64.4%, respectively. The area under the curve, obtained from receiver operating characteristic analysis, indicated the lack of significant difference among all the kits used.
Conclusion
The immunochromatographic assay demonstrated good diagnostic predictive values, comparable to those of the ELISA methods, and may represent a valid alternative in order to save operators' time. The test, in fact, has a short turnaround time and does not need a specific ELISA instrumentation.
Keywords: immunochromatographic assay, immunoenzymatic assay, fecal marker, gastrointestinal diseases, children
INTRODUCTION
Ulcerative colitis (UC) and Crohn's disease (CD) are two of the major forms of chronic intestinal inflammatory disease, characterized by a heavy and diffuse infiltration of neutrophil polymorphs cells. Calprotectin is a calcium‐binding protein, present in large amount in neutrophil cytoplasm, and represents more than 60% of cytosolic proteins 1. Consequently, fecal calprotectin concentration may be related to an inflammation of the bowel mucosa in inflammatory bowel disease (IBD) 2 and represents the main noninvasive fecal marker for “neutrophilic intestinal inflammation” in bowel disease 3. It is very useful to follow disease activity in CD and UC, largely applied in the pediatric clinical practice 2, 4, 5. Many studies reported that calprotectin concentrations correlate with the clinical activity indices 6, 7, and, even better, with both endoscopic and histological results for both UC 8, 9 and CD 7, 10. It is considered that calprotectin, together with lactoferrin, is the best noninvasive marker for serial monitoring of disease activity and treatment success, and can even serve in predicting clinical relapse in unsymptomatic patients or sustained remission 11. Furthermore, Schoepfer et al. reported that calprotectin was able to discriminate endoscopically inactive from mild, moderate, and severely active disease 12.
It is well known that pediatric patients suffering from IBDs have high levels of fecal calprotectin, and in these subjects the sensitivity and specificity of the calprotectin determination are more reliable than other biochemical parameters normally used to check the presence of an inflammatory condition of the intestine 2. By contrast, in the newborn, in the first days of life, there are very high levels of calprotectin, which normalize after the first few days of life. Subjects affected by inflammatory bowel syndrome (IBS), on the other hand, have a normal value of fecal calprotectin 13.
Calprotectin is a very stable molecule in fecal samples and its concentration remains constant for 12 months at –20°C and for 7 days at room temperature 14. Fecal samples can be easily obtained from pediatric and adult subjects. Calprotectin can be determined by several immunoenzymatic methods. The quantification of this protein can be performed in virtually all laboratories, mainly using enzyme‐linked immunosorbent assay (ELISA) techniques 15, 16. Quantitative rapid assays are generally available and are frequently used as point‐of‐care test (POCT) in clinical procedures 17.
The ELISA method using monoclonal or polyclonal antibodies is the oldest test for calprotectin determination. Recently, the new laboratory kit CalFast has been developed and offered to clinical laboratories, proposing a simplified quantitative immunochromatographic fecal assay that uses a proper mix of monoclonal and polyclonal antibodies. The aim of this study is to evaluate the diagnostic accuracy of the CalFast immunochromatographic assay, compared to that of two routine ELISA methods, in a pediatric cohort for screening of patients with IBDs.
MATERIALS AND METHODS
Patients and Stool Collection
The study was conducted at the Gastroenterology Unit and Laboratory of Molecular Medicine of the Institute of Maternal and Child Health—IRCCS Burlo Garofolo of Trieste, Germany. It was conducted according to the guidelines laid down in the Declaration of Helsinki protocol and all the procedures were approved by the Ethical Committee of our institute (REC number: 34/11). All major‐age subjects (two subjects) were informed about the aims and procedures of the study, and signed the specific informed consent. Otherwise, written informed consent was obtained from all the parents to permit the participation of the subjects of minor age.
Fecal samples were obtained from 148 pediatric subjects (ranging in ages from 2 to 18 years, 70 males and 78 females), 44 of which were affected by intestinal diseases. A total of 104 control subjects were enrolled: exclusion criteria were reported abdominal complaints, assessed by questionnaire prior to inclusion; gastrointestinal illness (GI) history; and any other medical condition that could influence the experimental results. The subjects with GI were consecutively enrolled at the Gastroenterology Unit of the hospital from November 2011 to December 2012. Twenty‐nine were affected by CD and 15 by UC, who were clinically classified following the multiparameter clinical score PCDAI (Pediatric Crohn's Disease Activity Index) for CD 18 and PUCAI (Pediatric Ulcerative Colitis Activity Index) for UC 19. All patients were diagnosed in acute phase. All stool samples were collected in plastic containers and frozen at –20°C without urine contamination until use.
Reagents
Commercial kits were used according to the manufacturer's instructions and applying the suggested cutoffs: PhiCal Calprotectin ELISA based on two‐site sandwich technique with two selected monoclonal antibodies, cutoff of 50 mg/kg (Pantec Immunodiagnostic, Milano, Italy); Calprest ELISA based on polyclonal antibodies against fecal calprotectin, cutoff of 100 mg/kg (Eurospital, Trieste, Italy); CalFast Immunochromatographic assay with a cutoff of 100 mg/kg combined with a mixture of anticalprotectin polyclonal and monoclonal antibodies (Eurospital, Trieste, Italy).
Test Procedure
One hundred milligrams of stool samples were carefully weighed for every single measurement, and treated according to the manufacturer's instructions. After the weighing, the stool samples were inserted into the tubes containing the extraction solution and were shaken by Vortex for 30 to 60 s to properly homogenize the content. Subsequently, the tube containing the homogenate was centrifuged at 3,0 ×g for 5 min and the supernatant collected. Supernatants were assayed in the ELISA automatic processor TEK4 (Alifax, Padova, Italy) using ELISA plates PhiCal or Calprest. Calprotectin concentration was calculated from the standard curve using the kit reagents.
Conversely, 100 μl of fecal supernatant was transferred in the immunochromatographic disposable device and incubated at room temperature for 20 min. The reaction was evaluated using CalFast reader (Eurospital, Trieste, Italy) after the proper calibration and identification of the reagent lot. The detectable range of calprotectin concentration, obtained using a CalFast Reader, was between 15 and 300 mg/kg.
Statistical Analysis
Fecal calprotectin results were analyzed using PRISM 5 (GraphPad Software, San Diego, CA) and SigmaPlot (Systat Software, San Jose, CA) software to calculate specificity, sensitivity, positive predictive value (PPV), and negative predictive value (NPV) and perform correlation studies, receiver operating characteristic (ROC) analysis, and chi‐square tests 20, 21.
RESULTS
Data from 148 fecal samples were elaborated to obtain specificity, sensitivity, PPV, and NPV (see Table 1. The diagnostic accuracy of each commercial kit was performed using an ROC curve. The corresponding areas under the curve (AUC) indicate the best accuracy and are shown in supporting Figure 1. The AUC values and the statistic results obtained from pair comparison are shown in Table 2 and indicate the absence of significant difference between the CalFast method and the two ELISA methods used for the assay of calprotectin.
Table 1.
Specificity, Sensitivity, PPV, and NPV (and Their 95% CI) for Fecal Calprotectin Assays in 148 Samples
| Kit | Sensitivity% (95% CI) | Specificity% (95% CI) | PPV% | NPV% | Cutoff (mg/kg) |
|---|---|---|---|---|---|
| CalFast | 86.4 (72.6–94.8) | 86.5 (78.5–92.4) | 73.1 | 93.8 | 100 |
| CalPrest | 88.6 (75.4–96.2) | 74 (64.5–82.1) | 59.1 | 93.9 | 100 |
| PhiCal | 93.2 (81.3–98.6) | 64.4 (54.5–73.6) | 53.6 | 95.7 | 50 |
Figure 1.
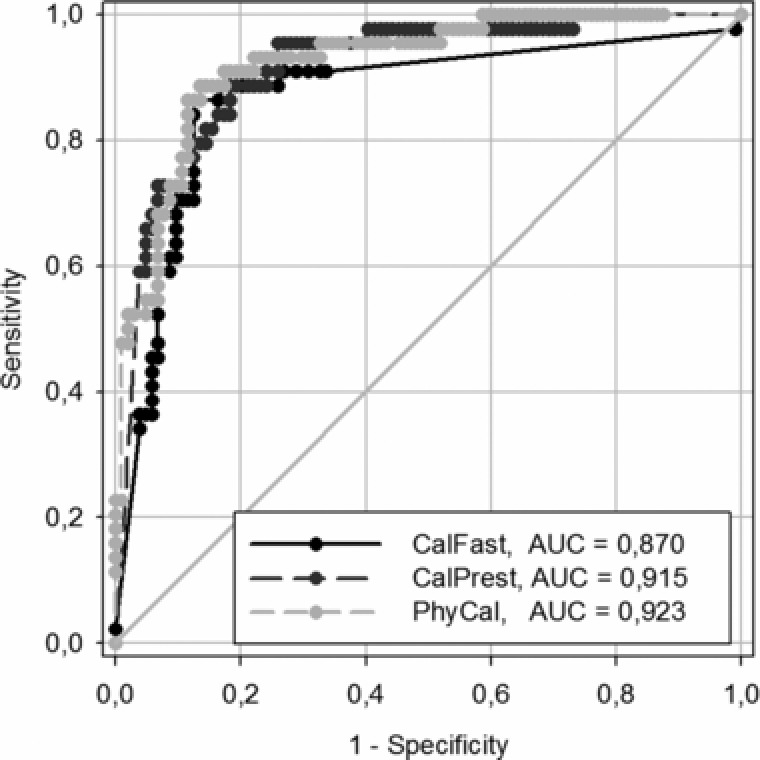
ROC analysis: Comparative study between three commercial kits for calprotectin assay.
Table 2.
ROC Curve Areas and Comparison
| CalFast | Phical | CalPrest | |
|---|---|---|---|
| AUC | 0.8703 | 0.9227 | 0.9152 |
| 0.0358 | 0.0232 | 0.0250 | |
| 95% CI | 0.7966 to 0.9420 | 0.6772 to 0.9683 | 0.8662 to 0.9643 |
| ROC curve areas’ comparison | |||
| Pair | CalFast–CalPrest | CalFast–Phical | CalPrest–Phical |
| Areas' difference | –0.04491 | –0.05244 | –0.0075 |
| SE | 0.0363 | 0.0358 | 0.0208 |
| 95% CI | –0.1161 to 0.0263 | –0.1228 to 0.0179 | –0.0483 to 0.0333 |
| P‐value | 0.2166 | 0.1439 | 0.7178 |
In Figure 2, we report the diagnostic agreement between the POCT and each ELISA method. The agreement between the two ELISA tests has been measured too. When considering the diseased patients, although there is a similar sensitivity for the three methods, the accord on detected calprotectin levels was poor for POCT versus ELISA tests (Pearson's coefficient < 0.4). A better agreement was found for the values in healthy subjects comparing CalFast to PhiCal. Nevertheless, the best agreement results when comparing the two ELISA methods, especially in healthy subjects. In addition, it is interesting to note that the Phical cutoff seems underestimated for our pediatric court. In line with published data 4 and manufacturer's instructions, a positive calprotectin result with Phical was considered to be greater than 50 μg/g; however, we note that we could improve specificity by moving this limit to 100 μg/g, similarly to the limit suggested and applied for the other two kits used. A similar change, in our patient groups, would increase the specificity of Phical to 84.1 (sensitivity of 86.3; PPP of 67.7, PPN of 94.1). Alternatively, in order to exclude false positives when a low calprotectin cutoff is used 13, we suggest to repeat testing in children with borderline results before referral.
Figure 2.
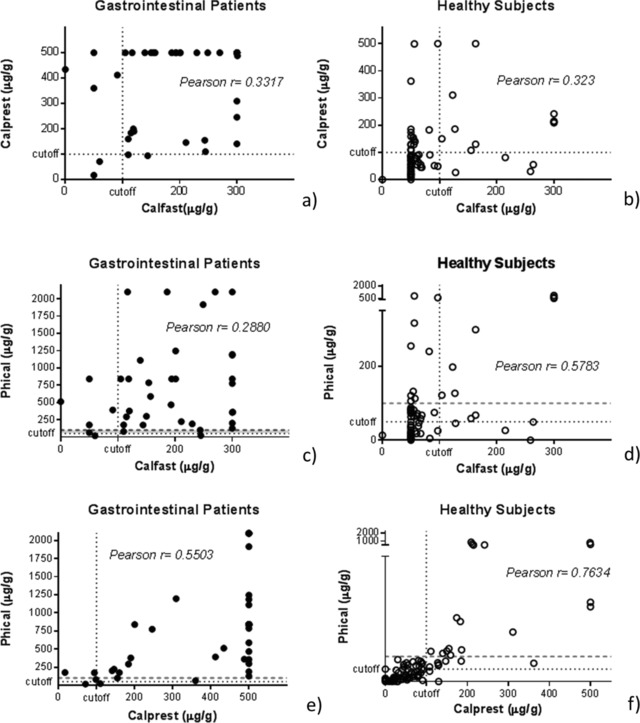
Diagnostic agreement in calprotectin measures between POCT and ELISA methods in GI patients (a, c, e) and healthy subjects (b, d, f). Dashed lines indicate adopted cutoffs; dotted red lines indicate suggested cutoff of 100 μg/g for Phical.
DISCUSSION
Calprotectin is the most commonly prescribed test for the differentiation between gastrointestinal functional disorders and inflammatory conditions such as UC and CD 22, although calprotectin is elevated in other conditions with gut inflammation as well 23. The fecal concentration of this protein is regularly elevated in subjects with active IBD and its measurement may be also a useful tool for monitoring the response to clinical treatments 7, 10, 11.
The meta‐analyses of diagnostic accuracy studies based on six fecal calprotectin studies for screening of adults with suspected IBD, in a recent review by van Rheenen et al., found out a pooled sensitivity and specificity of 93% (95% CI 85–97%) and 96% (79–99%), respectively 23. The reliability of the test was found to be lower in the studies on pediatric patients with a pooled sensitivity and specificity of 92% (84–96%) and 76% (62–86%) respectively, for seven studies on children and teenagers 24. This was confirmed by other review analyses comparing six selected studies in children with IBD and reporting a sensitivity ranging from 65% to 100%, a specificity ranging from 48% to 100%, together with PPV ranging from 54% to 100% and NPV from 67% to 100% 25. The high variability of accuracy found comparing pediatric studies may be due to different causes. The accuracy of a test is greatly influenced by preanalytical variables, which in the case of pediatric fecal analyses greatly exist in the sample collection procedures 24. To reduce this possible bias, in the present study, we carefully weighed the stool samples instead of using the disposable sampling devices suggested by the kit manufacturer. In our opinion, this caution reduced the number of false‐negative results.
Today, the dosages of calprotectin are performed by using ELISA methods, which are currently the laboratory gold standard 26. However, these methods have some limitations such as being excessive time‐consuming, needing experienced staff and specific instrumentation, high cost, and delayed reporting results. In addition, to be economically viable, ELISA tests require the simultaneous setting up of a calibration curve, appropriate controls, and series of fecal samples. Few new immunochromatographic tests are appearing on the market, proposing economic and practical advantages. Among them, the immunochromatographic CalFast is a valuable mono test that uses the same fecal sample extraction method of the ELISA test, while for quantification simply needs the specific instrument CalFast Reader, provided by the commercial company and requiring simple handling procedures.
From the data we obtained, there are marginal differences in terms of accuracy between the POCT and two ELISA kits we used. PhiCal test, when using the cut‐off value of 50 μg/g suggested by the manufacturer, has the highest sensitivity (93.2%) but the lowest specificity (64.4%); with CalPrest test the specificity increases slightly (74%), but the sensitivity is lower (88.6). Finally, CalFast demonstrates a sensitivity of 86.4%, specificity of 86.5%, but with a maximum of PPV of 73.1% and an NPV of 93.8%. To note, since the results did not show any significant differences between the ROC curves’ AUC, we definitely conclude that the three tests have a very similar assay accuracy, at least in our pediatric cohort. Our data are in agreement with pediatric literature, with sensitivity and specificity values that fall within the ranges of the most rigorous studies 24, 25.
When testing the diagnostic agreement for the POCT and ELISA measurements (Fig. 2, we note that, as expected, the two Elisa methods have the highest accord in terms of relative calprotectin values. Differently, when comparing POCT to each Elisa, as demonstrated by the Pearson's coefficients, there is a good agreement only between CalFast and Phical assays in the healthy subjects (Pearson's r = 0.578).
Our results are only marginally in accord with those recently published by Benahmed et al. 27, reporting a high diagnostic agreement between POCT and ELISA kits tested in 60 IBD manly adult patients. The discrepancy may reside in some technical differences, or in the limited sample size. Interestingly, our correlation analyses disclosed that the cutoff of 50 μg/g of calprotectin suggested for Phical by the manufacturer is not totally adequate in our pediatric population: the predictive values are improved by moving the cut‐off limit to 100 μg/g of calprotectin, similarly to what suggested for the POCT and other ELISA kit (Calprest).
Since the testing of calprotectin is a useful tool for identifying patients who are most likely to need an endoscopy for suspected IBD, the highest predictive values reduce the possibility of unnecessary invasive procedures, particularly troublesome for children. Notably, CalFast demonstrated the best balance between PPV and PNV values. In conclusion, CalFast test can be defined as a new smart assay that assures accuracy and analytical performance similar to the conventional ELISA tests but with improved practicability, which is especially useful for sporadic operators and nonspecialized laboratories.
CONFLICT OF INTEREST
No author has competing interests.
ABBREVIATIONS
- AUC
area under the curve
- CD
Crohn's disease
- GI
gastrointestinal illness
- IBD
inflammatory bowel disease
- NPV
negative predictive value
- POCT
point‐of‐care test
- PPV
positive predictive value
- ROC
receiver operating characteristic
- UC
ulcerative colitis
ACKNOWLEDGMENTS
The assay CalFast kits used in this study were supplied by Eurospital (Trieste, Italy), which did not play any role in the study regarding interpretation of data and the drafting of the manuscript.
Grant sponsor: Institute for Maternal and Child Health—IRCCS “Burlo Garofolo”.
REFERENCES
- 1. Røseth AG, Fagerhol MK, Aadland E, Schjønsby H. Assessment of the neutrophil dominating protein calprotectin in feces. A methodology study. Scand J Gastroenterol 1992;27:793–798. [DOI] [PubMed] [Google Scholar]
- 2. Bunn SK, Bisset MW, Main MJC, Golden BE. Fecal calprotectin as a measure of disease activity in childhood inflammatory bowel disease. J Pediatr Gastroenterol Nutr 2001;32:171–177. [DOI] [PubMed] [Google Scholar]
- 3. Abraham BP, Kane S. Fecal markers: Calprotectin and lactoferritin. Gastroenterol Clin North Am 2012;41:483–495. [DOI] [PubMed] [Google Scholar]
- 4. Quail MA, Russell RK, van Limbergen JE, et al. Fecal calprotectin complements routine laboratory investigations in diagnosing childhood inflammatory bowel disease. Inflamm Bowel Dis 2009;15:756–759. [DOI] [PubMed] [Google Scholar]
- 5. Aomatsu T, Yoden A, Matsumoto K, et al. Fecal calprotectin is a useful marker for disease activity in pediatric patients with inflammatory bowel disease. Dig Dis Sci 2011;56:2372–2377. [DOI] [PubMed] [Google Scholar]
- 6. Gaya D, Lyon T, Duncan A, et al. Faecal calprotectin in the assessment of Crohn's disease activity. QJM 2005;98:435–441. [DOI] [PubMed] [Google Scholar]
- 7. Sipponen T, Käkkäinen P, Savilahti E, et al. Correlation of faecal calprotectin and lactoferrin with an endoscopic score for Crohn's disease and histological findings. Aliment Pharmacol Ther 2008;28:1221–1229. [DOI] [PubMed] [Google Scholar]
- 8. Schoepfer A, Trummler M, Seeholzer P, Criblez D, Seibold F. Accuracy of four fecal assays in the diagnosis of colitis. Dis Colon Rectum 2007;50:1697–1706. [DOI] [PubMed] [Google Scholar]
- 9. Schoepfer A, Trummler M, Seeholzer P, Seibold‐Schmid B, Seibold F. Discriminating IBD from IBS: Comparison of the test performance of fecal markers, blood leukocytes, CRP, and IBD antibodies. Inflamm Bowel Dis 2008;14:32–39. [DOI] [PubMed] [Google Scholar]
- 10. Langhorst J, Elsenbruch S, Koelzer J, et al. Noninvasive markers in the assessment of intestinal inflammation in inflammatory bowel diseases: Performance of fecal lactoferrin, calprotectin, and PMN‐elastase, CRP, and clinical indices. Am J Gastroenterol 2008;103:162–169. [DOI] [PubMed] [Google Scholar]
- 11. Sipponen T. Diagnostics and prognostics of inflammatory bowel disease with fecal neutrophil‐derived biomarkers calprotectin and lactoferrin. Dig Dis 2013;31:336–344. [DOI] [PubMed] [Google Scholar]
- 12. Schoepfer A, Beglinger C, Straumann A, et al. Fecal calprotectin more accurately reflects endoscopic activity of ulcerative colitis than the Lichtiger Index, C‐reactive protein, platelets, hemoglobin, and blood leukocytes. Inflamm Bowel Dis 2013;19:332–341. [DOI] [PubMed] [Google Scholar]
- 13. Waugh N, Cummins E, Royle P, et al. Faecal calprotectin testing for differentiating amongst inflammatory and non‐inflammatory bowel diseases: systematic review and economic evaluation. Health Technol Assess 2013;xv–xix:1–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tøn H, Brandsnes, Dale S , et al. Improved assay for fecal calprotectin. Clin Chim Acta 2000;292:41–54. [DOI] [PubMed] [Google Scholar]
- 15. Summerton CB, Longlands MG, Wiener K, Shreeve DR. Fecal calprotectin: A marker of inflammation throughout the intestinal tract. Eur J Gastroenterol Hepatol 2002;14:841–845. [DOI] [PubMed] [Google Scholar]
- 16. Fagerberg UL, Lööf L, Merzoug RD, Hansson LO, Finkel Y. Fecal calprotectin levels in healthy children studied with an improved assay. J Pediatr Gastroenterol Nutr 2003;37:468–472. [DOI] [PubMed] [Google Scholar]
- 17. Dolci A, Panteghini M. Comparative study of a new quantitative rapid test with an established ELISA method for faecal calprotectin. Clin Chim Acta 2012;413:250–351. [DOI] [PubMed] [Google Scholar]
- 18. Hyams J, Markowitz J, Otley A, et al. Evaluation of the pediatric crohndisease activity index: A prospective multicenter experience. J Pediatr Gastroenterol Nutr 2005;41:416–421. [DOI] [PubMed] [Google Scholar]
- 19. Turner D, Hyams J, Markowitz J, et al. Appraisal of the pediatric ulcerative colitisactivity index (PUCAI). Inflamm Bowel Dis 2009;15:1218–1223. [DOI] [PubMed] [Google Scholar]
- 20. DeLong ER, DeLong DM, Clarke‐Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: A nonparametric approach. Biometrics 1988;44:837–845. [PubMed] [Google Scholar]
- 21. Akobeng AK. Understanding diagnostic test 3: Receiver operating characteristic curves. Acta Paediatrica 2007;96:644–647. [DOI] [PubMed] [Google Scholar]
- 22. Henderson P, Anderson NH, Wilson DC. The diagnostic accuracy of fecal calprotectin during the investigation of suspected pediatric inflammatory bowel disease: A systematic review and meta‐analysis. Am J Gastroenterol 2012;107:941–949. [DOI] [PubMed] [Google Scholar]
- 23. Vaos G, Kostakis ID, Zavras N, Chatzemichael A. The role of calprotectin in pediatric disease. Biomed Res Int 2013;2013:542363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. van Rheenen PF1, van de Vijver E, Fidler V. Faecal calprotectin for screening of patients with suspected inflammatory bowel disease: diagnostic meta‐analysis. BMJ 2010;341:c3369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Burri E, Beglinger C. Faecal protectin – A useful tool in the management of inflammatory bowel disease. Swiss Med Wkly 2012;142:1–12. [DOI] [PubMed] [Google Scholar]
- 26. Angriman I, Scarpa M, D'Incà R, et al. Enzymes in feces: Useful markers of chronic inflammatory bowel disease. Clin Chim Acta 2007;381:63–68. [DOI] [PubMed] [Google Scholar]
- 27. Benahmed NA, Manéné D, Barbot‐Trystram L, Kapel N. Evaluation of Calfast® immunochromatographic quantitative assay for the measurement of calprotectin in faeces. Clin Chem Lab Med 2014;52:143–145. [DOI] [PubMed] [Google Scholar]


