Abstract
Background
The accuracy of 25‐hydroxyvitamin D3 (25OHD3) measurement on specimens collected into serum separator tubes (SSTs) has been questioned because of possible interference by the gel. Possible interference was investigated in SSTs from Becton Dickinson (BD).
Design and methods
Blood specimens were collected simultaneously from 50 normal subjects into plain tubes and SSTs. 25OHD3 was assayed on serum using high performance liquid chromatography (Chromsystems), and Architect (Abbott) and Liaison (Diasorin) immunoassays.
Results
There were no significant differences between 25OHD3 results (means ± SE, nmol/l) obtained from specimens collected into plain tubes and SSTs assayed by HPLC (39.0 ± 2.7 vs. 39.3 ± 2.7), Liaison (32.9 ± 2.2 vs. 32.8 ± 2.3), or Architect (43.1 ± 2.8 vs. 43.2 ± 2.8). In specimens collected into plain tubes and SSTs, 25OHD3 measurements by HPLC correlated significantly (P < 0.0001) with those from the Architect (r = 0.895, r = 0.908) and Liaison (r = 0.907, r = 0.913), respectively.
Conclusions
The gel in SSTs (BD) does not interfere with the measurement of 25OHD3 by HPLC or common immunoassays. This important finding may enable clinical laboratories to make cost savings by using SSTs without concerns about inaccuracy.
Keywords: 25OHD3, Becton Dickinson, plain tubes, SSTs, vitamin D
Vitamin D has a physiological role in calcium homeostasis and bone turnover 1. Its insufficiency is implicated in numerous clinical disorders 2. The main vitamin D metabolites are cholecalciferol (25‐hydroxyvitamin D3 (25OHD3)) and ergocalciferol (25‐hydroxyvitamin D2 (25OHD2)). 25OHD3 is produced from 7‐dehydrocholesterol in the skin 3 whereas 25OHD2 is derived from plants. Both 25OHD3 and 25OHD2 are used as supplements but 25OHD3 is considered the more potent 4. HPLC and liquid chromatography‐tandem mass spectrometry (LC‐MS/MS) are still considered the reference methods for vitamin D measurement. Commercial immunoassays were later developed to satisfy increased demand for 25OHD measurement.
It has recently become common practice to collect blood into serum separator tubes (SSTs) containing a barrier gel that separates the blood clot from serum following centrifugation. Although this method is convenient, there have been concerns about possible analytical interference from the gel. Manufacturers of SSTs do not always provide information on the effect of the gel on 25OHD3 measurement by HPLC 5, 6. However, there have been reports of gel interference with measurement of 25OHD by both HPLC 7 and LC‐MS 8. No study has carried out a comprehensive investigation of the effect of SSTs on the estimation of 25OHD3 by HPLC or immunoassay methods. The purpose of the present study was therefore to determine whether SSTs influenced the 25OHD3 concentration. A secondary aim of the study was to evaluate bias of the immunoassay techniques compared to HPLC.
Blood specimens were collected from 50 healthy volunteers (22 males, 28 females, aged 42.6 ± 11.2 and BMI 27.4 ± 5.2 kg/m2), ten of whom were taking 25OHD3 supplements. All subjects gave signed informed consent for participation and the study was approved by the Research Ethics Committee at King Abdullah International Medical Research Center, Jeddah, Saudi Arabia. Blood specimens were simultaneously collected into plain vacutainer tubes and SST II advance vacutainer tubes using a standard venesection procedure. Vacutainers were from Becton Dickinson (BD). Specimens were centrifuged within 1 h of blood collection and the serum transferred into secondary tubes. Serum was stored at −80°C until analysis 6 months later. Previous studies have observed that vitamin D is stable under these conditions 9, 10.
The HPLC instrument was from Waters Corporation, Milford, MA (Alliance) using a Chromsystems reagent kit (Chromsystems Instruments and Chemical GmbH, Munich, Germany), which enables simultaneous chromatographic determination of 25OHD2 and 25OHD3 using UV detection. Calibrators, controls, precipitation solution, wash buffers, elution buffer, mobile phase, and internal standard (IS) were also from Chromsystems. Solid‐phase extraction was used to remove interferents and concentrate the analytes. Retention times of 25OHD2, 25OHD3, and IS were approximately 4.2, 4.6, and 7.1 min, respectively. Chromatographic separation required about 12 min. The manufacturer's intra‐assay coefficient of variation (CV) was 0.9–3.0% and interassay CV was 2.3–3.3%. The Liaison (Diasorin Inc., Stillwater, Italy) measures 25OHD by chemiluminescent immunoassay (CLIA). It is a direct competitive assay that measures active vitamin D forms (25OHD2 and 25OHD3) equally (intra‐assay CV = 2.9–5.5% and interassay CV = 6.3–12.9%). The Architect 25OHD method uses a chemiluminescent microparticle immunoassay (CMIA; Abbott Laboratories, Wiesbaden, Germany) for the quantitative determination of total vitamin D metabolites in human serum (intra‐assay CV = 1.4–3.7% and interassay CV = 2.7–4.6%).
SPSS version 20 was used for statistical analysis. Data were transformed using natural logarithm (Ln) to approximate a normal distribution. Associations between variables were examined using Pearson's correlation coefficients. Comparisons between means were performed by paired Student's t‐tests at 95% confidence interval. Slope, intercept, and correlation coefficients were calculated using the same program. Method comparisons were based on the Bland and Altman 11 and analyzed using MedCalc statistical software version 12.7.5.
There were no significant differences between 25OHD3 results (means ± SE, nmol/l) obtained from specimens collected into plain tubes and SSTs assayed by HPLC (39.0 ± 2.7 vs. 39.3 ± 2.7), Liaison (32.9 ± 2.2 vs. 32.8 ± 2.3), or Architect (43.1 ± 2.8 vs. 43.2 ± 2.8). On examining results obtained from specimens collected into plain tubes, there was a significant correlation (P < 0.0001) between those from HPLC and from the Architect analyzer (r = 0.895; intercept = 1.733; slope = 0.864) and between HPLC and the Liaison analyzer (r = 0.907; intercept = 3.378; slope = 1.083). Similarly, when SSTs were used, there was a significant correlation (P < 0.0001) between HPLC and the Architect analyzer (r = 0.908; intercept = 1.099; slope = 0.883) and between HPLC and the Liaison analyzer (r = 0.913; intercept = 5.154; slope = 1.042). When compared to HPLC, results from the Liaison analyzer correlated more strongly than did those form the Architect, irrespective of whether plain tubes or SSTs were used. In specimens collected into plain tubes (Fig. 1A), there was good agreement between the Liaison assay and HPLC with little variation at high concentrations. The Architect assay showed a clear positive bias at high 25OHD3 concentrations. Similar results were obtained for SSTs (Fig. 1B).
Figure 1.
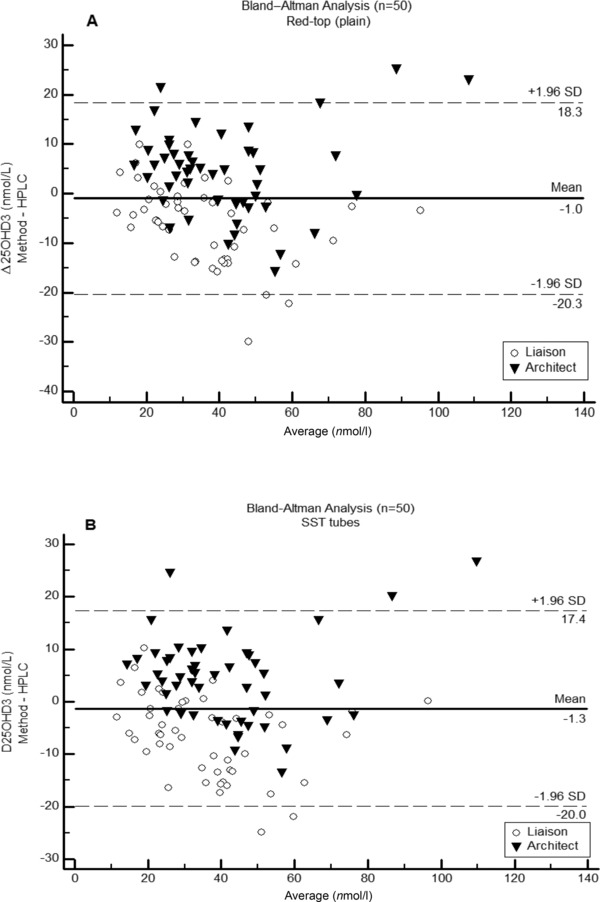
Bland–Altman plot for 25OHD3 results obtained from 50 subjects using the Liaison (Diasorin) and Architect (Abbott) immunoassays compared to HPLC (Chromsystems). (A) Plain tubes: HPLC versus Liaison and Architect. (B) SSTs: HPLC versus Liaison and Architect.
The use of SSTs is logistically advantageous because use of the primary tube for testing reduces consumable costs and processing time. However, it is important to exclude the possibility of gel interference with measurement. The data showed clearly that SSTs had no effect on serum 25OHD3 concentrations as measured by HPLC or either of the two immunoassays (Liaison and Architect). We conclude therefore that it is appropriate to collect blood into a single SST for both routine biochemical analyses and measurement of 25OHD3. This is an important finding because of the cost savings that can be made by using a single tube for multiple tests, particularly in view of the increase in 25OHD3 requests in recent years 12, 13.
The correlation between 25OHD concentrations estimated by LC‐MS or HPLC, and various immunoassay platforms have been investigated previously 14, 15, 16, 17. The results of our correlation analyses agreed well with those reported by Ferrell et al. (18), and with evaluation reports on the Liaison 19 and Architect analyzers 20. Although there were small differences in the strength of correlation of results from HPLC and immunoassay instruments, it appears that sample collection into either tube type is acceptable, irrespective of the analytical method used. Bland–Altman plot analysis showed that the Liaison analyzer agreed better with HPLC with very little variation at high levels. The Architect analyzer agreed well with HPLC at low 25OHD3 but with more variation at high concentrations. The observed differences between these methods emphasize the importance of interpreting results using method‐specific reference ranges.
Future studies are warranted to investigate the effect of SSTs on 25OHD2 measurement so that guidance can be provided to clinical laboratories in countries where this form of the vitamin is taken supplementally. Another factor reported to influence 25OHD3 measurement is vitamin D binding protein (DBP) 17. This also demands further study. A limitation of the present study was that LC‐MS measurement of 25OHD3 was not included in the comparison. Its principle of measurement is different from those of the methods used in this study. In order to ensure wide applicability of the findings, the study was performed on the commonly used BD tubes. However, it should be emphasized that the results are not necessarily applicable to gel tubes produced by other manufacturers.
BD gel separators do not interfere with the measurement of 25OHD3 when the analysis is carried out by HPLC or common immunoassay methods. This important finding may enable clinical laboratories to make cost savings without concerns about inaccuracy of measurements.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
ACKNOWLEDGMENTS
The authors acknowledge the unlimited support received from King Abdullah International Medical Research Center (KAIMRC), Jeddah, Saudi Arabia. The authors are also grateful to Abbott and Diasorin whose representatives in Saudi Arabia kindly provided vitamin D assay materials used in the study (Medi‐Serve and Abdullah Fouad Co.).
Ethical approval: The study was approved by the Research Ethics Committee at King Abdullah International Medical Research Center, Jeddah, Saudi Arabia.
Grant sponsor: King Abdullah International Medical Research Center.
REFERENCES
- 1. Holick MF. Vitamin D deficiency. N Engl J Med 2007;357:266–281. [DOI] [PubMed] [Google Scholar]
- 2. Zhang R, Naughton DP. Vitamin D in health and disease: Current perspectives. Nutr J 2010;9:1475–2891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Holick MF. Environmental factors that influence the cutaneous production of vitamin D. Am J Clin Nutr 1995;61:638S–645S. [DOI] [PubMed] [Google Scholar]
- 4. Armas LA, Hollis BW, Heaney RP. Vitamin D2 is much less effective than vitamin D3 in humans. J Clin Endocrinol Metab 2004;89:5387–5391. [DOI] [PubMed] [Google Scholar]
- 5.25‐OH Vitamin D3 HPLC Assay KEB, Inc. Available from: http://stores.eaglebio.com/images/VD331‐H100.pdf. Accessed on October 2013.
- 6.Evacuated Blood Collection System For In Vitro Diagnostic Use Gb‐o. Available from: http://www.gbo.com/documents/980200_IFU_VenousBloodCollection_rev11_GB.pdf. Accessed on October 2013.
- 7. Lensmeyer GL, Wiebe DA, Binkley N, et al. HPLC method for 25‐hydroxyvitamin D measurement: Comparison with contemporary assays. Clin Chem 2006;52:1120–1126. [DOI] [PubMed] [Google Scholar]
- 8. Elder PA, Lewis JG, King RI, Florkowski CM. An anomalous result from gel tubes for vitamin D. Clin Chim Acta 2009;410:95. [DOI] [PubMed] [Google Scholar]
- 9. Colak A, Toprak B, Dogan N, Ustuner F. Effect of sample type, centrifugation and storage conditions on vitamin D concentration. Biochem Med (Zagreb) 2013;23:321–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wielders JP, Wijnberg FA. Preanalytical stability of 25(OH)‐vitamin D3 in human blood or serum at room temperature: Solid as a rock. Clin Chem 2009;55:1584–1585. [DOI] [PubMed] [Google Scholar]
- 11. Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1986;1:307–310. [PubMed] [Google Scholar]
- 12. Mula‐Abed WA. 25‐ hydroxyvitamin d: Explosion in clinical interest and laboratory requests. Oman Med J 2009;24:239–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Krasowski MD. Pathology consultation on vitamin D testing. Am J Clin Pathol 2011;136:507–514. [DOI] [PubMed] [Google Scholar]
- 14. Hermida FJ, Fernandez M, Laborda B, et al. Assessment of ADVIA Centaur analyzer for the measurement of 25‐OH vitamin D. Clin Lab 2012;58:987–995. [PubMed] [Google Scholar]
- 15. Mochizuki A, Kodera Y, Saito T, et al. Preanalytical evaluation of serum 25‐hydroxyvitamin D3 and 25‐hydroxyvitamin D2 measurements using LC‐MS/MS. Clin Chim Acta 2013;420:114–120. [DOI] [PubMed] [Google Scholar]
- 16. Koivula MK, Matinlassi N, Laitinen P, Risteli J. Four automated 25‐OH total vitamin D immunoassays and commercial liquid chromatography tandem‐mass spectrometry in Finnish population. Clin Lab 2013;59:397–405. [DOI] [PubMed] [Google Scholar]
- 17. Heijboer AC, Blankenstein MA, Kema IP, Buijs MM. Accuracy of 6 routine 25‐hydroxyvitamin D assays: Influence of vitamin D binding protein concentration. Clin Chem 2012;58:543–548. [DOI] [PubMed] [Google Scholar]
- 18. Farrell CJ, Martin S, McWhinney B, Straub I, Williams P, Herrmann M. State‐of‐the‐art vitamin D assays: A comparison of automated immunoassays with liquid chromatography‐tandem mass spectrometry methods. Clin Chem 2012;58:531–542. [DOI] [PubMed] [Google Scholar]
- 19.D‐Facts Vitamin D Competitive Evaluation. Available at: http://wwwilmarorgil/diasorin/D‐fact_Competitive_Eval0112pdf. Accessed on October 2013.
- 20. Wallace AM, Gibson S, de la Hunty A, Lamberg‐Allardt C, Ashwell M. Measurement of 25‐hydroxyvitamin D in the clinical laboratory: Current procedures, performance characteristics and limitations. Steroids 2010;75:477–488. [DOI] [PubMed] [Google Scholar]


