Abstract
Background
Onychomycosis is principally caused by dermatophyte species, but nondermatophyte molds and yeasts have also been involved, causing different clinical manifestations. The aim of this investigation is to determine the clinicomycological and epidemiological profile of the etiologic agents of onychomycosis.
Methods
The study population included 9,785 suspected cases of onychomycosis referred to the Medical Mycology Reference Laboratory in Isfahan, Iran, during 2007–2014. Nail clipping was collected in sterile Petri dishes for direct microscopic examination and culture. Clinical isolates were identified by using phenotypic tests and molecular techniques.
Results
Of total 9,785 cases with clinical suspicion of onychomycosis comprised in the present study, 1,284 patients (13.1%) were positive by direct microscopy. Age range of patients was between 1 and 82 years. Housewives were the commonest infected population. Candida albicans was the most prevalent species isolated from patients in this study (34.9%) followed by Trichophyton interdigitale (11.7%) and Aspergillus flavus (9.1%).
Conclusion
The pattern of causative agents and clinical signs of onychomycosis is altering region to region, so repeated epidemiological surveys of onychomycosis seems to be fundamental. The present study provides novel and appropriate epidemiologic data of onychomycosis for the better prevention and treatment of this fungal infection.
Keywords: identification, causative agents, onychomycosis
Introduction
Onychomycosis is a fungal nail infection caused by yeasts, dermatophytes, and some nondermatophyte molds. It is the most common nail disease in adults, which is responsible for almost 50% of all nail disorders 1. Its incidence is estimated at more than 10% among the healthy population and 40% in the elderly individuals, maybe associated with lack of maintain good foot care, impaired immune system, and decreased growth of the nail plate throughout the life 2. The following forms of onychomycosis are recognized: distal and lateral subungual, superficial, endonyx, proximal subungual, mixed, totally dystrophic, and secondary onychomycosis. The nails become yellowish‐white, permeable, and fragile. Predisposing factors contain diabetes, increasing age, peripheral arterial disease, immunosuppression, trauma, poor peripheral circulation, and sports activities 2, 3, 4. The etiologic agent of onychomycosis varies in different countries 4, 5, 6, 7, 8, nevertheless few data are available in Iran 9, 10, 11 especially in Isfahan 12, 13. It is the purpose of this study to describe the prevalence of onychomycosis, and the range of fungal species isolated from nail infections in Isfahan, compared with other parts of Iran.
Materials and Methods
The study was performed between June 2007 and June 2014 in Isfahan, Iran. A total of 9,785 suspected cases (3,295 male and 6,490 female) referred to the Shefa Mycology Reference Laboratory, Center for Medical Mycology in Isfahan, Iran. All individuals were asked to participate in this research. Patients endorsed the consent form and completed a comprehensive questionnaire including demographics, a summary of medical history, and personal habits. Patients who had taken antifungal agents throughout the last week were kept out from the research. Nail clippings were taken from fingernails or/and toenails and collected in sterile Petri dishes for direct microscopic examination with 15% potassium hydroxide and culture on sabouraud dextrose agar with chloramphenicol and cycloheximide (Mycosel agar; Difco, Detroit, MI), and sabouraud glucose agar (Difco). Some additional tests like culture on Trichophyton agars (BIOMARK, India), hair penetration test, urea hydrolysis (QUELAB, Canada), growth on rice grains, germ‐tube test, chlamydoconidia production test using corn meal agar supplemented by 1% Tween 80 (BD, Maryland), CHROMagar Candida (CHROMagar Microbiology, Paris, France), API 20C AUX (bioMerieux, France), czapek dox agar (Merck, Germany) were used to confirm the fungal identification 13, 14, 15, 16, 17, 18. The polymerase chain reaction restriction fragment length polymorphism (PCR‐RFLP) was applied for identification of some Candida spp. and dermatophyte spp. isolated from onychomycosis in the present investigation.
PCR‐RFLP for Candida species
Genomic DNA of each strain was extracted using FTA® Elute MicroCards (Whatman Inc., Clifton, NJ) according to the manufacturer's instructions 19. Molecular identification of Candida strains was performed by using already delineated PCR‐RFLP profiles 20, 21. The ITS1‐5.8SrDNA‐ITS2 (where ITS is internal transcribed spacer) region was amplified using PCR mixture including 5 μl of 10× reaction buffer, 0.4 mM dNTPs, 1.5 mM MgCl2, 2.5 U of Taq polymerase, 30 pmol of both ITS1 (5′‐TCC GTA GGT GAA CCT GCG G‐3′) and ITS4 (5′‐TCC TCC GCT TAT TGA TAT GC‐3′) primers 21, and 2 μl of extracted DNA in a final volume of 50 μl. The PCR cycling conditions comprised: initial denaturation at 94°C for 5 min, followed by 30 cycles of denaturation at 94°C for 30 s, annealing at 55°C for 45 s, and extension at 72°C for 1 min, with a final extension at 72°C for 7 min. During the second step, PCR products were digested with the restriction enzyme HpaII (Fermentas, Vilnius, Lithuania). Identification of newly described species of C. parapsilosis complex (C. parapsilosis, C. orthopsilosis, and C. methapsilosis) was performed by amplification of SADH gene using the SADHF (5′‑GTT GAT GCT GTT GGA TTG T‑3′) and SADHR (5′‑CAA TGC CAA ATC TCC CAA‑3′) primers 22, followed by digestion with restriction enzyme Hin1II (NlaIII) (Fermentas). Five microliters of each PCR amplicons and 12 μl of RFLP products were separated by gel electrophoresis on 1.5% and 2% agarose gel (including 0.5 μg/ml ethidium bromide), respectively.
PCR‐RFLP for dermatophyte species
DNA extraction was performed by phenol/chloroform method 23, 24. ITS rDNA region was amplified using universal fungal primers ITS1 (5′‐TCCGTAGGTGAACCTGCGG‐3′) and ITS4 (5′‐TCCTCCGCTTATTGATATGC‐3′) (Sina Gene, Iran) 24. PCR amplicons were digested with the restriction enzyme MvaI (Fast digest; Fermentas) 25.
Results
One thousand two hundred eighty‐four patients of 9,785 (13.1%) had positive direct examination; 527 male (238 fingernail, 289 toenail) and 757 female (473 fingernail, 284 toenail). A total of 671 of 1,284 patients (52.2%) claimed for culture. Twenty‐three samples (3.4%) did not grow due to unknown factors like washing the lesions, insufficient sample, and use of antifungal drugs. The disease period was from a week to 3 years. Age range of patients was between 1 and 82 years (mean age, 45 years). Housewives were the commonest infected population (Fig. 1). Four hundred and sixty‐seven patients (36.4%) had distal onychomycosis, 438 patients (34.1%) had proximal, and 379 patients (29.5%) had lateral onychomycosis. Nine patients (0.7%) had both fingernail and toenail onychomycosis, and the etiologic agents of fingernail and toenail in six of nine patients (66.6%) were same. Three hundred and thirty‐one Candida spp. (51.1%), 174 dermatophyte spp. (26.8%), and 143 nondermatophyte spp. (22%) were isolated from nail infection in this investigation (Table 1). Candida albicans was the most prevalent species isolated from patients in this study (34.9%) followed by Trichophyton interdigitale (11.7%), Aspergillus flavus (9.1%), Epidermophyton floccosum (8.5%), C. parapsilosis (6.9%), and T. rubrum (5.8%) (Table 1). Two hundred of Candida spp. were identified by PCR‐RFLP technique (Fig. 2) and 131 strains with phenotypic methods such as API 20C AUX and culture on CHROMagar Candida. Fifty‐one dermatophyte spp. were identified by using phenotypic tests and 123 isolates with PCR‐RFLP method (Fig. 3). Two hundred and ninety‐four patients (22.9%) had diabetes mellitus, 133 patients (10.3%) presented wearing tight shoes, 107 cases (8.3%) had a history of trauma, 82 patients (6.4%) had contact with different animals, and 668 patients (52%) mentioned no predisposing factor in their medical history form. Figure 4 divided the frequency of onychomycosis into the seven stages in this study.
Figure 1.
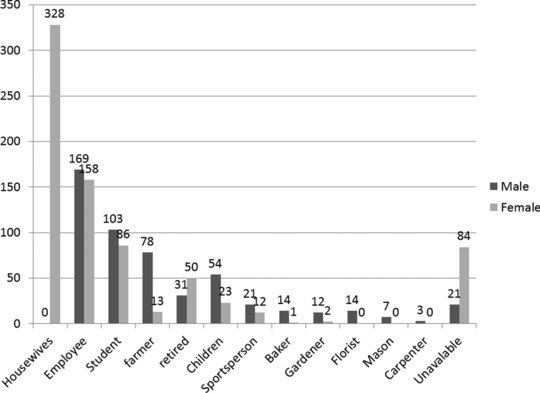
Distribution of patients with onychomycosis according to their occupation.
Table 1.
Clinical Isolates Obtained from Onychomycosis in the Present Study
| Isolates | Fingernail | Toenail | Total |
|---|---|---|---|
| Candida spp. | |||
| C. albicans | 219 | 7 | 226 |
| C. parapsilosis | 43 | 2 | 45 |
| C. tropicalis | 30 | 0 | 30 |
| C. kefyr | 9 | 0 | 9 |
| C. krusei | 7 | 1 | 8 |
| C. glabrata | 6 | 0 | 6 |
| C. guilliermondii | 5 | 0 | 5 |
| C. orthopsilosis | 2 | 0 | 2 |
| Total | 321 | 10 | 331 |
| Dermatophyte spp. | |||
| T. interdigitale | 27 | 49 | 76 |
| E. floccosum | 19 | 36 | 55 |
| T. rubrum | 13 | 25 | 38 |
| T. violaceum | 2 | 1 | 3 |
| M. gypseum | 1 | 0 | 1 |
| M. canis | 1 | 0 | 1 |
| Total | 63 | 111 | 174 |
| Nondermatophyte spp. | |||
| A. flavus | 26 | 33 | 59 |
| A. nidulans | 6 | 8 | 14 |
| A. fumigatus | 3 | 6 | 9 |
| A. terreus | 2 | 5 | 7 |
| Aspergillus spp. | 8 | 11 | 19 |
| Scopulariopsis spp. | 5 | 3 | 8 |
| Fusarium spp. | 2 | 5 | 7 |
| Acremonium spp. | 0 | 3 | 3 |
| Penicillium spp. | 1 | 2 | 3 |
| Alternaria spp. | 1 | 2 | 3 |
| Cladosporium spp. | 0 | 2 | 2 |
| Unknown spp. | 4 | 5 | 9 |
| Total | 58 | 85 | 143 |
| Total clinical isolates | 442 | 206 | 648 |
Figure 2.
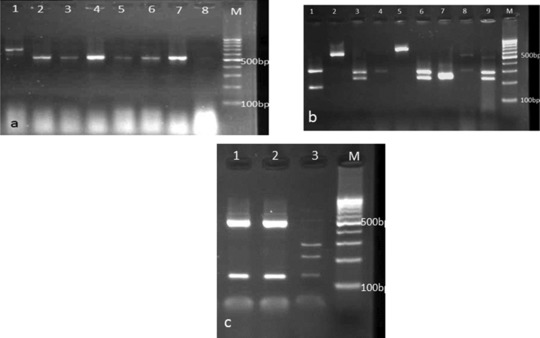
(a) PCR products of various Candida species: lane 1: C. guilliermondii, lanes 2, 6, 7: C. albicans, lanes 3, 5: C. krusei, lane 4: C. tropicali, and lane M: 100 bp DNA size marker. (b) Agarose gel electrophoresis of ITS‐PCR products of Candida species after digestion with HpaII. Lane 1: C. tropicalis, lane 2: C. parapsilosis, lanes 3, 6, 9: C. albicans, lanes 4, 8: C. glabrata, lane 5: C. kefyr, lane 7: C. krusei, and lane M: 100 bp DNA size marker. (c) Agarose gel electrophoresis of SADH‐PCR products after digestion with NlaIII. Lanes 1, 2: C. parapsilosis, lane 3: C. orthopsilosis, and lane M: 100 bp DNA size marker.
Figure 3.
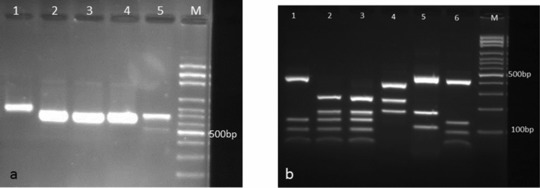
(a) PCR products of various dermatophyte species: Lane 1: E. floccosum, lanes 2–4: T. interdigitale, lane 5: T. rubrum, lane M: 100 bp DNA size marker. (b) Electrophoretic profile of ITS‐PCR products of dermatophyte species digested with MvaI restriction enzyme. Lanes 1—3, 6: T. interdigitale, lane 4: E. floccosum, lane 5: M. canis, and lane M: 100 bp DNA size marker.
Figure 4.
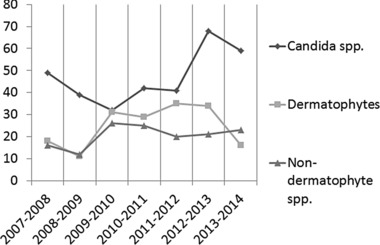
Fungal agents of onychomycosis between 2007 and 2014 in seven periods.
Discussion
The first step for treatment of onychomycosis is to make a precise diagnosis. This needs both clinical manifestations (such as discoloration, thickened nail plate, subungual hyperkeratosis, and onycholysis) and laboratory corroboration by using microscopy, culture, and some specific tests. Although 30–50% of cultures of nails yield false‐negative results, but most guidelines suggest fungal culture to approve recognition of onychomycosis before treatment initiation 5. Due to these facts that choice of drug depends on the causative agent of fungal infection, treatment duration of onychomycosis is prolonged, and extended systemic therapy with antifungal agents has potential side effects, consequently accurate identification of etiologic agents of nail infection can lead to an appropriate management of onychomycosis. If positive direct microscopy is taken as the exclusive criterion, the occurrence of onychomycosis would be 13.1% in this investigation, and it is higher than those formerly reported in the Spain (2.6%) 6, United States (11.8%) 4, Finland (8.4%), and United Kingdom (2.7%) 26. Aghamirian et al. 10 showed 40.2% of positive cases of onychomycosis in Qazvin (northwest of Tehran), Iran. They revealed lateral subungual onychomycosis as the most common clinical type of infection in 48.4% of patients, whereas we showed (29.5%) lateral onychomycosis in the present study as the rarest clinical form of the infection. They also reported T. rubrum as the most prevalent species (48.4%), but it was isolated from only 5.8% of patients in the present investigation. In agreement with findings in this study, they showed that females were affected more often than males, and fingernails were affected more frequently than toenails. Zaini et al. 27 reported 47.9% of onychomycosis in Tehran (capital and center of Iran). They showed C. albicans,
A. flavs, and T. mentagrophytes as the most prevalent species by using traditional methods. Same to the present study, they revealed that finger nail infection was most common in females while toenail infection was prevalent among male population. They also disclosed A. niger, Rhizopus spp., and C. humicola as causative agents of onychomycosis, whereas we did not isolate these species in the present study. Ghannoum et al. 4 and Heikkila and Stuff 7 disclosed that male subjects were twice and three times as likely to have fungal nail infection as female subjects, respectively, in contrast to the present survey. They ascribed to the suggestion that “men exercise more.” They reported that T. rubrum and Fusarium spp. were the most prevalent dermatophyte and nondermatophyte spp. isolated from the nails, while T. interdigitale and A. flavus were the most common dermatophyte and nondermatophyte isolates in the present study. Regression of onychomycosis after treatment is completely usual occurring in about 10–53% of cases, which is caused by insufficient or improper therapy or new nail infection after completing medicinal treatment 5, 28. One hundred and thirty‐four patients (10.4%) mentioned recurrence of infection after treatment in this study. Recurrence of onychomycosis as a result of the nondermatophyte molds is frequent due to the lack of definitive therapy for this group. Seventy‐six patients (53.1%) that infected to nondermatophyte species reminded relapse of the infection. This rate for dermatophytes and yeasts was 13.8% and 15.1%, respectively. For this reason their physicians had asked for culture of the clinical specimens. During 2003–2012, Afshar et al. 9 based on conventional techniques detected 56.8% of positive cases of onychomycosis in Sari (northeast city of Iran). Similar to the present study, they reported Candida spp. as the most common causative agents of onychomycosis (49.4%) and A. flavus as the most prevalent nondermatophyte agent (50%). Furthermore, they obtained an isolate of Trichosporon spp. and one Geotrichum spp. (0.2%) among clinical strains. In agreement with the present investigation and by means of molecular techniques, Mohammadi et al. 29 reported C. albicans as the most commonly clinical yeast strain (41.1%), followed by C. parapsilosis (21.4%) and C. tropicalis (12.8%). They reported 15 cases of C. orthopsilosis (4.1%), the newly described Candida species, whereas we found only two isolates (0.3%) in this investigation. Their data revealed from Alborz (northwest of Tehran), Kashan (north city of Isfahan), Tehran, Isfahan (south of Tehran), and Mazandaran provinces of Iran. Kafaie et al. 30 determined the prevalence of onychomycosis in diabetic patients in Yazd (southeast of Isfahan), Iran. They reported 6.9% of onychomycosis among diabetic patients, whereas 22.9% of diabetic patients were infected to onychomycosis in the present investigation. Asadi et al. 11 reported 18.9% of onychomycosis in Kashan, Iran between 2001 and 2003. They showed T. violaceum as the most prevalent species among isolates. Chadeganipour et al. 12 reported 39.8% of the occurrence rate of onychomycosis that it is higher than the incidence of onychomycosis in the present survey. In accordance with our findings, C. albicans was the most prevalent yeast (84%) in their study. In agreement with the present investigation, they reported housewives as the commonest infected population. Similar to our findings they showed distal and proximal subungual onychomycosis as the majority of fungal nail infections. The occurrence of toenail infection was same in males and females. Interestingly, almost all isolates in the present study are similar with previous one 12 except for C. kefyr, C. orthopsilosis, T. violaceum, and Microsprum canis. In the study that was performed by Khosravi et al. 31 in Tehran, Iran, Scopolariopsis brevicaulis was the most common nondermatophyte molds observed, but it was ranked sixth in the present study. In conformity with the present research, they revealed that females were affected more frequently than males, but opposing to this study, they showed that toenails were affected more often than fingernails. Surprisingly, in comparison with other study that was performed in Tehran, Iran 27, Khosravi et al. 31 reported T. violaceum as the third fungal spp. (17.8%) isolated from onychomycosis, whereas there was not any T. violaceum among isolates reported by Zeini et al. 27. We only identified three T. violaceum strains (0.5%) in the present study. In an investigation that was performed by Mohammadi et al. 32, T. interdigitale, E. floccosum, and T. rubrum were the most common dermatophytes isolated from tinea unguium. They isolated one T. violaceum strain from nail infection. Bassiri‐Jahromi et al. 33 reported 1,466 nail infection in 5 years in Tehran, Iran. They showed T. rubrum as the predominant etiologic agent of onychomycosis (73.9%). Candida species were responsible for 38% of all cases of onychomycosis and were more isolated from fingernail infections. They also isolated one A. niger from toenail infection, while we did not identify any A. niger from clinical isolates. Hashemi et al. 34 reported 76.2% of onychomycosis due to the Candida species, however the strains did not divide to the species level. In accordance with Zaini et al. 27, they revealed that T. mentagrophytes was the most commonly dermatophyte involved, however in disagreement with Khosravi et al. 31, T. violaceum was not obtained from clinical specimens. In a study that was carried out by Mikaeili et al. 35 in Kermanshah (western part of Iran), C. albicans, T. rubrum, and A. flavus were the most prevalent yeast, dermatophyte, and nondermatophytic molds, respectively. Moghaddami et al. 36 reported C. albicans and T. rubrum as the most frequently occurring species of onychomycosis. They showed that children were the commonest infected population, whereas the most patients in the present study were housewives. Similar to the present study, Ghasemi et al. 37 indicated that distal subugual onychomycosis was predominant clinical forms of onychomycosis. Based on nucleotide sequencing of 28S region, Ahmadi et al. 38 reported a case of onychomycosis caused by A. candidus. Falahati et al. 39 reported the first case of onychomycosis due to Exophiala (Wangiella) dermatitidisin in Iran, by using sequence analysis of the ITS region of rDNA. Mousavi et al. 40 isolated Fusarium spp. from chronic fingernail infection of a 51‐year‐old patient. Zarei Mahmoudabadi and Zarrin 41 reported A. flavus as the causative agent of onychomycosis in a 60‐year‐old female. It is remarkable that
A. flavus was the most common nondermatophyte mold in all studies that were performed in Iran except for Khosravi et al. 31, Bassiri‐Jahromi et al. 33, and Aghamirian et al. 10 that isolated S. brevicaulis, Acremonium spp., and A. niger, respectively. Table 2 summarizes published investigations related to onychomycosis that have been done in different provinces in Iran. Soltani et al. 42 reported 56.4% of onychomycosis in Tehran. They showed that yeasts were the most common pathogens isolated from patients (71.4%), followed by nondermatophytic molds (17.1%) and dermatophytes (11.5%). Gerami shoar et al. 43 isolated C. albicans as the most prevalent spp. among patients with onychomycosis in Tehran by phenotypic tests. Kazemi revealed 7% of tinea unguium in the north‐west of Iran 44. He showed that 66% of tinea unguium infections were caused by zoophilic drematophytes, 31% by anthropophilic drematophytes, and 3% by geophilic species.
Table 2.
Epidemiological Data of Onychomyosis in Iran
| City | Year(s) | Method(s) | Occurrence | Predominant spp. (Y/D/NDM) | Age range | CIP | M/F ratio | Author reference |
|---|---|---|---|---|---|---|---|---|
| Tehran | 1987–1988 | DM + C + PT | 28.9% | C. albicans/T. rubrum/A. flavus | 0 up to 42 | Housewives | 100/168 | Moghaddami 36 |
| Tehran | 1996–1997 | DM + C + PT | 61.5% | C. albicans/T. interdigitale/ S. brevicaulis | 10–65 | N/A | 37/60 | Khosravi 31 |
| Tehran | 2000–2005 | DM + C | 30% | C. albicans/E. floccosum/ Acremonium spp. | N/A | N/A | 2,705/3,978 | Bassiri‐Jahromi 33 |
| Kashan | 2001–2003 | DM + C + PT | 19% | C. albicans/T. violaceum/ A. flavus | N/A | Students | 11/15 | Asadi 11 |
| Tehran | 2004–2005 | DM + C + PT | 47.9% | C. albicans/T. mentagrophytes/ A. flavus | 1–83 | N/A | 89/174 | Zaini 27 |
| Qazvin | 2004–2007 | DM + C | 40.2% | C. albicans/T. rubrum/A. niger | 1–79 | N/A | 56/68 | Aghamirian 10 |
| Isfahan | 2006–2007 | DM + C + PT | 39.8% | C. albicans/T. interdigitale/ A. flavus | 1–80 | Housewives | 178/310 | Chadeganipour 12 |
| Yazd | 2008–2009 | DM + C | 3.8% | N/A | 35–95 | N/A | 7/3 | Kafaie 30 |
| Tehran, Isfahan, Alborz, Kashan, and Mazandaran | 2009–2011 | DM + C + PCR‐RFLP | N/A | C. albicans/N/A/N/A | 7–88 | N/A | 69/291 | Mohammadi 29 |
| Tehran | 2007 | DM + C | 42.8% | C. albicans/T. mentagrophytes/ A. flavus | N/A | N/A | 57/159 | Hashemi 34 |
| Sari | 2003–2012 | DM + C | 56.8% | C. albicans/T. mentagrophytes/ A. flavus | 1–88 | N/A | 393/232 | Afshar 9 |
| Kermanshah | 1994–2010 | DM + C | 45.2% | C. albicans/T. rubrum/ A. flavus | N/A | Housewives | 278/808 | Mikaeili 35 |
| Tehran | N/A | DM + C | 56.4% | N/A | N/A | N/A | N/A | Soltani 42 |
| Tehran | 2001–2002 | DM + C | 50.4% | C. albicans/T. mentagrophytes/ A. flavus | 0–70 | N/A | 36/91 | Gerami shoar 43 |
| Tabriz | 1996–2004 | DM + C | 7% | N/A/T. mentagrophytes/N/A | 1–70 | N/A | 25/16 | Kazemi 44 |
| Isfahan | 2007–2014 | DM + C + PT + PCR‐RFLP | 13.1% | C. albicans/T. interdigitale/ A. flavus | 1–82 | Housewives | 527/757 | Present study |
| Case reports | ||||||||
| City | Year | Method(s) | Toe/finger nail | Etiologic agent | Age (year) | Occupation | M/F | Author reference |
| Tehran | 2012 | Sequencing of the 28S regions of | Toenail | Aspergillus candidus | 60 | Housewife | F | Ahmadi (38) |
| Tehran | 2014 | Sequence analysis of internal transcribed spacer (ITS) domain | Toenail | Exophiala dermatitidis | 54 | Mountaineer | F | Falahati 39 |
| Ahvaz | 2005 | DM + C | Fingernail | Aspergillus favus | 60 | Housewife | F | Zarei Mahmoudabadi 41 |
| Kerman | 2007 | DM + C | Fingernail | Fusarium spp. | 51 | Garden worker | M | Mousavi 40 |
Y, yeast; D, dermatophyte; NDM, nondermatophyte mold; CIP, commonest infected population; DM, direct microscopy; C, culture; PT, physiological tests; N/A, nonavailable.
Conclusion
Precise diagnosis of onychomycosis is critical for excellent management of infection that is based on clinical signs and laboratory tests. The profile of the fungal nail infection is changing in different areas, so repeated studies on the prevalence of onychomycosis and the etiologic agents of the infection seems to be essential. The present study provides novel and appropriate epidemiologic data of onychomycosis, which can lead to the better prevention and treatment of this fungal infection.
Acknowledgment
The authors expressed their appreciation to the Shefa laboratory personnel for their helpful cooperation.
References
- 1. Mügge C, Haustein UF, Nenoff P. Causative agents of onychomycosis—A retrospective study. J Dtsch Dermatol Ges 2006;4:218–228. [DOI] [PubMed] [Google Scholar]
- 2. Shemer A, Trau H, Davidovici B, Amichai B, Grunwald MH. Onychomycosis: Rationalization of topical treatment. Isr Med Assoc J 2008;10:415–416. [PubMed] [Google Scholar]
- 3. Tosti A, Hay R, Arenas‐Guzmán R. Patients at risk of onychomycosis—Risk factor identification and active prevention. J Eur Acad Dermatol Venereol 2005;19:13–16. [DOI] [PubMed] [Google Scholar]
- 4. Ghannoum M, Hajjeh R, Scher R, et al. A large‐scale North American study of fungal isolates from nails: The frequency of onychomycosis, fungal distribution, and antifungal susceptibility patterns. J Am Acad Dermatol 2000;43:641–648. [DOI] [PubMed] [Google Scholar]
- 5. Leelavathi M, Noorlaily M. Onychomycosis nailed. Malays Fam Physician 2014;9:2–7. [PMC free article] [PubMed] [Google Scholar]
- 6. Sais G, Jugglág A, Peyri J. Prevalence of dermatophyte onychomycosis in Spain: A cross‐sectional study. Br J Dermatol 1995;132:758–761. [DOI] [PubMed] [Google Scholar]
- 7. Heikkilå H, Stubb S. The prevalence of onychomycosis in Finland. Br J Dermatol 1995;133:699–703. [DOI] [PubMed] [Google Scholar]
- 8. Garg A, Venkatesh V, Singh M, et al. Onychomycosis in central India: A clinicoetiologic correlation. Inter J Dermatol 2004;43:498–502. [DOI] [PubMed] [Google Scholar]
- 9. Afshar P, Khodavaisy S, Kalhori S, Ghasemi M, Razavyoon T. Onychomycosis in North‐East of Iran. Iranian J Microbiol 2014;6:98–103. [PMC free article] [PubMed] [Google Scholar]
- 10. Aghamirian MR, Ghiasian SA. Onychomycosis in Iran: Epidemiology, causative agents and clinical features. Jpn J Med Mycol 2010;51:23–29. [DOI] [PubMed] [Google Scholar]
- 11. Asadi MA, Dehghani R, Sharif MR. Epidemiologic study of onychomycosis and tinea pedis in Kashan, Iran. Jundishapur J Microbiol 2009;2:61–64. [Google Scholar]
- 12. Chadeganipour M, Nilipour S, Ahmadi G. Study of onychomycosis in Isfahan, Iran. Mycoses 2010;53:153–157. [DOI] [PubMed] [Google Scholar]
- 13. Chadeganipour M, Mohammadi R, Shadzi S. A 10‐year study of dermatophytoses in Isfahan, Iran. J Clin Lab Anal 2015. doi: 10.1002/jcla.21852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Weitzman I, Summerbell RC. The dermatophytes. Clin Microbiol Rev 1995;8:240–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Silva L, Oliveira D, Silva B, et al. Identification and antifungal susceptibility of fungi isolated from dermatomycoses. J Eur Acad Dermatol Venereol 2014;28:633–640. [DOI] [PubMed] [Google Scholar]
- 16. Marinho SA, Teixeira AB, Santos OS, et al. Identification of Candida spp. by phenotypic tests and PCR. Braz J Microbiol 2010;41:286–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Diba K, Kordbacheh P, Mirhendi S, Rezaie S, Mahmoudi M. Identification of Aspergillus species using morphological characteristics. Pak J Med Sci 2007;23:867–872. [Google Scholar]
- 18. Cetinkaya Z, Altindiş M, Aktepe O, Karabicak N. [Comparison of different methods for the identification of Candida species isolated from clinical specimens]. Mikrobiyol Bul 2003;37:269–276. [PubMed] [Google Scholar]
- 19. Borman AM, Linton CJ, Miles S‐J, Campbell CK, Johnson EM. Ultra‐rapid preparation of total genomic DNA from isolates of yeast and mould using Whatman FTA filter paper technology—A reusable DNA archiving system. Med Mycol 2006;44:389–398. [DOI] [PubMed] [Google Scholar]
- 20. Mohammadi R, Mirhendi H, Rezaei‐Matehkolaei A, et al. Molecular identification and distribution profile of Candida species isolated from Iranian patients. Med Mycol 2013;51:657–663. [DOI] [PubMed] [Google Scholar]
- 21. Mirhendi H, Makimura K, Khoramizadeh M, Yamaguchi H. A one‐enzyme PCR‐RFLP assay for identification of six medically important Candida species. Nihon Ishinkin Gakkai Zasshi 2006;47:225–229. [DOI] [PubMed] [Google Scholar]
- 22. Tavanti A, Davidson AD, Gow NA, Maiden MC, Odds FC. Candida orthopsilosis and Candida metapsilosis spp. nov. to replace Candida parapsilosis groups II and III. J Clin Microbiol 2005;43:284–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Liu D, Coloe S, Baird R, Pedersen J. Rapid mini‐preparation of fungal DNA for PCR. J Clin Microbiol 2000;38:471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Raeder U, Broda P. Rapid preparation of DNA from filamentous fungi. Appl Microbiol 1985;1:17–20. [Google Scholar]
- 25. Jackson CJ, Barton RC, Evans EGV. Species identification and strain differentiation of dermatophyte fungi by analysis of ribosomal‐DNA intergenic spacer regions. J Clin Microbiol 1999;37:931–936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Roberts D. Prevalence of dermatophyte onychomycosis in the United Kingdom: Results of an omnibus survey. Br J Dermatol 1992;126:23–27. [DOI] [PubMed] [Google Scholar]
- 27. Zaini F, Mahmoudi M, Mehbod A, Kordbacheh P, Safara M. Fungal nail infections in Tehran, Iran. Iran J Pub Heal 2009;38:46–53. [Google Scholar]
- 28. Sigurgeirsson B, Ólafsson JH, þ Steinsson J, Paul C, Billstein S, Evans EGV. Long‐term effectiveness of treatment with terbinafine vs itraconazole in onychomycosis: A 5‐year blinded prospective follow‐up study. Arch Dermatol 2002;138:353–357. [DOI] [PubMed] [Google Scholar]
- 29. Mohammadi R, Badiee P, Badali H, et al. Use of restriction fragment length polymorphism to identify Candida species, related to onychomycosis. Adv Biomed Res 2015;4:95. doi: 10.4103/2277-9175.156659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kafaie P, Noorbala MT. Evaluation of onychomycosis among diabetic patients of Yazd diabetic center. J Pak Assoc Dermatol 2010;20:217–221. [Google Scholar]
- 31. Khosravi A, Mansouri P. Onychomycosis in Tehran, Iran: Prevailing fungi and treatment with itraconazole. Mycopathologia 2001;150:9–13. [DOI] [PubMed] [Google Scholar]
- 32. Mohammadi R, Abastabar M, Mirhendi H, et al. Use of restriction fragment length polymorphism to rapidly identify dermatophyte species related to dermatophytosis. Jundishapur J Microbiol 2015;8:e17296. doi: 10.5812/jjm.8(5)2015.17296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Bassiri‐Jahromi S, Khaksari AA. Epidemiological survey of dermatophytosis in Tehran, Iran, from 2000 to 2005. Indian J Dermatol Venereol Leprol 2009;75:142–147. [DOI] [PubMed] [Google Scholar]
- 34. Hashemi S, Gerami M, Zibafar E, Daei M, Moazeni M, Nasrollahi A. Onychomycosis in Tehran: Mycological study of 504 patients. Mycoses 2010;53:251–255. [DOI] [PubMed] [Google Scholar]
- 35. Mikaeili A, Karimi I. The incidence of onychomycosis infection among patients referred to hospitals in Kermanshah province, Western Iran. Iran J Pub Heal 2013;42:320–325. [PMC free article] [PubMed] [Google Scholar]
- 36. Moghaddami M, Shidfar MR. A study of onychomycosis in Tehran. Med J Islam Repub Iran 1989;3:143–149. [Google Scholar]
- 37. Ghasemi Z, Falahati M, Sadri M, et al. Report of 34 cases of saprophytic fungi isolated from dystrophic nails of patients referred to Razi Hospital (2010‐2011). Razi J Med Sci 2012;18:8–14. [Google Scholar]
- 38. Ahmadi B, Hashemi SJ, Zaini F, et al. A case of onychomycosis caused by Aspergillus candidus . Med Mycol Case Rep 2012;1:45–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Falahati M, Ghasemi Z, Zaini F, et al. The first case of Onychomycosis due to Exophiala dermatitidis in Iran: Molecular identification and Antifungal Susceptibility. Bull Environ Pharmacol Life Sci 2014;3:125–129. [Google Scholar]
- 40. Mousavi S, Esfandiarpour I, Salari S, Shokri H. Onychomycosis due to Fusarium spp. in patient with squamous cell carcinoma: A case report from Kerman, Iran. J Mycol Méd 2009;19:146–149. [Google Scholar]
- 41. Mahmoudabadi AZ, Zarrin M. Onychomycosis with Aspergillus flavus: A case report from Iran. Pak J Med Sci 2005;21:497–498. [Google Scholar]
- 42. Soltani M, Khosravi AR, Shokri H, et al. A study of onychomycosis in patients attending a dermatology center in Tehran, Iran. J Mycol Méd 2015;25:e81–e87. [DOI] [PubMed] [Google Scholar]
- 43. Gerami shoar M, Zomorodian K, Emami M, et al. Study and identification of the etiological agents of onychomycosis in Tehran, capital of Iran. Iran J Pub Heal 2002;31:100–104. [Google Scholar]
- 44. Kazemi A. Tinea unguium in the North‐West of Iran (1996‐2004). Rev Iberoam Micol 2007;24:113–117. [DOI] [PubMed] [Google Scholar]


