Abstract
Objective
To explore the clinical significance of serum levels of IL‐6/10/18 in sepsis.
Methods
Sixty‐six patients with sepsis were selected to be the case group. Additionally, 42 healthy adults were selected to be the control group. ELISA was used to measure the serum levels of IL‐6/10/18, and ROC was utilized to evaluate the diagnostic values of IL‐6/10/18 in sepsis.
Results
The heart rate, respiratory rate, WBC count and APACHE II score in the sepsis group were significantly higher than those in the control group, and these indexes were increased in turn in the mild sepsis group, severe sepsis group, and septic shock group (all P < 0.05 after correction). The serum IL‐6/18 levels in sepsis patients were significantly higher than those in the control group, and both of the levels were increased in turn in the mild sepsis group, severe sepsis group, and septic shock group (both P < 0.05). However, no significant difference was found in serum IL‐10 level between groups (P > 0.05). The cut‐off points of IL‐6 and IL‐18 were 109.19 pg/ml (sensitivity: 94.4%; specificity: 83.3%) and 116.01 pg/ml (sensitivity: 77.8%; specificity: 83.3%), respectively. Serum IL‐6 levels were positively correlated with the APACHE II score and heart rate (both P < 0.001).
Conclusion
Serum levels of IL‐6/8 are up‐regulated in sepsis patients. Additionally, IL‐6 has a greater sensitivity than IL‐18. Serum IL‐6 levels were positively correlated with the APACHE II score and heart rate, indicating that IL‐6 could be used as a potential biomarker for sepsis.
Keywords: interleukin 10, interleukin 18, interleukin 6, receiver operating characteristic curve, sepsis, serum level
Introduction
Sepsis is characterized by a systemic inflammatory response caused by bacterial infections and non‐infectious conditions that is mediated by the activation of various proinflammatory mediators 1. Sepsis is estimated to be responsible for approximately 6 million victims per year, which represents the major cause of death in intensive care units (ICU) worldwide 2. In the US, the hospitalization rate of patients with sepsis or even septicemia has increased from 221 to 377 per 100,000, and the prevalence of severe postoperative sepsis has increased from 0.3% to 0.9% 3. Patients with sepsis are apt to develop an inflammatory response on account of microbes in their blood, skin, lungs, urine, or other tissues, and severe sepsis can even contribute to multiple organ failure as a result of blood clotting in finer blood vessels 4. Additionally, sepsis is the major 3 cause of acute renal failure related to hypoperfusion, followed by tissue injury as well as subsequent organ damage 5. There is an interaction between various pathways involved in the pathogenesis of sepsis, such as inflammation, coagulation, immunity, and the neuroendocrine system 6. As expected, the worldwide incidence will increase with aging, greater antimicrobial resistance, greater access to medical technology and interventions, and a wider use of immunosuppressive therapies 7. Increasing release of systemic cytokines such as interleukin‐6 (IL‐6), interleukin‐10 (IL‐10), interleukin‐18 (IL‐18), and tumor necrosis factor (TNF) has been well documented in the septic human response and in relevant animal models 6, 8.
Human IL‐6 is a type of cytokine that is composed of 184 amino acids with a molecular weight of 21–26 kDa, and its gene is located on chromosome 7 9. The IL‐6 receptor is produced by several cell types, such as B and T lymphocytes, monocytes/macrophages, and in turn stimulates T and B lymphocytes and other cells involved in cell proliferation and differentiation and enhances their functionality 10, 11. Additionally, IL‐10 is a potent regulatory cytokine that functions as an anti‐inflammatory that inhibits the synthesis of a number of cytokines, such as interferon (IFN)‐γ, TNF and other pro‐inflammatory cytokines produced by activated macrophages and by helper T cells 12, 13. The human IL‐10 molecule is composed of 160 amino acids with a molecular weight of 18.5 kDa, whereas murine IL‐10 consists of 157 amino acids with a molecular weight of 18.5 kDa 14. IL‐18, which belongs to the IL‐1 super‐family, is a pro‐inflammatory cytokine that induces the synthesis of TNF‐α, IL‐1B, IL‐13, and other cytokines and is produced by macrophages as well as other cells 15, 16. In addition, IL‐18 has the capability of inducing chemokines such as MIP‐1α, MIP‐1b, and MCP‐1, which are attributed to the infection of monocytes and macrophages, thus initiating the inflammatory process 17. IL‐6 has been reported to be involved in various physiological and immunological processes, and it plays an important role in SIRS, sepsis, and other diseases 18. Cytokines (both pro‐inflammatory and anti‐inflammatory) present a double‐edged sword in sepsis 2: they are critical to eliminate the infection 2, yet their overproduction can cause tissue or organ damage 19. Ocuin et al. 20 found that IL‐10 can inhibit the occurrence of TNF‐α, IL‐1, IL‐6, and IL‐8, and that it plays an important regulatory role in the progression of sepsis. Several studies have indicated that IL‐18 participates in the development of sepsis and is involved in the severity of sepsis 21, 22. A previous study has identified several important roles of individual cytokines, and an analysis of the cytokines (e.g., IL‐6 and IL‐18) involved in sepsis is essential to understand the mechanisms of sepsis to offer better treatment approaches and may also be helpful for identifying biomarkers of sepsis 23. As there have been no studies conducted that compare the effects of these three inflammatory factors on sepsis, our study aims to investigate the associations between the inflammatory factors IL‐6, IL‐10, and IL‐18 and the development of sepsis.
Materials and methods
Ethics Statement
The study was reviewed and approved by the Institutional Review Board of Jining NO.1 People's Hospital. All subjects gave their written informed consent, and the conduct of the study complied with the latest revision of the Declaration of Helsinki 24.
Study Subjects
Sixty‐six patients with sepsis who were admitted to the intensive care unit (ICU) of Jining NO.1 People's Hospital from December 2011 to December 2013 were selected to be the case group; this group included 39 males and 27 females with ages ranging from 19 to 83 years and had a mean age of 51.18 ± 16.17 years old. All of the patients promptly completed the Acute Physiology and Chronic Health Evaluation II (APACHE II) within 24 hours of being admitted to the hospital. Additionally, 42 healthy adults who received a physical examination in the same hospital during the same period were selected to be in the control group, including 25 males and 17 females ranging from 22 to 67 years old with a mean of 47.57 ± 11.81 years old. None of the healthy controls had trauma, infections, burns, pancreatitis, or non‐specific inflammatory processes resulting from any other disease.
The diagnostic criteria of sepsis referred to the systemic inflammatory response syndrome (SIRS) criteria formulated by the American College of Chest Physicians and Society of Critical Care Medicine (ACCP/SCCM) in 1991 and the diagnostic criteria of the International Conference on Sepsis constituted in 2001 1, 25. The diagnostic criteria of SIRS included meeting two or more of the following items at the same time: (1) body temperature >38°C or <36°C; (2) heart rate >90 beats/min; (3) respiratory rate >20 breaths/min or arterial partial pressure of carbon dioxide (PaCO2) < 32 mmHg (4.3 kPa); and (4) peripheral blood white blood cell (WBC) count >12,000/mm3, <4,000/mm3, or immature granulocyte >10%. The diagnostic criteria for sepsis included meeting the diagnostic criteria of SIRS and a clear focus of infection. The diagnostic criteria for severe sepsis were systemic infection accompanied by organ dysfunction, poor tissue perfusion, or low blood pressure. The diagnostic criteria for septic shock included: (1) clear clinical infection, (2) existence of SIRS, (3) systolic pressure <90 mmHg or decreased more than 40 mmHg compared with a baseline value within at least 1 hour or blood pressure dependent on infusion or drug maintenance, and (4) the existence of poor tissue perfusion, such as less urine (<30 ml/h) for more than 1 hour or an acute mental disorder. According to the severity of the disease, the patients were divided into a mild sepsis group (n = 26), a severe sepsis group (n = 22), and a septic shock group (n = 18).
The exclusion criteria of this study were as follows: (1) age >83 or <19 years old; (2) a history of autoimmune disease, acquired immunodeficiency syndrome (AIDS), acute cerebral vascular accident, active bleeding, chronic renal failure or cancers, or the use of an immunosuppressant; (3) presence of any acute or chronic infectious disease; (4) history of chest compressions, defibrillation, direct current cardioversion, chest trauma, or left bundle branch block 7 days before being admitted to the hospital; (5) presence of acute myocardial infarction (AMI) symptoms and/or an atypical electrocardiogram (ECG) when admitted into the hospital; and (6) discontinuation of systemic therapy.
Measurements of serum levels of IL‐6, IL‐10, and IL‐18
After sepsis diagnosis based on APACHE II score, approximately 5 ml samples of venous blood were collected from all subjects in the morning of the first, third, and seventh day. Additionally, approximately 5 ml samples of venous blood were collected from the healthy controls. The venous blood samples were preserved in the laboratory by ethylene diamine tetraacetic acid‐k2 (EDTA‐K2) anticoagulants for 30 min and then centrifuged for 10 min at 3,000 rpm/min at 4°C to separate plasma and blood cells, which were then stored at −80°C for later use. A double antibody sandwich enzyme‐linked immunosorbent assay (ELISA) was used to measure the serum levels of IL‐6, IL‐10, and IL‐18. The kits were provided by the American R&D Company, and the device was an ELx800 enzyme‐labeled meter (Bio‐Tek Instruments, Inc., Winooski, VT). The conduct of the experiments strictly complied with the manufacturers' instructions, and all tests were performed by specialists. A receiver operating characteristic curve (ROC) and the area under the curve (AUC) were utilized to evaluate the clinical values of serum IL‐6, IL‐10, and IL‐18 in the diagnosis of different severities of sepsis.
Statistical analysis
SPSS 19.0 (Science Chicago, SPSS, Chicago, IL, USA) software was used for statistical analysis. The numerical data were presented as mean ± standard deviation ( ± s), with t‐tests used for the comparisons between two groups and one‐way analysis of variance (ANOVA) used for the comparisons between three or more groups. The categorical data were presented as the rate or composition ratio, with the chi‐squared test used for comparisons between groups. Pearson's test was adopted for correlation analysis. ROC curves were implemented to evaluate the diagnostic value of IL‐6, IL‐10, and IL‐18 in determining the severity of sepsis by comparing the AUC of each factor. All statistical tests were two‐sided. All of the P values were Bonferroni‐corrected, and the P values displayed in the table were corrected P values; a corrected P < 0.05 was considered statistically significant.
Results
Baseline characteristics of study subjects
The baseline characteristics of the patient groups with different severities of sepsis and the control group are presented in Table 1. No statistical significance between the control group and the sepsis group was observed in relation to gender, age, or body temperature (P > 0.05), whereas the heart rate, respiratory rate, WBC count, and APACHE II score in the sepsis group were significantly higher than those in the control group (all P < 0.05 after correction). Gender, age, and body temperature were not significantly different between the patients with different severities of sepsis (all P > 0.05), whereas the heart rate, respiratory rate, WBC count, and APACHE II score of the patients were increased in turn in the mild sepsis group, severe sepsis group, and septic shock group; there were significant differences between the mild sepsis group and the severe sepsis group as well as between the mild sepsis group and the septic shock group (all P < 0.05 after correction).
Table 1.
Comparison of the baseline characteristics of the control group and the patients with different severities of sepsis
| Age (years) | Gender (M/F) | Temperature (°C) | Heart rate (time/min) | Respiratory rate (time/min) | WBC count (×109 L) | APACHE II score | |
|---|---|---|---|---|---|---|---|
| Control group (n = 42) | 47.57 ± 11.81 | 25/17 | 36.9 ± 7.62 | 89.12 ± 14.06 | 17.22 ± 7.42 | 11.99 ± 3.78 | 10.2 ± 7.75 |
| Sepsis group (n = 66) | 51.18 ± 16.17 | 39/27 b | 38.97 ± 5.91 | 111.18 ± 11.46 a | 24.01 ± 7.00 a | 14.80 ± 4.73 a | 21.15 ± 7.75 a |
| Mild sepsis group (n = 26) | 53.58 ± 21.30 | 14/12 | 38.6 ± 5.47 | 103.02 ± 10.42 | 20.21 ± 6.34 | 12.12 ± 3.22 | 18.7 ± 5.70 |
| Severe sepsis group (n = 22) | 50.55 ± 17.99 | 13/9 | 39.1 ± 6.44 | 112.54 ± 10.32 b | 24.35 ± 7.01 b | 14.89 ± 4.80 b | 20.7 ± 6.50 |
| Septic shock group (n = 18) | 51.06 ± 17.89 | 10/8 | 39.8 ± 6.06 | 118.06 ± 11.33 b | 26.78 ± 7.19 b | 16.13 ± 5.67 b | 26.20 ± 9.70 b |
M, male; F, female; WBC, while blood cell; APACHE II, Acute Physiology and Chronic Health Evaluation II.
aRefers to P < 0.05 when compared with the control group; brefers to P < 0.05 when compared with the mild sepsis group.
Associations between serum levels of IL‐6, IL‐10, and IL‐18 and sepsis and severity of sepsis
The serum IL‐6, IL‐10, and IL‐18 levels in the control group and the case groups with different severities of sepsis are presented in Table 2. Serum IL‐6 and IL‐18 levels in the sepsis group were significantly higher than those in the control group (both P < 0.05 after correction), but there was no statistical significance in the serum level of IL‐10 between the two groups (P > 0.05). The serum levels of IL‐6 and IL‐18 in patients in the mild sepsis group, the severe sepsis group, and the septic shock group showed an increasing trend, and there were significant differences between the groups (both P < 0.05 after correction); the serum levels of IL‐10 in the patients in these three groups had no significant difference (P > 0.05).
Table 2.
Association between serum levels of IL‐6, IL‐10, and IL‐18 and sepsis and severity of sepsis
| IL‐6 (pg/ml) | IL‐10 (pg/ml) | IL‐18 (pg/ml) | |
|---|---|---|---|
| Control group (n = 42) | 23.65 ± 19.32 | 96.68 ± 19.32 | 32.51 ± 16.21 |
| Sepsis group (n = 66) | 102.69 ± 29.69a | 106.32 ± 30.68 | 119.30 ± 29.33a |
| Mild sepsis group (n = 26) | 84.15 ± 15.82 | 96.67 ± 28.66 | 89.96 ± 10.58 |
| Severe sepsis group (n = 22) | 105.83 ± 13.44b | 101.51 ± 24.281 | 109.30 ± 13.35b |
| Septic shock group (n = 18) | 131.42 ± 20.01b , c | 113.24 ± 32.62 | 122.21 ± 14.34b , c |
Refers to P < 0.05 when compared with the control group.
Refers to P < 0.05 when compared with the mild sepsis group.
Refers to P < 0.05 when compared with the severe sepsis group.
The sensitivity and specificity of serum IL‐6 and IL‐18 levels in diagnosing the severity of sepsis
As shown in Figure 1, the AUC of IL‐6 was 0.935, which was significantly higher than the AUC of IL‐18 (0.881). At the upper left corner of the curve, the sensitivity and the specificity of IL‐6 were 94.4% and 83.3%, respectively, and the corresponding optimal cut‐off point of IL‐6 that achieved the highest values of sensitivity and specificity was 109.19 pg/ml. Similarly, at the upper left corner of the curve, the sensitivity and the specificity of IL‐18 were 77.8% and 83.3%, respectively, and the corresponding optimal cut‐off point of IL‐18 that achieved the highest values of sensitivity and specificity was 116.01 pg/ml. The results indicated that IL‐6 was more promising than IL‐18 in the diagnosis of sepsis.
Figure 1.
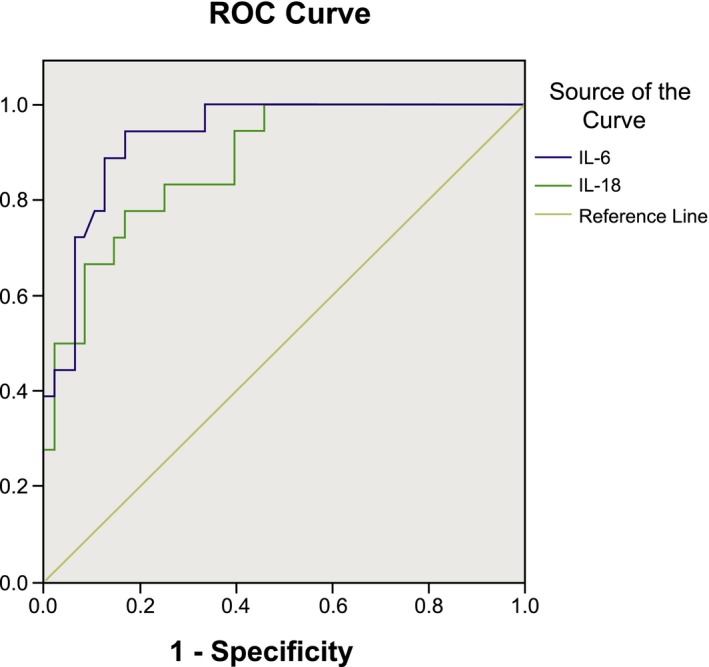
The receiver operating characteristic curve of serum levels of IL‐6 and IL‐18 in diagnosing the severity of sepsis.
Correlation analysis
Correlation analysis values between the serum levels of IL‐6 and IL‐18 in sepsis patients and body temperature, heart rate, respiratory rate, WBC count, and APACHE II score are displayed in Table 3. The results showed that there was a strong positive correlation between IL‐6 and APACHE II score as well as heart rate (APACHE II score: r = 0.454; heart rate: r = 0.551; both P < 0.001, Fig. 2), but no correlation was found for WBC count, respiratory rate, or body temperature (all P > 0.05). Furthermore, no significant correlation was detected between IL‐18 and any of the other indices (all P > 0.05).
Table 3.
Correlation analysis of serum levels of IL‐6 and IL‐18 with body temperature, heart rate, respiratory rate, WBC count, and APACHE II score
| IL‐6 (pg/ml) | IL‐18 (pg/ml) | |
|---|---|---|
| Body temperature | −0.009 | −0.046 |
| Heart rate | 0.551a | 0.211 |
| Respiratory rate | 0.192 | −0.058 |
| WBC count | 0.167 | 0.142 |
| APACHE II score | 0.454b | 0.151 |
WBC, while blood cell; APACHE II, Acute Physiology and Chronic Health Evaluation II.
Refers to P < 0.05 for the correlation between serum IL‐6 level and heart rate.
Refers to P < 0.05 for the correlation between serum IL‐6 level and APACHE II score.
Figure 2.
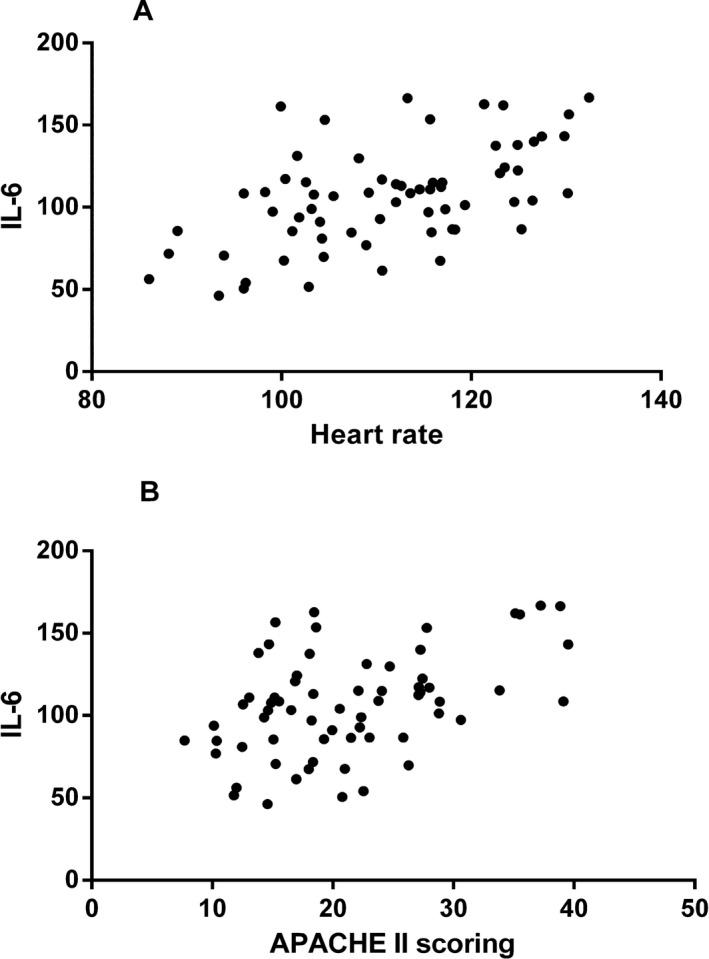
Correlation analysis results. Correlation between (A) serum IL‐6 level and heart rate, and (B) serum IL‐6 level and APACHE II score.
Discussion
Our results indicated that IL‐6 and IL‐18 were highly expressed in the serum of sepsis patients, suggesting that serum levels of IL‐6 and IL‐18 may play a vital role in the development of sepsis. As a multifunctional cytokine involved in pro‐inflammatory and anti‐inflammatory processes, IL‐6 can regulate the immune response, hematopoiesis, and the involvement of inflammation 26, 27. The importance of IL‐6 is its presence in the early stages of the host response to infection, and IL‐6 stimulation has been related to the increased migration of activated T cells in vitro, as IL‐6 has a significant function in stimulating the production of C‐reactive protein but is known to precede this increase 28, 29. Essentially, the concentration of IL‐6 clearly increases after exposure to bacterial products in the early stages of infection 30. It is well known that IL‐18 plays a central role in regulating immune and inflammatory responses to infections and sterile tissue damage 10. IL‐18 has the ability to induce the production of IFN‐γ in the presence of IL‐12 and also to induce chemokines attracting monocytes and macrophages to the sites of infection, thereby initiating the inflammatory process linked to the development of sepsis 31. Moreover, IL‐18 may induce IL‐2 and granulocyte‐macrocyte colony‐stimulating factor produced by antigenically stimulated T cells and may enhance natural killer cell activity as well as Fas ligand expression in Th1 cells 32. Notably, specific blockade of IL‐6 or IL‐18 can alleviate the effects of sepsis on organ damage or improve host survival, and their concentrations fall abruptly with specific treatment, thus supporting the idea that serum IL‐6 and IL‐18 levels participate in the pathologic processes of sepsis and that both of them may be helpful for the early diagnosis of sepsis 18, 33. Additionally, in our study, the septic shock group had the highest serum IL‐6 and IL‐18 levels, followed by the severe sepsis group, the mild sepsis group, and the control group, which can be explained by the fact that serum IL‐6 levels in patients with septic shock are higher when compared to those in patients without septic shock as well as in patients who have died from severe sepsis 34. In addition, an elevated level of IL‐6 has been suggested to be related to the highest risk of mortality in patients with sepsis, which suggests that IL‐6 may constitute a candidate biomarker for sepsis 35. A previous study has shown that elevated plasma IL‐18 concentrations are associated with poor clinical outcomes in severe inflammatory and septic conditions, and thus IL‐18 may also constitute a candidate biomarker for sepsis 23.
Our study also indicated that IL‐6 had a higher sensitivity and specificity than IL‐18, which suggests that IL‐6 can be used as a potential diagnostic indicator of sepsis. To date, many researchers have attempted to associate cytokine levels with the development of sepsis, but no single biological or clinical indicator of sepsis has gained unanimous acceptance 36, 37. High levels of inflammation parameters, such as IL‐6, IL‐8, procalcitonin (PCT), and C‐reactive protein (CRP), have been recognized as the most promising candidates of the potential sepsis markers 38. Of these, as the most well‐known marker, IL‐6 has been reported to act as an early indicator of sepsis due to its rapid increase after endotoxin challenge; IL‐6 is a potent inflammatory mediator, and its plasma concentrations have typically been tested as prognostic factors in severe infections and septic shock 39. IL‐6 can induce the differentiation of T and B cells, stimulate synthesis of the acute phase reaction protein in liver cells, and catalyze and amplify the inflammatory reaction and toxic effects, which results in damage to the tissue cells, thus reflecting severity of the disease 30. Basu et al. found that when compared to sepsis patients in the recovery period controls, there were significantly elevated IL‐6 levels at the onset of sepsis in septic preterm newborns 40. In another study, Cernada et al. proposed that plasma IL‐6 detection was an ideal indicator to diagnose the early onset of sepsis, and the combination of IL‐6 and CRP detection may have the same result 41. The results of our study also suggest that IL‐6 is positively correlated with heart rate and APACHE II score, which further confirms the diagnostic value of IL‐6. Thus, persistently high IL‐6 is a very sensitive marker of inflammation and is likely to be of prognostic value in determining the severity of sepsis.
There were also some limitations to our study. First, due to the restrictions on the number of cases and funds, the sample size of the study was one of the limitations of this paper. Second, this paper only explored the associations of serum IL‐6, IL‐10, and IL‐18 levels with different degrees of sepsis and concluded that IL‐6 had a better diagnostic value for patients with sepsis; the other factors related to sepsis diagnosis require further investigation. Finally, the relationship between serum levels of IL‐6, IL‐10, and IL‐18 in patients with sepsis and the efficacy of the treatment remains to be seen.
In conclusion, serum levels of IL‐6 and IL‐18 are up‐regulated in sepsis patients. The ROC curve is a commonly used measure to assess the functions of a diagnostic biomarker in predicting a binary disease outcome. Generally, the ROC curve displays the sensitivity and specificity for different cut‐off values that classify an individual as healthy or diseased 42. In our study, IL‐6 had a higher sensitivity and specificity than IL‐18, and IL‐6 level was positively correlated with APACHE II score and heart rate; this indicates that IL‐6 can be used as a potential diagnostic biomarker for sepsis. A reliable biomarker for the diagnosis and prognosis of sepsis is critical; for one, the inhibition of excessive cytokine production or removal of cytokines may suppress systemic inflammation during sepsis and improve patient outcomes. Furthermore, clinical trials have demonstrated that treatment with long courses of low doses of corticosteroids significantly reduces mortality in patients with severe sepsis and septic shock 43. Therefore, the measurement of IL‐6 in patients with suspected sepsis could increase the precision of diagnosis and improve patient management.
Acknowledgments
We acknowledge the reviewers for their helpful comments on this paper.
Mingchen Feng and Tingting Sun contributed equally to this work.
References
- 1. Bone RC, Balk RA, Cerra FB, et al. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine. 1992. Chest 2009;136(5 Suppl.):e28. [DOI] [PubMed] [Google Scholar]
- 2. Leentjens J, Kox M, Koch RM, et al. Reversal of immunoparalysis in humans in vivo: a double‐blind, placebo‐controlled, randomized pilot study. Am J Respir Crit Care Med 2012;186:838–845. [DOI] [PubMed] [Google Scholar]
- 3. Reinhart K, Bauer M, Riedemann NC, et al. New approaches to sepsis: molecular diagnostics and biomarkers. Clin Microbiol Rev 2012;25:609–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Marti‐Carvajal AJ, Sola I, Gluud C, et al. Human recombinant protein C for severe sepsis and septic shock in adult and paediatric patients. Cochrane Database Syst Rev 2012;12:CD004388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Haase M, Bellomo R, Baldwin I, et al. Hemodialysis membrane with a high‐molecular‐weight cutoff and cytokine levels in sepsis complicated by acute renal failure: a phase 1 randomized trial. Am J Kidney Dis 2007;50:296–304. [DOI] [PubMed] [Google Scholar]
- 6. Diao H, He J, Zheng Q, et al. A possible role for NKT‐like cells in patients with chronic hepatitis B during telbivudine treatment. Immunol Lett 2014;160:65–71. [DOI] [PubMed] [Google Scholar]
- 7. Iskander KN, Osuchowski MF, Stearns‐Kurosawa DJ, et al. Sepsis: multiple abnormalities, heterogeneous responses, and evolving understanding. Physiol Rev 2013;93:1247–1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Angus DC. The search for effective therapy for sepsis: back to the drawing board? JAMA 2011;306:2614–2615. [DOI] [PubMed] [Google Scholar]
- 9. Hirano T. Interleukin 6 (IL‐6) and its receptor: their role in plasma cell neoplasias. Int J Cell Cloning 1991;9:166–184. [DOI] [PubMed] [Google Scholar]
- 10. Sims JE, Smith DE. The IL‐1 family: regulators of immunity. Nat Rev Immunol 2010;10:89–102. [DOI] [PubMed] [Google Scholar]
- 11. Kim HJ, Kim MY, Hwang JS, et al. PPARdelta inhibits IL‐1beta‐stimulated proliferation and migration of vascular smooth muscle cells via up‐regulation of IL‐1Ra. Cell Mol Life Sci 2010;67:2119–2130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Henry CJ, Huang Y, Wynne AM, et al. Peripheral lipopolysaccharide (LPS) challenge promotes microglial hyperactivity in aged mice that is associated with exaggerated induction of both pro‐inflammatory IL‐1beta and anti‐inflammatory IL‐10 cytokines. Brain Behav Immun 2009;23:309–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sanjabi S, Zenewicz LA, Kamanaka M, et al. Anti‐inflammatory and pro‐inflammatory roles of TGF‐beta, IL‐10, and IL‐22 in immunity and autoimmunity. Curr Opin Pharmacol 2009;9:447–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Conti P, Kempuraj D, Kandere K, et al. IL‐10, an inflammatory/inhibitory cytokine, but not always. Immunol Lett 2003;86:123–129. [DOI] [PubMed] [Google Scholar]
- 15. van de Veerdonk FL, Netea MG, Dinarello CA, et al. Inflammasome activation and IL‐1beta and IL‐18 processing during infection. Trends Immunol 2011;32:110–116. [DOI] [PubMed] [Google Scholar]
- 16. Volin MV, Koch AE. Interleukin‐18: a mediator of inflammation and angiogenesis in rheumatoid arthritis. J Interferon Cytokine Res 2011;31:745–751. [DOI] [PubMed] [Google Scholar]
- 17. Fortin CF, Ear T, McDonald PP. Autocrine role of endogenous interleukin‐18 on inflammatory cytokine generation by human neutrophils. FASEB J 2009;23:194–203. [DOI] [PubMed] [Google Scholar]
- 18. Celik IH, Demirel FG, Uras N, et al. What are the cut‐off levels for IL‐6 and CRP in neonatal sepsis? J Clin Lab Anal 2010;24:407–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Fjell CD, Thair S, Hsu JL, et al. Cytokines and signaling molecules predict clinical outcomes in sepsis. PLoS One 2013;8:e79207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ocuin LM, Bamboat ZM, Balachandran VP, et al. Neutrophil IL‐10 suppresses peritoneal inflammatory monocytes during polymicrobial sepsis. J Leukoc Biol 2011;89:423–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Waikar SS, Bonventre JV. Biomarkers for the diagnosis of acute kidney injury. Curr Opin Nephrol Hypertens 2007;16:557–564. [DOI] [PubMed] [Google Scholar]
- 22. Zaki Mel S, Elgendy MY, El‐Mashad NB, et al. IL‐18 level correlates with development of sepsis in surgical patients. Immunol Invest 2007;36:403–411. [DOI] [PubMed] [Google Scholar]
- 23. Chaudhry H, Zhou J, Zhong Y, et al. Role of cytokines as a double‐edged sword in sepsis. In Vivo 2013;27:669–684. [PMC free article] [PubMed] [Google Scholar]
- 24. Pn M. World Medical Association publishes the Revised Declaration of Helsinki. Natl Med J India 2014;27:56. [PubMed] [Google Scholar]
- 25. Levy MM, Fink MP, Marshall JC, et al. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Crit Care Med 2003;31:1250–1256. [DOI] [PubMed] [Google Scholar]
- 26. Sibbing D, Koch W, Massberg S, et al. No association of paraoxonase‐1 Q192R genotypes with platelet response to clopidogrel and risk of stent thrombosis after coronary stenting. Eur Heart J 2011;32:1605–1613. [DOI] [PubMed] [Google Scholar]
- 27. Turpie AG, Esmon C. Venous and arterial thrombosis–pathogenesis and the rationale for anticoagulation. Thromb Haemost 2011;105:586–596. [DOI] [PubMed] [Google Scholar]
- 28. Dienz O, Rincon M. The effects of IL‐6 on CD4 T cell responses. Clin Immunol 2009;130:27–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kimura A, Kishimoto T. IL‐6: regulator of Treg/Th17 balance. Eur J Immunol 2010;40:1830–1835. [DOI] [PubMed] [Google Scholar]
- 30. Jekarl DW, Lee SY, Lee J, et al. Procalcitonin as a diagnostic marker and IL‐6 as a prognostic marker for sepsis. Diagn Microbiol Infect Dis 2013;75:342–347. [DOI] [PubMed] [Google Scholar]
- 31. Vanden Berghe T, Demon D, Bogaert P, et al. Simultaneous targeting of IL‐1 and IL‐18 is required for protection against inflammatory and septic shock. Am J Respir Crit Care Med 2014;189:282–291. [DOI] [PubMed] [Google Scholar]
- 32. Novick D, Kim S, Kaplanski G, et al. Interleukin‐18, more than a Th1 cytokine. Semin Immunol 2013;25:439–448. [DOI] [PubMed] [Google Scholar]
- 33. Anas AA, Wiersinga WJ, de Vos AF, et al. Recent insights into the pathogenesis of bacterial sepsis. Neth J Med 2010;68:147–152. [PubMed] [Google Scholar]
- 34. Wu HP, Chen CK, Chung K, et al. Serial cytokine levels in patients with severe sepsis. Inflamm Res 2009;58:385–393. [DOI] [PubMed] [Google Scholar]
- 35. Kellum JA, Kong L, Fink MP, et al. Understanding the inflammatory cytokine response in pneumonia and sepsis: results of the Genetic and Inflammatory Markers of Sepsis (GenIMS) Study. Arch Intern Med 2007;167:1655–1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Schulte W, Bernhagen J, Bucala R. Cytokines in sepsis: potent immunoregulators and potential therapeutic targets–an updated view. Mediators Inflamm 2013;2013:165974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kothari N, Bogra J, Abbas H, et al. Tumor necrosis factor gene polymorphism results in high TNF level in sepsis and septic shock. Cytokine 2013;61:676–681. [DOI] [PubMed] [Google Scholar]
- 38. Schrag B, Roux‐Lombard P, Schneiter D, et al. Evaluation of C‐reactive protein, procalcitonin, tumor necrosis factor alpha, interleukin‐6, and interleukin‐8 as diagnostic parameters in sepsis‐related fatalities. Int J Legal Med 2012;126:505–512. [DOI] [PubMed] [Google Scholar]
- 39. Lin S, Huang Z, Wang M, et al. Interleukin‐6 as an early diagnostic marker for bacterial sepsis in patients with liver cirrhosis. J Crit Care 2015;30:732–738. [DOI] [PubMed] [Google Scholar]
- 40. Basu S, Dewangan S, Anupurva S, Kumar A. Statistical validity of interleukin‐6 as a biomarker for the diagnosis of early‐onset neonatal sepsis. Microbiol Res 2012;3:5. [Google Scholar]
- 41. Cernada M, Badia N, Modesto V, et al. Cord blood interleukin‐6 as a predictor of early‐onset neonatal sepsis. Acta Paediatr 2012;101:e203–e207. [DOI] [PubMed] [Google Scholar]
- 42. Rodriguez‐Alvarez MX, Meira‐Machado L, Abu‐Assi E, et al. Nonparametric estimation of time‐dependent ROC curves conditional on a continuous covariate. Stat Med 2015;35:1090–1102. [DOI] [PubMed] [Google Scholar]
- 43. Annane D, Bellissant E, Bollaert PE, et al. Corticosteroids in the treatment of severe sepsis and septic shock in adults: a systematic review. JAMA 2009;301:2362–2375. [DOI] [PubMed] [Google Scholar]


