Abstract
Background
The pathophysiology of preeclampsia is not clearly understood worldwide. Hypoxia inducible factor 1α (HIF‐1α) is thought to be the preliminary factor for the hypoxic conditions prevailing in preeclampsia, which causes imbalance in the expression of angiogenic proteins. A proangiogenic protein, placental growth factor (PIGF), is reported to be dysregulated in preeclampsia. Therefore, this study focuses on the investigation of HIF‐1α and PIGF in preeclamptic conditions and a possible molecular association between them.
Methods
Placental tissue (n = 45 + 45) and serum samples (n = 80 + 80) of preeclamptic patients and healthy control were collected and processed for the analysis of HIF‐1α and PIGF by immunohistochemistry and enzyme‐linked immunosorbent assay (ELISA).
Results
In preeclamptic group, the significant nuclear and cytoplasmic expression of HIF‐1α was noticed in syncytiotrophoblast (P = 0.0001) but in control placenta, it was localized to cytoplasm (P = 0.0001). The intensity of PIGF expression was lower in syncytiotrophoblast cytoplasm (P = 0.0001) in preeclamptic cases as compared with control. Also, the significant upregulated concentration of HIF‐1α and downregulated PIGF was observed in serum samples of preeclamptic woman (P = 0.0001). Thus, there was a significant direct negative correlation between HIF‐1α and PIGF both at tissue and serum level (P < 0.01).
Conclusion
The direct inverse association between HIF‐1α and PIGF in serum and placental tissues may be responsible for the low oxidative stress and endothelial dysfunction, leading to the pathogenesis of preeclampsia.
Keywords: preeclampsia, hypoxia, HIF‐1α, PIGF
INTRODUCTION
Preeclampsia is a heterogeneous pregnancy disorder that is characterized by hypertension and proteinuria mostly developing in late pregnancy. It affects 2–8% of pregnancies worldwide 1, 2. The incident rate of preeclampsia is 2.8% higher in developing countries like India as compared to that of developed nations 3. In spite of extensive research in the pathophysiology of this disease, the etiology is still poorly understood. It may be due to insufficient adaptation of decidual and intramyometrial portion of spiral arterioles or due to shallow trophoblastic invasion, resulting in reduced uteroplacental blood flow leading to placental hypoxia 4. Placental hypoxia results in the release of several mediators into maternal circulation, which causes endothelial dysfunction prevailing in preeclampsia. The key mediator of the hypoxic condition is the hypoxia inducible factor 1 (HIF‐1). HIF‐1 is involved in transcription of many oxygen‐dependent genes that encode for proteins associated with angiogenesis and cell metabolism. HIF‐1 consists of two dimeric subunits α and β. HIF‐1α is hypoxia inducible, while HIF‐1 β is constitutively expressed 5. During low oxygen conditions, HIF‐1α is highly expressed and helps in development of placenta in early gestation. Overexpression of HIF‐1 α has been observed in many inflammatory disorders, including cancer and preeclampsia 6. Hypoxia induces angiogenesis by regulating angiogenic proteins such as vascular endothelial growth factor (VEGF), placental growth factor (PIGF) and FLT‐1. The change in concentration of these proteins causes angiogenic imbalance, leading to endothelial damage and the onset of preeclampsia. PIGF is a proangiogenic protein and member of the vascular endothelial growth factor (VEGF) family. It is one of the key molecules in angiogenesis and vasculogenesis especially during embryogenesis and placental trophoblast is the main source of PIGF throughout the gestational period of pregnancy 7. It shares structural as well as amino acid sequence similarity with VEGF, but PIGF has binding affinity only for VEGF receptor 1 (VEGFR‐1; 8). The inter‐ and intramolecular cross‐talk between the VEGFR‐1 and VEGFR‐2 is regulated by PIGF. It binds to VEGFR‐1 and displaces VEGF from this receptor, which results in activation and intermolecular transphosphorylation of VEGFR‐2 thereby amplify the VEGF‐induced angiogenesis 9. But soluble form of VEGFR‐1 (known as sFLT‐1) inhibit the interaction of PIGF and its receptor that causes endothelial dysfunction, a manifestation of preeclampsia 10. Although extensive studies have been conducted to investigate the expression of these two proteins in preeclampsia, but hardly any report is available regarding the serum analysis of HIF‐1α in preeclamptic patients as well as the association of HIF‐1α with the angiogenic protein PIGF at tissue and serum level. Thus, this study focused on the quantification of serum levels of HIF‐1α and finding the possible molecular link between the expression of HIF‐1α and PIGF in preeclamptic Indian women compared with normotensive pregnancy.
MATERIAL AND METHODOLOGY
Sample
Placental tissue as well as serum samples of preeclamptic patients and age‐matched controls were retrieved from the department of Obstetrics and Gynaecology, Safdarjung Hospital, New Delhi, India. All the preeclamptic patients included in our study had hypertension (systolic BP > 140 mmHg and diastolic BP > 90 mmHg) and proteinuria exceeding 0.3 g/day after 20th week of gestation. Ethical approval was obtained from the institutional ethics committee of VMMC and Safdarjung Hospital, New Delhi, India and written informed consent was obtained from all the patients prior to sample collection. The placental samples were rinsed with normal saline to remove excess blood followed by perfusion with buffered 10% formalin (pH 7.0) and then processed for paraffin embedding sectioning for Immunohistochemistry (IHC). The serum was separated from blood samples by centrifugation at 12,000 rpm for 15 min for enzyme‐linked immunosorbent assay (ELISA) analysis and stored at −20°C. The clinicopathological parameters of patients are summarized in Table 1. All the placental samples (n = 45 + 45) and serum samples (n = 80 + 80) were processed for IHC and ELISA, respectively.
Table 1.
Clinicopathological Characteristics of the Study Groups
| Clinicopathological parameters | Control (n = 45) | Preeclampsia (n = 45) | P‐value |
|---|---|---|---|
| Maternal age (years; mean ± SE) | 21.53 ± 0.410 | 22.29 ± 0.446 | 0.216 |
| Gestational age (weeks; mean ± SE) | 37.62 ± 0.485 | 38.58 ± 0.366 | 0.120 |
| Gravidity (no. /%) | |||
| 1 | 22/48.9 | 23/51.1 | 0.891 |
| 2 | 18/40.0 | 16/35.6 | |
| 3 | 5/11.1 | 6/13.3 | |
| Parity (no. /%) | |||
| 0 | 22/48.9 | 21/46.7 | 0.945 |
| 1 | 18/40.0 | 18/40.0 | |
| 2 | 5/11.1 | 6/13.3 | |
| Systolic blood pressure (mmHg; mean ± SE) | 117.73 ± 0.368 | 145.07 ± 1.730 | 0.0001* |
| Diastolic blood pressure (mmHg; mean ± SE) | 77.40 ± 0.520 | 100.13 ± 1.247 | 0.0001* |
| Urine albumin (no. /%) | |||
| 0 | 45/100 | 0/0.0 | 0.0001* |
| 1 | 0/0.0 | 15/33.3 | |
| 2 | 0/0.0 | 19/42.2 | |
| 3 | 0/0.0 | 11/24.4 | |
| Edema (no. /%) | |||
| 0 | 45/100 | 31/68.9 | 0.0001* |
| 1 | 0/0.0 | 14/31.1 | |
| Baby weight (kg; mean ± SE) | 3.133 ± 0.0360 | 2.016 ± 0.0360 | 0.0001* |
| Mode of delivery (no. /%) |
|
|
0.292 |
Data are represented as mean ± standard error or number/percentage as per requirement. Mann–Whitney U‐test and Wilcoxon W test (Asymp. sig. (two‐tailed]).
*P < 0.05 is considered significant.
Immunohistochemistry
Formalin‐fixed and paraffin‐embedded tissue sections of 5 μ thickness were collected and processed for conventional histological assessment by Haematoxylin and Eosin (H & E) staining. The immunoexpression of HIF‐1α and PIGF was analyzed by immunohistochemistry. In brief, the sections were deparaffinized in xylene, dehydrated by graded alcohols followed by blocking of endogenous peroxidises activity by 0.03% H2O2 in methanol for 15 min. The slides were then retrieved for antigen in 10 mM citrate buffer (pH 6.0) by heating the sections at 900 W for 15 min and at 360 W for 10 min in microwave oven. Further, the sections were incubated overnight with primary antibodies for HIF‐1α (concentration 1:70 [mouse anti‐human monoclonal HIF‐1α antibody ab1–250; Abcam, Inc., Cambridge, UK]) and PIGF (concentration 1:40 [rabbit anti‐human polyclonal PIGF antibody ab97618; Abcam, Inc.) in humid chamber, at 4°C. Next day, after brief washing with TBS (Tris buffer saline, pH 7.4) , the serial sections were incubated with Envision™ (dextran conjugated with peroxidase and incorporated with molecules of secondary antibody against both immunoglobulins of mouse and rabbit, Dako Cytomation, Glostrup, Denmark) for 1 hr at room temperature. The 3,3‐diaminobenzidine hydrochloride (DAB) was used for the chromogenic visualization reaction, counterstained with Mayer's hematoxylin, and mounted. The serial sections were then examined under light microscope (Olympus BX‐51, Japan). In the negative control, isotype‐specific immunoglobulin G was used in place of primary antibody.
ELISA
The serum samples of control (n = 80) and preeclampsia (n = 80) were quantitatively analyzed for both HIF‐1α and PIGF by sandwich ELISA. For HIF‐1α, Cusabio ELISA kit was used and manufacturer's protocol was followed. The minimum detectable dose of human HIF‐1α was typically less than 15.6 pg/ml. For PIGF, the ELISA kit of R&D System (Minneapolis, Minnesota) was used and manufacturer's protocol was followed. The minimum detectable dose of human PIGF was typically less than 7 pg/ml.
Scoring of Immunohistochemical Staining
Evaluation of immunohistochemistry for all proteins was performed on two arbitrarily chosen slide of each case (both preeclamptic and control). Result of immunohistochemistry was evaluated by two observers and followed the scoring criterion given by Tripathi et al. 11. Protein expression was semiquantified with regard to the intensity of cell staining graded as 0–3(“0” for negative staining if there is total absence, “1” for mild staining, “2’ for moderate, “3” for intense staining). H‐score was calculated for both preeclampsia and control using the formula, H‐score = P(S + 1), where P represent the aggregate percentage of stained cells and S represents the intensity of the cell.
Statistical Analysis
Statistical analysis was performed using SPSS 18.0 statistics software (SPSS, Inc., Chicago, IL). These data are represented as mean ± standard error. Chi‐square test was carried out to determine the significance of protein expression among control and preeclamptic placenta. The significance of clinicopathological parameters of preeclamptic patients and control was determined with Wilcoxon's W‐test and Mann–Whitney's U‐test (Asymp. sig. [two‐tailed]). The associations between proteins HIF‐1α and PIGF was explored using Pearson correlation test (two‐tailed). The P values less than 0.05 were regarded as significant for Mann–Whitney's U‐test (Asymp. sig. [two‐tailed]) and less than 0.01 was considered significant for Pearson correlation test [two‐tailed].
The receiver operating characteristic (ROC) analysis was also carried out to find the significance of immunohistochemistry result. For ELISA results, Wilcoxon's W‐test and Mann–Whitney's U‐test (Asymp. sig. [two‐tailed]) as well as box plot were performed. Results were considered significant, when P was less than 0.05.
RESULTS
The clinicopathological characteristics, such as baby weight, urine albumin, edema, systolic and diastolic blood pressure of preeclamptic mother, were found to be statistically significant (P = 0.000), while there was no significant difference between control and preeclamptic group in terms of maternal age, gestational age, gravidity, and parity (P = 0.216, P = 0.120, P = 0.0.891, P = 0.945, respectively; Table 1).
Immunohistochemistry
The IHC analysis revealed that HIF‐1α was localized both in nucleus and cytoplasm of syncytiotrophoblast in preeclamptic placental tissue. The nuclear expression was intense in 64.4% (29/45), moderate in 22.2% (10/45), and mild in 13.3% (6/45) cases. However, the intensity of HIF‐1α in cytoplasm was moderate in 8.9% (4/45) and mild in 91.1% (41/45) of preeclamptic group. While in the control group, cytoplasmic expression was noticed. The expression was intense in 42.2% (19/45), moderate in 40.0% (18/45), and mild in 17.8% (8/45). And only 6.6% (3/45) cases showed the nuclear expression of HIF‐1α in control. Both nuclear and cytoplasmic expression was found to be significant in preeclampsia as well as in control ones (P = 0.0001; Fig. 1A to C; Table 2).
Figure 1.
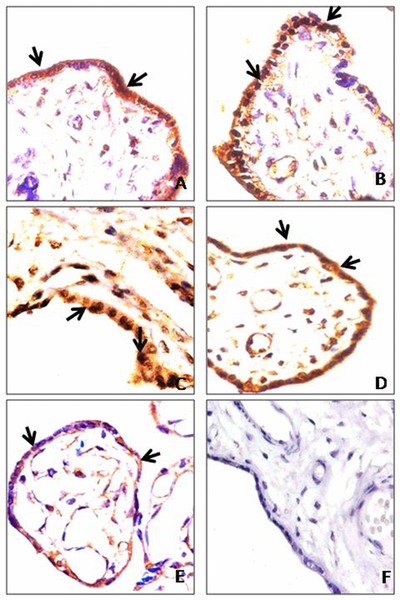
(A) Control placenta (40 weeks) showing moderate cytoplasmic expression of HIF‐1α in syncytiotrophoblast; (B and C) preeclamptic placenta (38 and 36 weeks) showing nuclear accumulation of HIF‐1α in syncytiotrophoblast; (D) control placenta (32 weeks) showing moderate cytoplasmic expression of PIGF in syncytiotrophoblast; (E) preeclamptic placenta (32 weeks) showing mild cytoplasmic expression of PIGF in syncytiotrophoblast; (F) negative control incubated with IgG showing placental villi. Arrow shows the expression of protein. Magnification: 400×.
Table 2.
Assessment of IHC for HIF‐1 α and PIGF in Control and Preeclamptic Placenta
| Protein | Expression | Preeclampsia (N = 45) n (%) | Control (N = 45) n (%) | P values |
|---|---|---|---|---|
| HIF‐1α nuclear | No staining | 0 (0.0) | 42(93.3) | 0.0001* |
| Mild | 06 (13.3) | 3 (6.6) | 0.0001* | |
| Moderate | 10 (22.2) | 0 (0.0) | 0.0001* | |
| Intense | 29 (64.4) | 0 (0.0) | 0.0001* | |
| HIF‐1α cytoplasmic | No staining | 4(8.9) | 0(0.0) | 0.0001* |
| Mild | 41 (91.1) | 8 (17.8) | 0.0001* | |
| Moderate | 0 (0.0) | 18 (40.0) | 0.0001* | |
| Intense | 0 (0.0) | 19(42.2) | 0.0001* | |
| PIGF cytoplasmic | Mild | 43(95.6) | 0 (0.0) | 0.0001* |
| Moderate | 2 (4.4) | 20(44.4) | 0.0001* | |
| Intense | 0 (0.0) | 25 (55.6) | 0.0001* |
Pearson chi‐square test (Asymp. sig. [two‐tailed]).
* P < 0.05 is considered significant.
N, no. of cases.
The immunoreactivity of PIGF revealed a significant downregulation in the cytoplasm of syncytiotrophoblast of preeclamptic placenta. The intensity was mild in 95.6% (43/45) and moderate in 4.4% (2/45) of the study group. However, the cytoplasmic expression was moderate in 44.4% (20/45) and intense in 55.6% (25/45) of the control placenta. In both control and preeclamptic cases, PIGF expression was found to be significant (P = 0.0001; Fig. 1D and E; Table 2). Although cytotrophoblast was present in some of the studied preeclamptic cases, the immunoexpression of HIF‐1α and PIGF was not significant.
The H‐score analysis was carried out for control as well as experimental group to assess the intensity of HIF‐1α and PIGF at nuclear and cytoplasmic level. The mean and standard error value of HIF‐1 α was higher (312.22 ± 11.132) for nuclear expression in preeclamptic group, but in control the mean and standard error was more for cytoplasmic expression (275.69 ± 11.872; Table 3, Fig. 2A). In case of PIGF, the preeclamptic cytoplasmic expression was lower (177.51 ± 3.275) than the control group (314.20 ± 7.305; Table 3, Fig. 2B).
Table 3.
H‐Score Assessment of HIF‐1 α and PIGF in Control and Preeclamptic Placenta
| HIF‐1 α | PIGF | ||
|---|---|---|---|
| Study group | Nucleus (mean ± SD) | Cytoplasm (mean ± SD) | Cytoplasm (mean ± SD) |
| Control (N = 45) | 102.13 ± 4.875 | 275.69 ± 11.872 | 314.20 ± 7.305 |
| Preeclampsia (N = 45) | 312.22 ± 11.132 | 167.62 ± 4.353 | 177.51 ± 3.275 |
| P–value | 0.0001 | 0.0001 | 0.0001 |
Mann–Whitney U‐test and Wilcoxon W test (Asymp. sig. [two‐tailed]).
P < 0.05 is considered significant.
N, no. of cases.
Figure 2.
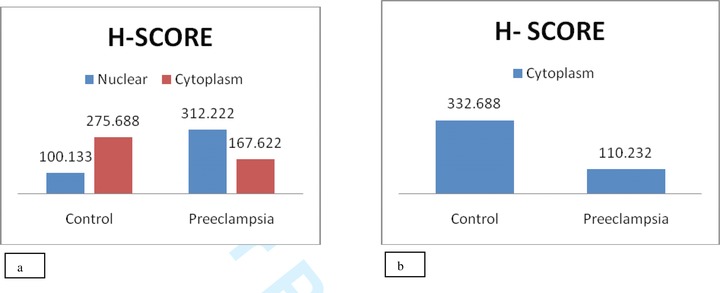
Comparison of immunohistochemical H‐score analysis. (A) HIF‐1α expression in cytoplasm and nucleus of syncytiotrophoblast cells of control and preeclamptic patients. (B) PIGF expression in cytoplasm of syncytiotrophoblast cells of control and preeclamptic group.
The ROC curve analysis revealed the significant nuclear expression of HIF‐1α in preeclamptic group as compared with controls (P = 0.0001, Fig. 3A) with area under curve of 0.989, specificity 88.9%, and sensitivity 88.9%. However, PIGF was significantly downregulated in preeclamptic group as compared with controls (P = 0.0001, Fig. 3B) with area under curve of 0.962, specificity 82.2%, and sensitivity 82.2%.
Figure 3.
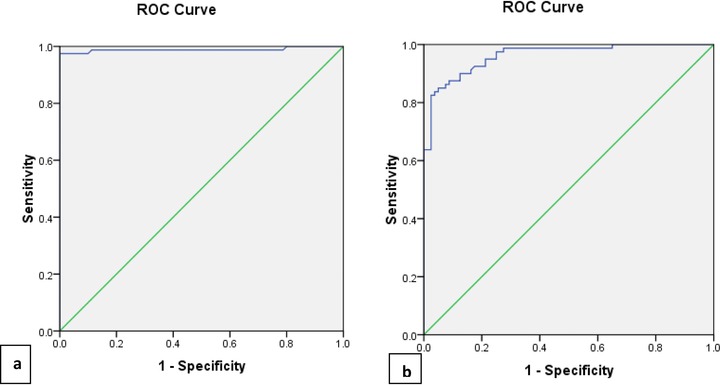
ROCs curve showing the expression of HIF‐1α and PIGF to differentiate preeclamptic group from control. (A) HIF‐1α (AUC = 0.989, sensitivity = 88.9%, specificity = 88.9%). (B) PIGF (AUC = 0.962, Sensitivity = 82.2%, specificity = 82.2%).
ELISA
The Mann–Whitney test showed that the serum concentration of HIF‐1α was higher (mean = 6.581 pg/ml) in preeclamptic cases than that of control group (mean= 4.947 pg/ml). For PIGF, the serum concentration of control was increased (mean = 6.333 pg/ml), while that of preeclampsia was lower (mean = 3.939 pg/ml). The concentration of HIF‐1α and PIGF in serum of control and preeclamptic patients was represented as mean ± standard error in Table 4.
Table 4.
HIF‐1 α and PIGF Concentration (pg/ml) in Serum of Control and Preeclamptic Cases
| Study group | HIF‐1 α (mean ± SE) | PIGF (mean ± SE) |
|---|---|---|
| Control (N = 80) | 4.947 ± 0.045 | 6.335 ± 0.093 |
| Preeclampsia (N = 80) | 6.581 ± 0.030 | 3.939 ± 0.179 |
| P‐value | 0.0001* | 0.0001* |
Mann–Whitney U‐test and Wilcoxon W test (Asymp. sig. [two‐tailed]).
*Significant.
P < 0.05 is considered significant.
N, no. of cases.
According to Box plot analysis, the serum levels of HIF‐1α were higher (median 6.640 pg/ml) in preeclamptic cases as compared with the control ones (median = 4.945 pg/ml; Fig. 4A); however, for PIGF, the serum levels were found to be downregulated significantly (median = 3.434 pg/ml) in preeclamptic group than that of control (median = 7.462 pg/ml; Fig. 4B).
Figure 4.
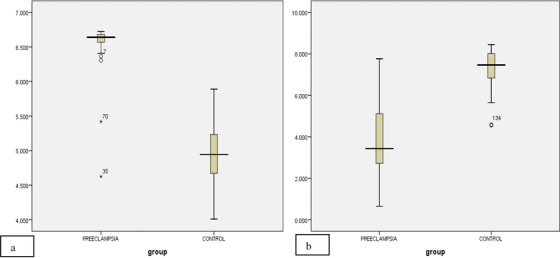
Box plot showing the serum concentration (pg/ml) of (A) HIF‐1α and (B) PIGF in control and preeclamptic patients. The solid bar indicates median, upper, and lower limits of box, 75th and 25th percentiles; upper and lower bars, maximum and minimum values (P < 0.05).
Correlation Between HIF‐1α and PIGF
The significant negative association was noted between HIF‐1α nuclear and PIGF cytoplasmic expression in patients suffering from preeclampsia (r = −0.196, P < 0.05; Table 5). There was also a significant inverse correlation between the serum levels of HIF‐1α and PIGF in preeclamptic group (r = −0.220, P < 0.05; Table 6).
Table 5.
Correlation of HIF‐1 α and PIGF in Placental Tissue of Preeclamptic Patients
| Protein | No. of cases | HIF‐1 α nuclear | PIGF cytoplasm |
|---|---|---|---|
| HIF‐1 α nuclear | 45 | 1 |
|
| PIGF cytoplasm | 45 |
|
1 |
Pearson correlation test (two‐tailed); r, correlation coefficient.
*Correlation is highly significant at 0.01 level.
Table 6.
Correlation of HIF‐1 α and PIGF in Serum Samples of Preeclamptic Group
Pearson correlation test (two‐tailed); r, correlation coefficient.
*Correlation is highly significant at 0.01 level.
DISCUSSION
Hypoxic environment is essential for the invasion and infiltration of cytotrophoblast into the maternal decidua for maintenance of materno‐fetal circulation at early periods of pregnancy. But its prevalence in later stages of pregnancy causes several complications that may lead to maternal and fetal morbidity and mortality 12, 13. Under hypoxic conditions, HIF‐1α plays a vital role in multiple physiological responses, such as erythropoiesis, glycolysis, and also affects the transcription of VEGF gene that counteract angiogenesis 14. It also has an important role in vascularization and survival of embryos, pulmonary vascular remodeling, and vascularization of tumors 15. Caniggia and Winter noticed the increased expression of HIF‐1α mRNA and protein in placental tissues of preeclamptic patients 16. Several researchers also showed the overexpression of HIF‐1α in preeclamptic human placenta that modulates the pathogenesis of preeclampsia 17, 18, 19. It has been observed that during hypoxic conditions, HIF‐1α gets stabilized and translocates to nucleus from cytoplasm 20, 21. In the present study, the immunoreactivity of HIF‐1α in preeclamptic placental tissue showed significant nuclear expression (P = 0.0001) in syncytiotrophoblast. However, cytoplasmic expression of HIF‐1α with low intensity was also observed in preeclamptic tissues (P = 0.0001). While in the control group, significant cytoplasmic immunoexpression was noticed in syncytiotrophoblast (P = 0.0001). Thus, there is possibility of significant translocation of HIF‐1α from cytoplasm to nucleus, leading to low oxidative stress prevailing in preeclampsia.
Many researchers have worked on serum concentration of HIF‐1α in different pathological conditions, such as cancer. Zhang et al. observed the overexpression of HIF‐1α in serum of primary hepatocellular carcinoma (PHC) 22. To the best of our knowledge, no investigator has evaluated the expression of HIF‐1α in serum samples of preeclamptic patients. Therefore, in this study quantification of the serum concentration of HIF‐1α in maternal circulation of both control and preeclamptic patients was carried out. The ELISA analysis showed that the mean serum concentration of HIF‐1α was higher (mean = 6.581 pg/ml) in preeclamptic cases than that of control group (mean = 4.947 pg/ml), which infer that the maternal serum levels of HIF‐1α also get significantly elevated during pathogenesis of preeclampsia (P < 0.05).
PIGF, an angiogenic protein, plays an important role in placental development and is expressed in villous cytotrophoblast and syncytiotrophoblasts in placenta 23. Various researchers had noticed the increased expression of PIGF gene in normal trophoblast, while the expression was reduced in preeclampsia 24. Torry et al. found in normal pregnancy PIGF is highly expressed in trophoblasts and its expression is significantly downregulated in preeclampsia by low oxygen tension 25. The same group in their another study observed the decreased PIGF mRNA expression in preeclamptic trophoblast 26. In the present study, the immunoreactivity of PIGF revealed a significant downregulation of PIGF (P = 0.0001) in the cytoplasm of syncytiotrophoblast of preeclamptic placenta. However, the intensity of cytoplasmic expression in the control placenta was lower in comparison to preeclamptic cases (P = 0.0001). Therefore, this study coincides with the reports provided by previous authors 24, 25, 26 regarding the decreased expression of PIGF in the preeclamptic conditions. Furthermore, maternal serum levels of PIGF were quantified to assess the quantity of PIGF in the preeclamptic conditions. Many researchers had suggested that the abnormal serum levels of PIGF in preeclampsia result in improper trophoblast invasion and the generalized maternal endothelial dysfunction, which leads to preeclampsia 27, 28. Some authors also noticed the increased serum concentration of PIGF in normal pregnancy and decreased concentration in preeclampsia 29, 30. Livingston et al. also observed that the median concentrations of PIGF were significantly lower in pregnancies complicated by severe preeclampsia than in control 31. Torry et al. also reported the significantly reduced concentration of maternal serum placental growth factor (P < 0.0001) in women with preeclampsia than normotensive controls 32. In this study, the significant low concentration of serum PIGF was noticed in preeclamptic group as compared to normal pregnant woman (P = 0.0001), which suggests that the decrease in the serum concentration of PIGF may be responsible for the improper trophoblast invasion, leading to endothelial dysfunction in preeclampsia.
Several researchers have proved that the overexpression of HIF‐1α is associated with the increased maternal serum concentration of soluble Fms‐like tyrosine kinase 1 (sFlt1) during hypoxic conditions 33. High circulating levels of sFlt1 exerts an antiangiogenic state that is associated with low levels of proangiogenic factors, such as PIGF, and inhibition of PIGF with its receptor VEGFR‐1, causing endothelial dysfunction in preeclampsia 34. Other researchers proved that VEGF and PIGF are dysregulated in preeclampsia due to high levels of sVEGFR‐1, which leads to impaired placental angiogenesis 35. The downregulation of trophoblast PIGF gene expression was mediated by hypoxia, which is differentially regulated by HIF‐1, and had been observed in previous studies 36, 37. HIF‐1α affects the expression of PIGF gene that is dependent on the type of cell and the conditions prevailing in a cell 38. Gobble et al. reported that hypoxia decreases PIGF gene transcription that results in the decreased value of PIGF via mechanisms independent of HIF‐1 24. In this study, the significant negative association was noted between HIF‐1α nuclear and PIGF cytoplasmic expression in patients suffering from preeclampsia (r = −0.752, P = 0.0001). Also, the statistical analysis of the serum levels of HIF‐1α and PIGF showed a significant negative correlation between the proteins in preeclampsia (r = −0.220, P < 0.05). The expression of VEGFR‐1 (sFLT‐1) was also studied in another study and it was upregulated both at tissue and serum level. There was no statistical association found between VEGFR‐1 and PIGF (data not shown in this report). Therefore, we hypothesized that the upregulation of HIF‐1α and downregulation of PIGF in serum and placental tissues may be directly associated with the pathogenesis of preeclampsia. Under pathological conditions, due to failure of replacement of vascular smooth muscle and endothelial cells of spiral artery by cytotrophoblast, the spiral artery remodeling do not occur, leading to the prevalence of hypoxic conditions in late gestation of pregnancy. During this period, HIF‐1α may be responsible for the molecular modification of several mediators involved in pathophysiology of pregnancy disorders. In addition, the abnormal expression of angiogenic factor, such as PIGF, may be associated with the hypoxic conditions prevailing in preeclampsia via activation of HIF signaling pathway.
In light of the present study on preeclampsia, the inverse correlation of PIGF and HIF‐1 α may offer tremendous promise in screening of preeclamptic patients. Several treatment strategies may be aimed at preventing endothelial dysfunction by using PIGF. Further studies on different angiogenic factors and hypoxic proteins have also been designed on larger cohorts to understand the exact molecular mechanisms regulating the pathogenesis of preeclampsia. This may allow the development of new preventive strategies for pregnancy complications, such as preeclampsia.
ACKNOWLEDGEMENT
The authors are grateful to Indian Council of Medical Research (Ref. No. 5/7/587/11‐RHN) New Delhi, India for providing the grants without which the work was not possible. They thank their technician Rajeshwar Singh for his support during the study.
Grant sponsor: Indian Council of Medical Research (ICMR).
REFERENCES
- 1. Altinbas S, Togrul C, Orhan A, Yucel M, Danisman N. Increased MPV is not a significant predictor for preeclampsia during pregnancy. J Clin Lab Anal 2012;26:403–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kaleli I, Kaleli B, Demir M, Yildirim B, Cevahir N, Demir S. Serum levels of neopterin and interleukin‐2 receptor in women with severe preeclampsia. J Clin Lab Anal 2005;19:36–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. World Health Organization . Make every mother and child count. The world health report 2005; Geneva, Switzerland. [Google Scholar]
- 4. Kanasaki K, Kalluri R. The biology of preeclampsia. Kidney Int 2009;76:831–837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Qingdong K, Costa M. Hypoxia‐inducible factor‐1 (HIF‐1). Mol Pharmacol 2006;70:1469–1480. [DOI] [PubMed] [Google Scholar]
- 6. Rajakumar A, Brandon HM, Daftary A, Ness R, Conrad KP. Evidence for the functional activity of hypoxia‐inducible transcription factors overexpressed in preeclamptic placentae. Placenta 2004;25:763–769. [DOI] [PubMed] [Google Scholar]
- 7. Iyer S, Leonidas DD, Swaminathan G, et al. The crystal structure of human placenta growth factor‐1 (PIGF‐1), an angiogenic protein, at 2.0 a resolution. J Biol Chem 2001;276:53–61. [DOI] [PubMed] [Google Scholar]
- 8. Park JE, Chen HH, Winer J, Houck KA, Ferrara N. Placenta growth factor. Potentiation of vascular endothelial growth factor bioactivity, in vitro and in vivo, and high affinity binding to Flt‐1 but not to Flk‐1/KDR. J Biol Chem 1994;269:46–54. [PubMed] [Google Scholar]
- 9. Autiero M, Waltenberger J, Communi D, et al. Role of PIGF in the intra‐ and intermolecular cross talk between the VEGF receptors FLT1 and FLK1. Nat Med 2003;9:936–943. [DOI] [PubMed] [Google Scholar]
- 10. Autiero M, Luttun A, Tjwa M, Carmeliet P. Placental growth factor and its receptor, vascular endothelial growth factor receptor‐1: Novel targets for stimulation of ischemic tissue revascularization and inhibition of angiogenic and inflammatory disorders. J Thromb Haemost 2003;1:1356–1370. [DOI] [PubMed] [Google Scholar]
- 11. Tripathi R, Rath G, Jain A, Salhan S. Soluble and membranous vascular endothelial growth factor receptor‐1 in pregnancies complicated by preeclampsia. Ann Anat 2008;190:477–489. [DOI] [PubMed] [Google Scholar]
- 12. Fox H. The villous trophoblasts as an index of placental ischaemia. J Obstet Gynaecol Brit Comm 1964;71:885–893. [DOI] [PubMed] [Google Scholar]
- 13. Kingdom JCP, Kaufmann P. Oxygen and placental villous development: Origins of fetal hypoxia. Placenta 1997;18:613–621. [DOI] [PubMed] [Google Scholar]
- 14. Semenza GL. Hypoxia‐inducible factor 1: Master regulator of O2 homeostasis. Curr Opin Genet Dev 1998;8:588–594. [DOI] [PubMed] [Google Scholar]
- 15. Semenza GL. Expression of hypoxia‐inducible factor 1: Mechanisms and consequences. Biochem Pharmacol 2000;59:47–53. [DOI] [PubMed] [Google Scholar]
- 16. Caniggia I, Winter JL. Adriana and luisa castellucci Award 2001. Hypoxia‐inducible factor 1: Oxygen regulation of trophoblast differentiation in normal and preeclamptic pregnancies‐a review. Placenta 2002;23:S47–S57. [DOI] [PubMed] [Google Scholar]
- 17. Rajakumar A, Brandon HM, Daftary A, Ness R, Conrad KP. Evidence for the functional activity of hypoxia‐inducible transcription factors overexpressed in preeclamptic placentae. Placenta 2004;25:763–769. [DOI] [PubMed] [Google Scholar]
- 18. Iwagaki S, Yokoyama Y, Tang L, Takahashi Y, Nakagawa Y, Tamaya T. Augmentation of leptin and hypoxia‐inducible factor 1α mRNAs in the preeclamptic placenta. Gynecol Endocrinol 2004;18:263–268. [DOI] [PubMed] [Google Scholar]
- 19. Tal R, Shaish A, Barshack I, Charcon SP, et al. Effects of hypoxia‐inducible factor‐1‐overexpression in pregnant mice. Am J Pathol 2010; 177:2950–2962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Huang LE, Arany Z, Livingston DM, Bunn HF. Activation of hypoxia inducible transcription factor depends primarily upon redox‐sensitive stabilization of its alpha subunit. J Biol Chem 1996;271:53–59. [DOI] [PubMed] [Google Scholar]
- 21. Kallio PJ, Okamoto K, O'Brien S, et al. Signal transduction in hypoxic cells: Inducible nuclear translocation and recruitment of the CBP/p300 coactivator by the hypoxia‐inducible factor‐1alpha. EMBO J 1998;17:6573–6586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zhang DH, Zhu WG, Yue S. Expression of HIF‐1alpha in serum of primary hepatocellular carcinoma and effects on tumor invasion and metastasis. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi 2011;27(5):551–552, 554. [PubMed] [Google Scholar]
- 23. Torry DS, Mukherjea D, Arroyo J, Torry RJ. Expression and function of placenta growth factor: Implications for abnormal placentation. J Soc Gynecol Investig 2003;10:178–188. [DOI] [PubMed] [Google Scholar]
- 24. Gobble RM, Groesch KA, Chang M, Torry RJ, Torry DS. Differential regulation of human PlGF gene expression in trophoblast and nontrophoblast cells by oxygen tension. Placenta 2009;30:869–875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Torry DS, Mukherjea D, Arroyo J, Torry RJ. Expression and function of placenta growth factor: Implications for abnormal placentation. J Soc Gynecol Investig 2003;10:178–188. [DOI] [PubMed] [Google Scholar]
- 26. Torry DS, Hinrichs M, Torry RJ. Determinants of placental vascularity. Am J Reprod Immunol 2004;51:257–268. [DOI] [PubMed] [Google Scholar]
- 27. Levine RJ, Maynard SE, Qian C, et al. Circulating angiogenic factors and the risk of preeclampsia. N Engl J Med 2004; 350:672–683. [DOI] [PubMed] [Google Scholar]
- 28. Baumann MU, Bersinger NA, Surbek DV. Serum markers for predicting pre‐eclampsia. Mol Aspects Med 2007;28:227–244. [DOI] [PubMed] [Google Scholar]
- 29. Robinson JC, Johnson DD, Chang EY, Armstrong DM, Wang W. Evaluation of placenta growth factor and soluble Fms‐like tyrosine kinase 1 receptor levels in mild and severe preeclampsia. Am J Obstet Gynecol 2006;255–259. [DOI] [PubMed] [Google Scholar]
- 30. Tidwell SC, Ho HN, Chiu WH, Torry RJ, Torry DS. Low maternal serum levels of placenta growth factor as an antecedent of clinical preeclampsia. Am J Obstet Gynecol 2001; 184:1267–1272. [DOI] [PubMed] [Google Scholar]
- 31. Livingston JC, Haddad B, Gorski LA, et al. Placenta growth factor is not an early marker for the development of severe preeclampsia. Am J Obstet Gynecol 2001; 184:1218–1220. [DOI] [PubMed] [Google Scholar]
- 32. Torry DS, Wang HS, Wang TH, Caudle MR, Torry RJ. Preeclampsia is associated with reduced serum levels of placenta growth factor. Am J Obstet Gynecol 1998;179:1539–1544. [DOI] [PubMed] [Google Scholar]
- 33. Nevo O, Soleymanlou N, Wu Y, et al. Increased expression of sFlt‐1 in in vivo and in vitro models of human placental hypoxia is mediated.by HIF‐1. Am J Physiol Regul Integr Comp Physiol 2006;291:1085–1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Maynard SE, Min JY, Merchan J, et al. Excess placental soluble fms‐like tyrosine kinase 1 (sFlt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia. J Clin Invest 2003;111:649–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ahmad S, Ahmed A. Elevated placental soluble vascular endothelial growth factor receptor‐1 inhibits angiogenesis in preeclampsia. Circ Res 2004;95:884–891. [DOI] [PubMed] [Google Scholar]
- 36. Li H, Gu B, Zhang Y, Lewis DF, Wang Y. Hypoxia‐induced increase in soluble Flt‐1 production correlates with enhanced oxidative stress in trophoblast cells from the human placenta. Placenta 2005;26:210–217. [DOI] [PubMed] [Google Scholar]
- 37. Ahmed A, Dunk C, Ahmad S, Khaliq A. regulation of placental vascular endothelial growth factor (VEGF) and placenta growth factor (PLGF) and soluble flt‐1 by oxygen‐ a review. Placenta 2000;21:16–24. [DOI] [PubMed] [Google Scholar]
- 38. Kelly BD, Hackett SF, Hirota K, et al. Cell type‐specific regulation of angiogenic growth factor gene expression and induction of angiogenesis in nonischemic tissue by a constitutively active form of hypoxia‐inducible factor 1. Circ Res 2003;93:1074–1081. [DOI] [PubMed] [Google Scholar]


