Abstract
Background
The cobas u 701, a new automated image‐based urine sediment analyzer, was introduced recently. In this study, we compared its performance with that of UF‐1000i flow cytometry and manual microscopy in the examination of urine sediments.
Methods
Precision, linearity, and carry‐over were determined for the two urine sediment analyzers. For a comparison of the method, 300 urine samples were examined by the automated analyzers and by manual microscopy using a KOVA chamber.
Results
Within‐run coefficients of variation (CVs) for the control materials were 7.0–8.8% and 1.7–5.7% for the cobas u 701 and UF‐1000i systems, respectively. Between‐run CVs were 8.5–9.8% and 2.7–5.4%, respectively. Both instruments showed good linearity and negligible carry‐over. For red blood cells (RBC), white blood cells (WBC), and epithelial cells (EPI), the overall concordance rates within one grade of difference among the three methods were good (78.6–86.0%, 88.7–93.8%, and 81.3–90.7%, respectively). The concordance rate for casts was poor (66.5–68.9%).
Conclusion
Compared with manual microscopy, the two automated sediment analyzers tested in this study showed satisfactory analytical performances for RBC, WBC, and EPI. However, for other urine sediment particles confirmation by visual microscopy is still required.
Keywords: automated urine sediment analyzer, cobas u 701, UF‐1000i, urine microscopy, urine sediment
INTRODUCTION
Routine urinalysis consists of two major components: physicochemical determinations (specific gravity and reagent strip measurements) and a microscopic examination of urine sediment for evidence of hematuria, pyuria, casts (cylindruria), crystalluria, etc. 1. However, microscopic analysis is labor intensive, time consuming, and necessitates a high level of expertise for accurate interpretations. Nonetheless, the precision of manual microscopy is low because of variations in sample preparation and particle‐counting techniques. Despite these disadvantages, microscopic analysis is still used worldwide and remains the reference method for examining the cells and particles in urine 2, 3.
Several automated urine sediment analysis systems based on technologies such as flow cytometry 4 and image‐based analysis systems 3, 5 are currently used in clinical laboratories. These automated systems save both labor and time, are more precise, and allow greater sample throughput than manual microscopic analysis 2, 6, 7. The cobas u 701 (Roche Diagnostics International, Rotkreuz, Switzerland) is a recently introduced automated urine sediment analysis system. It analyzes urine sediments from images captured by a digital camera.
In this study, we compared the analytical performances of the cobas u 710 urine analyzer and another automated system, the UF‐1000i (Sysmex Corporation, Kobe, Japan), which uses flow cytometry technology, with that of manual microscopic analysis using a standardized KOVA cell chamber (Hycor, California).
MATERIALS AND METHODS
Urine Specimens
This study was conducted using 300 fresh urine samples selected randomly from inpatients and outpatients and submitted to our laboratory for diagnostic urinalysis. After arrival in the laboratory, each sample was divided into three tubes, two of which were used for the cobas u 701 and UF‐1000i automated analyzers and the other for manual microscopic examination, as the reference method. All samples were analyzed within 2 h of their receipt in our laboratory. The design of the study was approved by our Hospital Ethics Committee.
Manual Microscopic Examination
Manual microscopic sediment examination was performed according to the CLSI guideline GP16‐A3 8. A standardized KOVA cell chamber system was used for microscopic counting. Each urine sample (10 ml) was centrifuged at 400 × g for 5 min, after which 9 ml of the supernatant was removed. The pellet was resuspended and one drop was placed in the KOVA cell chamber. Red blood cells (RBC), white blood cells (WBC), and epithelial cells (EPI) were counted in ten small grids of the middle section of the chamber at 400× magnification using bright‐field microscopy. Other particles were evaluated qualitatively in low‐power fields (LPFs) at 100× magnification. Each sample was counted twice by two independent, experienced laboratory technologists.
AUTOMATED URINE SEDIMENT ANALYSES
cobas u 701 System
The cobas u 701 system analyzes and counts urinary particles sedimented by centrifugation in a specially designed disposable cobas u cuvette (Roche Diagnostics International). The sample tubes are placed in racks and introduced into the cobas u 701 analyzer, where 200 μl of urine sample are transferred to the cuvette and then centrifuged at 260 × g for 10 s. A built‐in camera takes 15 digital images (∼400× magnification) from different locations within the cuvette. All particles in the 15 digital images represent those contained in 2.3 μl of native urine. The images are evaluated using high‐quality image Auto Image Evaluation Module (AIEM) processing software (version 8, Roche Diagnostics International). The results are classified within three different grading systems based on manufacturer‐defined criteria: (i) quantitative counting of RBC and WBC; (ii) semiquantitative counting of bacteria, squamous epithelial cells, nonsquamous epithelial cells, and hyaline casts; and (iii) qualitative determination of pathologic casts, crystals, yeast, sperm, and mucus. The quantitative results can be expressed as counts/μl or per high‐power field (HPF). The semiquantitative results can be expressed as −, 1+, 2+, or 3+. Samples with very high particle counts are flagged for review of the respective images by an operator. In this study, the raw data (counts/μl) of all urine particles were reviewed and compared with the data obtained from the other two methods. EPI counts were calculated as the sum of the number of squamous and nonsquamous epithelial cells; casts were calculated as the sum of hyaline and pathologic casts.
UF‐1000i System
The UF‐1000i is an automated flow cytometry based system for counting particles in native urine 9. The analyzer automatically aspirates urine samples and stains them with two different fluorochromes—one for bacteria, the other for leukocytes and yeast‐like cells—in two dedicated analytic channels. Irradiation of the stained urine samples by a laser beam results in the production of forward‐scattering light, side‐scattering light, and side‐scattering fluorescence signals by the particles in the samples. The measured parameters are converted into electrical signals that allow the identification of the different elements present in the urine sample. The UF‐1000i is capable of detecting and counting RBC, WBC, bacteria, EPI, casts, pathologic casts, crystals, yeast‐like cells, small round cells, mucus, and spermatozoa. Particles that cannot be classified within one of these categories are counted as “other cells.” The requirement for microscopic review is indicated by review messages (flags) generated by the software.
Precision, Linearity, and Carry‐over
The within‐run and between‐run precisions, linearity, and carry‐over of the two automated sediments analyzers were determined only for RBC and WBC. For the within‐run precision, two levels of quality‐control materials, recommended by each manufacturer, were analyzed 20 times during the same day: Liquicheck™ urinalysis control (Bio‐Rad, Hercules, CA) for the cobas u 701 and UF II control (Sysmex Corp) for the UF‐1000i system. Between‐run precision was analyzed at two levels for each control on 20 separate days throughout the course of the study. The precision of each measurement method was assessed as the coefficient of variation (CV).
To determine linearity, different pooled urines were used under each analytical measuring range (RBC, 1,800/μl, WBC, 900/μl for cobas u 701; RBC, 5,000/μl, WBC, 5,000/μl for UF‐1000i) as high‐level samples for each parameter. The supernatant of centrifuged normal urine was used as the low‐level sample. Low‐ and high‐level samples were mixed in the following ratios: 0:4, 1:3, 2:2, 3:1, and 4:0 and the five dilutions were analyzed in duplicate.
Carry‐over was determined by analyzing a series of four identical high‐level pooled samples (H1, H2, H3, and H4) immediately followed by a series of four identical low‐level pooled samples (L1, L2, L3, and L4). The relative percentage of carry‐over was calculated according to the formula: carry‐over % = [L1 − (L3 + L4) / 2] / [(H2 + H3) / 2 − (L3 + L4) / 2] × 100.
Analysis of the Results
For quantitative RBC, WBC, and EPI data, the normality of the parameters was tested by the Kolmogorov–Smirnov test. Spearmen's rank correlation was used to evaluate correlations (r, correlation coefficient) for the nonparametric data among the three urinary analysis methods. A P‐value < 0.05 was considered to indicate statistical significance. Passing–Bablok regression analysis was also performed. Bias and 95% limits of agreement were determined using a Bland–Altman analysis 10.
To compare the performances of the three methods, the results from each were converted into counts per field and then classified either within the appropriate range for each parameter or as negative or positive (Table 1). The results of RBC, WBC, EPI, and casts were considered concordant if they were within one grade of difference. The pairwise concordance rate among the three systems was defined by the percent of results within ±1 grade from the best‐fit line. Spearman's rank correlation was calculated for other particles. Statistical analysis was performed using SPSS v 18.0 (IBM Corp., Armonk, NY) and Microsoft Excel 2010 (Microsoft Corporation, Redmond, Washington) with Analyze‐it v.3.90.7 (Analyze‐it Software Ltd., Leeds, UK).
Table 1.
Grading Systems Used in the Analysis of Urine Particles by the Three Methods
| Parameters | Ranges | |||||
|---|---|---|---|---|---|---|
| RBC, WBC, EPI (counts/HPF) | 0–1 | 2–4 | 5–10 | 11–20 | 21–30 | >30 |
| Casts (counts/LPF) | 0–1 | 2–3 | 4–5 | 6–10 | 11–20 | >20 |
| Bacteria | – | 1+ | 2+ | 3+ | ||
| Others | – | + | ||||
RBC, red blood cells; WBC, white blood cells; EPI, epithelial cells; HPF, high‐power field; LPF, low‐power field.
RESULTS
Precision, Linearity, and Carry‐over
The between‐run and within‐run precisions of the two automated sediments analyzers for RBC and WBC are shown in Table 2. The standard deviations (SDs) and CVs of the low control for the cobas u 701 were not calculated because this sample served as the negative control sample and the cell counts were zero. The within‐run CVs for the high‐quality control materials as measured by the cobas u 701 were 7.0% and 8.8% for RBC and WBC, respectively. The CVs obtained with the UF‐1000i were lower, 2.6% and 1.7%, respectively. For the between‐run CVs, the results were similar (Table 2). Within each analytical measurement range, both automated sediment analyzers showed clinically relevant linearities for RBC and WBC (Fig. 1). The carry‐over rates for RBC and WBC were 0% and 0.22% using the cobas u 701 analyzer and −0.21% and 0.13% using the UF‐1000i, respectively.
Table 2.
Coefficient of Variations Obtained Using the cobas u 701 and UF‐1000i Automated Analyzers
| Within‐run precision | Between‐run precision | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Low | High | Low | High | ||||||
| Mean ± SD (counts/μl) | CV (%) | Mean ± SD (counts/μl) | CV (%) | Mean ± SD (counts/μl) | CV (%) | Mean ± SD (counts/μl) | CV (%) | ||
| cobas u 701 | RBC | 0.00 ± a | a | 452.11 ± 31.41 | 7.0 | 0.00 ± a | a | 417.13 ± 37.13 | 8.5 |
| WBC | 0.00 ± a | a | 104.87 ± 9.22 | 8.8 | 0.00 ± a | a | 103.40 ± 10.16 | 9.8 | |
| UF‐1000i | RBC | 40.07 ± 2.10 | 5.2 | 187.75 ± 4.81 | 2.6 | 41.19 ± 2.23 | 5.4 | 188.36 ± 6.68 | 3.6 |
| WBC | 42.22 ± 2.42 | 5.7 | 784.25 ± 13.41 | 1.7 | 40.94 ± 1.87 | 4.6 | 774.58 ± 21.20 | 2.7 | |
CV, coefficient of variation; SD, standard deviation.
SDs and CVs were not calculated because the mean RBC and WBC values were 0.
Figure 1.
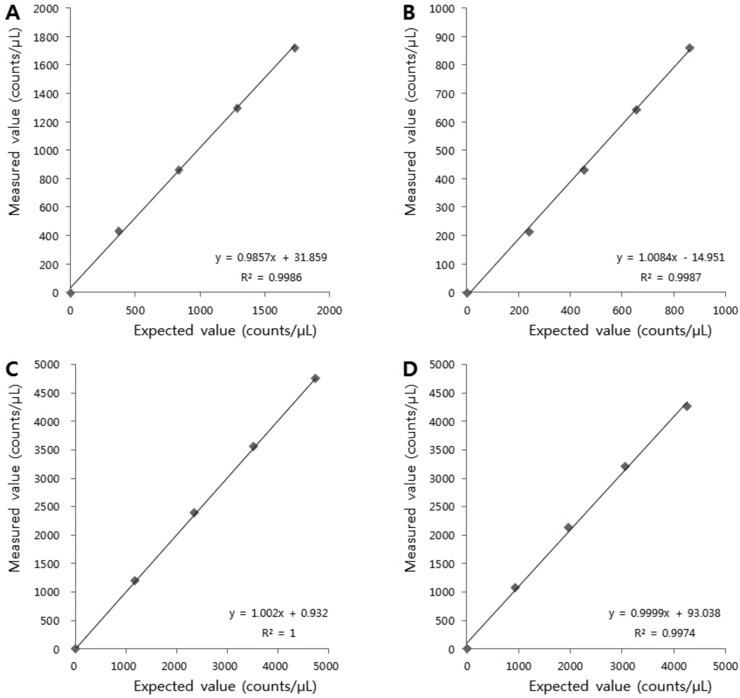
Linearities of RBC and WBC counts as analyzed by the cobas u 701 (A, RBC; B, WBC) and UF‐1000i (C, RBC; D, WBC) automated systems.
Comparison Study
Of the 300 urine samples, six samples with very high particle density (RBC, three samples; WBC, two samples; crystals, one sample) did not yield results by the cobas u 701. In an additional 37 samples, the counts were above the analytical measuring range (RBC, 15 samples; WBC, 22 samples) of the cobas u 701. Thus, 257 results were included in the final data processing.
The Passing–Bablok regression lines of the WBC, RBC, and EPI data from the two automated sediments analyzers and the manual microscopic analysis are listed in Table 3. The correlation coefficients for RBC, WBC, and EPI between the cobas u 701 and the manual microscopic analysis were 0.867, 0.893, and 0.867, respectively (Table 3). In a comparison of the UF‐1000i and the manual microscopic analysis, the correlation coefficients were 0.839, 0.928, and 0.710, respectively. Bland–Altman analysis revealed that slightly fewer cells were counted using the cobas u 701 analyzer than manually using the KOVA chamber (RBC, 14.2/μl; WBC, 15.3/μl; EPI, 17.5/μl) and the UF‐1000i (RBC, 9.1/μl; WBC, 16.0/μl; EPI, 6.3/μl) (Table 3).
Table 3.
Results of the Passing–Bablok Regression and Bland–Altman Analyses of RBC, WBC, and EPI for the Three Methods
| Limits of agreementc | |||||||
|---|---|---|---|---|---|---|---|
| Method y | Method x | Slope | Intercept (counts/μl) | r a | Biasb (counts/μl) | Low (counts/μl) | High (counts/μl) |
| RBC | |||||||
| Manual | cobas u 701 | 1.418 | 1.525 | 0.867 | 14.2 | −362.0 | 390.4 |
| Manual | UF‐1000i | 0.968 | −1.742 | 0.839 | 5.1 | −329.0 | 339.1 |
| cobas u 701 | UF‐1000i | 0.772 | −2.547 | 0.725 | −9.1 | −298.4 | 280.2 |
| WBC | |||||||
| Manual | cobas u 701 | 1.250 | 0.000 | 0.893 | 15.3 | −89.9 | 120.5 |
| Manual | UF‐1000i | 1.004 | −0.912 | 0.928 | −0.7 | −137.0 | 135.6 |
| cobas u 701 | UF‐1000i | 0.823 | −0.910 | 0.880 | −16.0 | −119.3 | 87.4 |
| EPI | |||||||
| Manual | cobas u 701 | 2.291 | −0.008 | 0.867 | 17.5 | −72.7 | 107.6 |
| Manual | UF‐1000i | 1.500 | −2.850 | 0.710 | 11.2 | −88.1 | 110.5 |
| cobas u 701 | UF‐1000i | 0.643 | −1.104 | 0.663 | −6.3 | −47.3 | 34.7 |
r, Spearman's correlation coefficient.
P < 0.01.
Mean difference between x and y.
Limits of agreement = bias ± 1.96 SD
The pairwise concordance rates within one grade of difference for RBC, WBC, EPI, and casts of the three methods are summarized in Table 4. For the RBC data, 86.0% of the samples were within one grade of difference when the manual analysis and the cobas u 701 system were compared, leaving 36 samples with substantial disagreement (Table 5). Among these latter samples, 28 were two or more grades lower than determined using manual microscopic analysis. In a comparison of the manual analysis and the UF‐1000i system, the concordance rate within one grade of difference was 83.7% and 16 of the 42 samples with substantial disagreement were two or more grades lower than determined manually.
Table 4.
Pairwise Concordance Rate (%) within One Grade Difference for the Urine Sediment Results Obtained Using the Three Methods
| Within one grade difference (%) | ||||
|---|---|---|---|---|
| RBC | WBC | EPI | Casts | |
| Manual vs. cobas u 701 | 86.0 | 88.7 | 83.3 | 68.9 |
| Manual vs. UF‐1000i | 83.7 | 93.8 | 81.3 | 67.3 |
| cobas u 701 vs. UF‐1000i | 78.6 | 92.2 | 90.7 | 66.5 |
Table 5.
Comparison of the Pairwise Results for RBC among the Two Automated Instruments and Manual Microscopy
| Manual microscopy (counts/HPF) | |||||||
|---|---|---|---|---|---|---|---|
| cobas u 701 (counts/HPF) | 0–1 | 2–4 | 5–10 | 11–20 | 21–30 | >30 | Sum |
| 0–1 | 88 | 39 | 9 | 2 | 0 | 0 | 138 |
| 2–4 | 2 | 7 | 11 | 4 | 0 | 0 | 24 |
| 5–10 | 1 | 1 | 8 | 6 | 4 | 2 | 22 |
| 11–20 | 0 | 1 | 0 | 10 | 5 | 7 | 23 |
| 21–30 | 0 | 0 | 1 | 1 | 2 | 4 | 8 |
| >30 | 0 | 1 | 1 | 3 | 1 | 36 | 42 |
| Manual microscopy (counts/HPF) | |||||||
| UF‐1000i (counts/HPF) | 0–1 | 2–4 | 5–10 | 11–20 | 21–30 | >30 | Sum |
| 0–1 | 64 | 10 | 3 | 2 | 0 | 0 | 79 |
| 2–4 | 13 | 21 | 10 | 1 | 0 | 0 | 45 |
| 5–10 | 8 | 12 | 11 | 9 | 2 | 2 | 44 |
| 11–20 | 5 | 4 | 4 | 8 | 5 | 6 | 32 |
| 21–30 | 1 | 1 | 2 | 2 | 4 | 9 | 19 |
| >30 | 0 | 1 | 0 | 4 | 1 | 32 | 38 |
| cobas u 701 (counts/HPF) | |||||||
| UF‐1000i (counts/HPF) | 0–1 | 2–4 | 5–10 | 11–20 | 21–30 | >30 | Sum |
| 0–1 | 72 | 3 | 2 | 1 | 0 | 1 | 79 |
| 2–4 | 34 | 8 | 2 | 1 | 0 | 0 | 45 |
| 5–10 | 19 | 8 | 10 | 5 | 1 | 1 | 44 |
| 11–20 | 10 | 4 | 3 | 9 | 3 | 3 | 32 |
| 21–30 | 2 | 1 | 4 | 4 | 4 | 4 | 19 |
| >30 | 1 | 0 | 1 | 3 | 0 | 33 | 38 |
( ) represents the number of cases within one grade difference and (
) represents the number of cases within one grade difference and ( ) the number with the same grade.
) the number with the same grade.
For the WBC data, 88.7% of the samples were within one grade of difference when the manual and the cobas u 701 systems were compared, leaving 29 samples with substantial disagreement (Table 6). Among these latter samples, 24 were two or more grades lower than determined using manual microscopic analysis. A comparison of the manual analysis and the UF‐1000i system showed that the concordance rate within one grade of difference was 93.8%. Seven of the 16 samples with substantial disagreement were two or more grades lower than determined manually.
Table 6.
Comparison of the Pairwise Results for WBC among the Two Instruments and Manual Microscopy
| Manual microscopy (counts/HPF) | |||||||
|---|---|---|---|---|---|---|---|
| cobas u 701 (counts/HPF) | 0–1 | 2–4 | 5–10 | 11–20 | 21–30 | >30 | Sum |
| 0–1 | 67 | 25 | 6 | 0 | 0 | 0 | 98 |
| 2–4 | 4 | 12 | 20 | 7 | 0 | 1 | 44 |
| 5–10 | 1 | 4 | 19 | 12 | 1 | 3 | 40 |
| 11–20 | 2 | 0 | 5 | 10 | 10 | 6 | 33 |
| 21–30 | 0 | 0 | 0 | 2 | 1 | 3 | 6 |
| >30 | 0 | 1 | 0 | 1 | 0 | 34 | 36 |
| Manual microscopy (counts/HPF) | |||||||
| UF‐1000i (counts/HPF) | 0–1 | 2–4 | 5–10 | 11–20 | 21–30 | >30 | Sum |
| 0–1 | 68 | 15 | 0 | 0 | 0 | 0 | 83 |
| 2–4 | 3 | 21 | 18 | 0 | 0 | 0 | 42 |
| 5–10 | 3 | 4 | 26 | 16 | 1 | 0 | 50 |
| 11–20 | 0 | 1 | 2 | 14 | 8 | 6 | 31 |
| 21–30 | 0 | 0 | 2 | 2 | 3 | 8 | 15 |
| >30 | 0 | 1 | 2 | 0 | 0 | 33 | 36 |
| cobas u 701 (counts/HPF) | |||||||
| UF‐1000i (counts/HPF) | 0–1 | 2–4 | 5–10 | 11–20 | 21–30 | >30 | Sum |
| 0–1 | 73 | 8 | 1 | 1 | 0 | 0 | 73 |
| 2–4 | 18 | 20 | 4 | 0 | 0 | 0 | 18 |
| 5–10 | 6 | 11 | 23 | 10 | 0 | 0 | 6 |
| 11–20 | 0 | 4 | 7 | 17 | 3 | 0 | 0 |
| 21–30 | 0 | 0 | 5 | 4 | 3 | 3 | 0 |
| >30 | 1 | 1 | 0 | 1 | 0 | 33 | 1 |
( ) represents the number of cases within one grade of difference and (
) represents the number of cases within one grade of difference and ( ) the number with the same grade.
) the number with the same grade.
For the EPI data, 83.3% of the samples from the manual analysis and the cobas u 701 system were within one grade of difference, leaving 43 samples with substantial disagreement (Table 7). Among these, 41 samples were two or more grades lower than determined using manual microscopic analysis. A comparison between the latter and UF‐1000i system showed that the concordance rate within one grade of difference was 81.3% and 35 of 48 samples with substantial disagreement were two or more grades lower than determined manually.
Table 7.
Comparison of the Pairwise Results for EPI among the Two Instruments and Manual Microscopy
| Manual microscopy (counts/HPF) | |||||||
|---|---|---|---|---|---|---|---|
| cobas u 701 (counts/HPF) | 0–1 | 2–4 | 5–10 | 11–20 | 21–30 | >30 | Sum |
| 0–1 | 128 | 35 | 11 | 2 | 0 | 0 | 176 |
| 2–4 | 1 | 5 | 14 | 8 | 2 | 0 | 30 |
| 5–10 | 1 | 1 | 2 | 10 | 4 | 4 | 22 |
| 11–20 | 0 | 0 | 0 | 2 | 9 | 10 | 21 |
| 21–30 | 0 | 0 | 0 | 0 | 1 | 4 | 5 |
| >30 | 0 | 0 | 0 | 1 | 0 | 2 | 3 |
| Manual microscopy (counts/HPF) | |||||||
| UF‐1000i (counts/HPF) | 0–1 | 2–4 | 5–10 | 11–20 | 21–30 | >30 | Sum |
| 0–1 | 97 | 22 | 2 | 2 | 0 | 0 | 123 |
| 2–4 | 23 | 13 | 19 | 5 | 3 | 1 | 64 |
| 5–10 | 8 | 3 | 5 | 13 | 8 | 11 | 48 |
| 11–20 | 2 | 3 | 1 | 3 | 3 | 3 | 15 |
| 21–30 | 0 | 0 | 0 | 0 | 2 | 5 | 7 |
| >30 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| cobas u 701 (counts/HPF) | |||||||
| UF‐1000i (counts/HPF) | 0–1 | 2–4 | 5–10 | 11–20 | 21–30 | >30 | Sum |
| 0–1 | 118 | 4 | 1 | 0 | 0 | 0 | 123 |
| 2–4 | 38 | 19 | 7 | 0 | 0 | 0 | 64 |
| 5–10 | 14 | 7 | 12 | 14 | 0 | 1 | 48 |
| 11–20 | 6 | 0 | 2 | 4 | 1 | 2 | 15 |
| 21–30 | 0 | 0 | 0 | 3 | 4 | 0 | 7 |
| >30 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
( ) represents the number of cases within one grade of difference and (
) represents the number of cases within one grade of difference and ( ) the number with the same grade.
) the number with the same grade.
For casts, the concordances among the three methods were poorer than for other particles (Table 4). In the manual microscopic analysis, 168 samples were classified as “0–1/HPF,” vs. 117 and 81 samples according to the cobas u 701 and UF‐1000i systems, respectively (Table 8).
Table 8.
Comparison of the Pairwise Results for Casts among the Two Instruments and Manual Microscopy
| Manual microscopy (counts/LPF) | |||||||
|---|---|---|---|---|---|---|---|
| cobas u 701 (counts/LPF) | 0–1 | 2–3 | 4–5 | 6–10 | 11–20 | >20 | Sum |
| 0–1 | 102 | 0 | 5 | 9 | 0 | 1 | 117 |
| 2–3 | 18 | 0 | 6 | 4 | 0 | 0 | 28 |
| 4–5 | 16 | 0 | 3 | 0 | 3 | 0 | 22 |
| 6–10 | 24 | 0 | 5 | 3 | 4 | 2 | 38 |
| 11–20 | 4 | 0 | 5 | 5 | 7 | 4 | 25 |
| >20 | 4 | 0 | 1 | 2 | 6 | 14 | 27 |
| Manual microscopy (counts/LPF) | |||||||
| UF‐1000i (counts/LPF) | 0–1 | 2–3 | 4–5 | 6–10 | 11–20 | >20 | Sum |
| 0–1 | 73 | 0 | 6 | 2 | 0 | 0 | 81 |
| 2–3 | 41 | 0 | 8 | 4 | 2 | 1 | 56 |
| 4–5 | 17 | 0 | 3 | 3 | 3 | 0 | 26 |
| 6–10 | 17 | 0 | 2 | 6 | 5 | 3 | 33 |
| 11–20 | 9 | 0 | 2 | 5 | 4 | 5 | 25 |
| >20 | 11 | 0 | 4 | 3 | 6 | 12 | 36 |
| cobas u 701 (counts/LPF) | |||||||
| UF‐1000i (counts/LPF) | 0–1 | 2–3 | 4–5 | 6–10 | 11–20 | >20 | Sum |
| 0–1 | 65 | 6 | 3 | 5 | 1 | 1 | 81 |
| 2–3 | 22 | 12 | 3 | 15 | 2 | 2 | 56 |
| 4–5 | 9 | 3 | 6 | 1 | 6 | 1 | 26 |
| 6–10 | 8 | 2 | 4 | 10 | 5 | 4 | 33 |
| 11–20 | 6 | 2 | 4 | 4 | 4 | 5 | 25 |
| >20 | 7 | 3 | 2 | 3 | 7 | 14 | 36 |
( ) represents the number of cases within one grade of difference and (
) represents the number of cases within one grade of difference and ( ) the number with the same grade.
) the number with the same grade.
The correlation coefficients and results of the comparisons for other particles are summarized in Tables 9 and 10. There was a good correlation between the cobas u 701 analyzer and manual microscopic analysis for bacteria, and moderate correlations for casts, crystals, yeast, sperm, and mucus.
Table 9.
Correlation Coefficients for Other Particles between Manual Microscopy and the Two Automated Sediment Analyzers
| cobas u 701 (postreview) | UF‐1000i | ||
|---|---|---|---|
| Parameters | r | r | |
| Manual microscopy | Casts | 0.561a | 0.509a |
| Bacteria | 0.648a | 0.636a | |
| Pathologic casts | NDb | ND | |
| Crystals | 0.563a (0.652a) | 0.460a | |
| Yeast | 0.404a (0.606a) | 0.297a | |
| Sperm | 0.533a (0.673a) | 0.529a | |
| Mucus | 0.435a (0.439a) | 0.297a |
r, Spearman's correlation coefficient.
P < 0.01.
ND, not determined. Pathologic casts were not differentiated by manual microscopy.
Table 10.
Analytic Comparisons of the Results Obtained for Other Particles using the cobas u 701 and the UF‐1000i Automated Systems Vs. Manual Microscopy
| Bacteriaa | Pathologic cast | Crystal | Yeast | Sperm | Mucus | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| cobas u 701 | UF‐1000i | cobas u 701 | UF‐1000i | cobas u 701 | UF‐1000i | cobas u 701 | UF‐1000i | cobas u 701 | UF‐1000i | cobas u 701 | UF‐1000i | |
| Sensitivity | 77.8 | 62.1 | NDb | NDb | 68.2 | 36.4 | 61.1 | 38.9 | 85.7 | 28.6 | 83.3 | 17.9 |
| Specificity | 84.6 | 90.4 | NDb | NDb | 94.5 | 98.3 | 91.2 | 93.7 | 95.6 | 100.0 | 75.5 | 98.7 |
| PPV | 88.1 | 90.5 | NDb | NDb | 53.6 | 66.7 | 34.4 | 31.8 | 35.3 | 100.0 | 30.9 | 62.5 |
| NPV | 72.1 | 61.8 | NDb | NDb | 96.9 | 94.3 | 96.9 | 95.3 | 99.6 | 98.0 | 98.3 | 90.8 |
Data are given in percentages. PPV, positive predictive value; NPV, negative predictive value.
Sensitivity, specificity, PPP, and NPV were calculated based on the qualitative results.
ND, not determined. Pathologic casts were not differentiated by manual microscopy.
DISCUSSION
The cobas u 701 system is a new, automated, digital imaging based microscopy system for urinalysis that operates according to principles similar to those used in manual microscopic analysis. After the centrifugation of native urine in the cobas u cuvette, 15 digital images are captured per sample at approximately 400× magnification and then stored. All particles in the images are analyzed by the AIEM software. The results can be reported automatically without review or after reclassification of the particles in the images by the operator.
The precisions of two automated urine sediments analysis systems were tested with control materials rather than pooled urines because of the instability of the latter. Thus, only RBC and WBC were analyzed, because there were no coexisting particles in the each control materials. Both the cobas u 701 and the UF‐1000i automated sediment analyzers demonstrated satisfactory within‐run and between‐run precisions, as reported previously 3, 7, 11. However, the between‐run and within‐run CVs were lower in the UF‐1000i than in the cobas u 701 system. Low‐level precisions could not be compared because the respective control materials for the u 701 were negative for any particles. Nevertheless, within‐run and between‐run precisions at lower concentrations were better with the UF‐1000i than with the cobas u 701 analyzer. In other reports comparing the performances of the UF‐100 (previous version of UF‐1000i) and the iQ‐200 (another automated urine sediment analysis system using image‐based analysis), the former also showed better precision, especially for low‐level samples 6, 7.
RBC and WBC are clinically more important than other particles and are frequently present in high numbers in urine samples. Therefore, linearity and carry‐over were determined only for these particles. Linearity was evaluated using the high‐level RBC and WBC samples, which reduced interference by other elements present in the samples. Both automated sediments analyzers showed excellent linearities within each analytical measurement range, with negligible carry‐over for RBC and WBC.
The correlations among the three methods for RBC, WBC, and EPI were good. For RBC and EPI, the correlation coefficients between the cobas u 701 system and manual microscopic analysis (0.867 and 0.867, respectively) were higher than those between the UF‐1000i system and the manual method (0.839 and 0.710, respectively). The differences were statistically significant. Bland–Altman analysis showed a tendency towards lower counts for the cobas u 701 than for the UF‐1000i and manual methods, but the reasons for these differences are unclear. Wah et al. compared the iQ200 system and manual microscopic analysis using Fuchs–Rosenthal counting chambers 11. Despite the good correlation between the two approaches for RBC, WBC, and EPI, fewer cells were detected by the iQ200 system than by manual microscopic analysis. By contrast, Chien et al. reported higher RBC and WBC counts with the iQ200 and UF‐100 systems than with manual microscopic analysis using a KOVA chamber 7. Because the inaccuracy of manual cell counting methods can contribute to these differences, in this study the imprecision in manual microscopic analysis was minimized by using a standardized cell chamber, as was the case in previous studies 4, 11, 12. Nonetheless, other sources of error, such as incomplete centrifugation and counting variations, also likely contributed to these differences 8. Unlike other automated sediments analyzers, the principle of the cobas u 701 is similar to that of manual microscopic analysis. In the latter, observers adjust the focus of the microscope to take into account all particles in the viewing field. However, microscopy relies on two‐dimensional images to recognize cellular components in urine sediment, whereas both the KOVA chamber and the cobas u cuvette enclose a three‐dimensional volume. Therefore, some particles that are out of focus in the microscopy image might not be counted. In our reviews of the stored images, this was indeed the case, especially with samples containing a high particle density. In addition, in the cobas u 701 analyzer, particles that resemble the target cells might not be accurately identified. We found that the cobas u 701 occasionally failed to recognize a ghost RBC or RBC clumping or misidentified yeast cells as RBC. Similar inaccuracies have been reported for other image analysis systems 3, 5. Zaman et al. showed that these could be resolved by cross‐checking both the sorted images and the strip reader results (RBC vs. hemoglobin; WBC vs. leukocyte esterase; casts vs. protein; bacteria vs. nitrite) 3.
In most clinical laboratories, the results of urine sediments are reported as counts per HPF or LPF. To enable more practical comparisons of the results from manual microscopic analysis and the two automated sediment analyzers, we converted the particle concentrations in urine into units counted per HPF or LPF according to the recommendations of each manufacturer and the CLSI GP16‐A3 8.
The overall concordance rates among the three methods for the major elements (RBC, WBC, and EPI) were good (78.6–86.0%, 88.7–93.8%, and 81.3–90.7%, respectively). Our data were similar to those reported elsewhere 13, 14. For RBC and EPI, agreement was better between the cobas u 701 analyzer and manual microscopy than between the UF‐1000i analyzer and the manual method (86.0% and 83.3% vs. 83.7% and 81.3%, respectively). For WBC, concordance was slightly lower for the former than for the latter comparison (88.7% vs. 93.8%). However, compared with the UF‐1000i, the results for the major elements obtained using the cobas u 701 system more frequently ranked lower than those derived from manual microscopic analysis. These findings were consistent with the results of the Bland–Altman analysis.
For the 37 samples with counts above the analytical measurement ranges of the cobas u 701 (15 RBC samples and 22 WBC samples), the system correctly classified the counts as “>30/HPF.” For the other major elements, concordance with manual microscopic analysis was similar for the cobas u 701 and the UF‐1000i systems (data not shown). However, using the cobas u 701, data could not be obtained for six samples with very high particle density, whereas the UF‐1000i showed good concordances for RBC, WBC, and EPI except in two samples (data not shown). Thus, an advantage of the UF‐1000i over the cobas u 701 is its wider analytical measuring ranges for RBC and WBC.
For casts, the agreement among the three methods was poor. This can be explained by the highly variable appearance, size, shape, and stability of urinary casts 1. In addition, the two automated sediments analyzers differ in their classification of casts. While the cobas u 710 recognizes hyaline casts and pathologic casts, the UF‐1000i recognizes casts and pathologic casts. Thus, direct comparisons are difficult. Our approach was to compare the sum of hyaline and pathologic casts of the cobas u 701 and the cast results of the UF‐1000i, although this may ultimately have influenced the poor concordance. Nonetheless, higher cast counts were detected by the two automated analyzers than by manual microscopic analysis, perhaps because of the inherent inconsistencies in manual microscopic analysis. Hyaline casts, for example, are translucent and thus might not be counted during bright‐field microscopic analysis. In addition, some casts might be destroyed during the centrifugation step in manual preparation 1, 14. Neither of the automated analyzers could identify the cast type 8; instead, they simply flagged the presence of pathologic casts. The respective manufacturers recommend that all casts be verified by manual review. In this study, we did not classify the cast type during manual microscopic analysis. However, among the 80 samples classified by the cobas u 701 as positive for pathologic casts, a review of the stored images confirmed only 41 as positive. An analysis of these 41 samples by the UF‐1000i identified only 24 as positive.
Bacteria, crystals, yeast, sperm, and mucus were classified semiquantitatively or qualitatively according to the cutoffs suggested by the manufacturers of the two automated analyzers. The correlation coefficients of the cobas u 701 system for bacteria, crystals, yeast, sperm, and mucus were higher than those of the UF‐1000 system (Table 9). After manual editing of the images assigned as positive by the cobas u 701 analyzer, the correlation coefficients for crystals, yeast, sperm, and mucus improved. The sensitivity of the cobas u 701 for these particles was also higher than that of the UF‐1000i (Table 10), although manual microscopic analysis identified only a small number of positive samples (crystals, 22 samples; yeast, 18 samples; sperm seven samples; mucus, 28 samples). Nevertheless, for crystals, yeast, sperm, and mucus, the negative predictive values of the two automated sediments analyzers were similar and better than the respective positive predictive values. This relatively high likelihood of false‐positive results points out the need for further improvements in particle recognition to obtain more accurate results.
Automated sediment analyzers have been widely used in clinical laboratories and are replacing traditional manual microscopic examination. Nonetheless, visual microscopic examination is still required in many cases, especially for pathologic casts and crystals 2, 5. The effective use of automated sediment analyzers in clinical laboratories requires a reduction in the total review rate to within reasonable limits. Recently, Yuan et al. compared the UF‐1000i system with manual microscopic analysis and subsequently proposed a classification algorithm, based on the supervised machine learning approach 15. This algorithm reduced the microscopic review rate to ∼30%. However, image analysis systems such as the cobas u 701 store all of the captured images of urine sediments, unlike the UF‐1000i. Thus, for review, operators can use these images and thereby avoid the need for manual microscopic re‐examination. This function is one of the advantages of the cobas u 701.
In summary, both automated urine sediment analyzers showed satisfactory analytical performances for RBC, WBC, and EPI and the results correlated well with those obtained manually by KOVA cell chamber counting. In addition, automated analysis was less time consuming and labor intensive than manual microscopic analysis, especially regarding specimen preparation. However, for other particles, such as pathologic casts and crystals, the data obtained using automated sediments analyzers must still be confirmed visually by microscopy.
CONFLICT OF INTEREST
No potential conflict of interest relevant to this article were reported.
Grant sponsor: Roche Diagnostics Korea.
REFERENCES
- 1. McPherson RA, Ben‐Ezra J. Basic examination of urine McPherson RA, Pincus MR. (eds.). Henry's Clinical Diagnosis and Management by Laboratory Methods, 22nd edition, Philadelphia: Saunders; 2011. p 445–479. [Google Scholar]
- 2. Yuksel H, Kilic E, Ekinci A, Evliyaoglu O. Comparison of fully automated urine sediment analyzers H800‐FUS100 and Labumat‐Urised with manual microscopy. J Clin Lab Anal 2013;27:312–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Zaman Z, Fogazzi GB, Garigali G, Croci MD, Bayer G, Kranicz T. Urine sediment analysis: Analytical and diagnostic performance of sediMAX®—A new automated microscopy image‐based urine sediment analyser. Clin Chim Acta 2010;411:147–154. [DOI] [PubMed] [Google Scholar]
- 4. Manoni F, Tinello A, Fornasiero L, et al. Urine particle evaluation: A comparison between the UF‐1000i and quantitative microscopy. Clin Chem Lab Med 2010;48:1107–1111. [DOI] [PubMed] [Google Scholar]
- 5. Altekin E, Kadicesme O, Akan P, et al. New generation IQ‐200 automated urine microscopy analyzer compared with KOVA cell chamber. J Clin Lab Anal 2010;24:67–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mayo S, Acevedo D, Quinones‐Torrelo C, Canos I, Sancho M. Clinical laboratory automated urinalysis: Comparison among automated microscopy, flow cytometry, two test strips analyzers, and manual microscopic examination of the urine sediments. J Clin Lab Anal 2008;22:262–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chien TI, Kao JT, Liu HL, et al. Urine sediment examination: A comparison of automated urinalysis systems and manual microscopy. Clin Chim Acta 2007;384:28–34. [DOI] [PubMed] [Google Scholar]
- 8. Clinical and Laboratory Standards Institute . Urinalysis; Approved Guideline—Third Edition. CLSI Document GP16‐A3. Wayne, PA: CLSI; 2009. [Google Scholar]
- 9. Wang J, Zhang Y, Xu D, Shao W, Lu Y. Evaluation of the Sysmex UF‐1000i for the diagnosis of urinary tract infection. Am J Clin Pathol 2010;133:577–582. [DOI] [PubMed] [Google Scholar]
- 10. Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1986;i:307–310. [PubMed] [Google Scholar]
- 11. Wah DT, Wises PK, Butch AW. Analytic performance of the iQ200 automated urine microscopy analyzer and comparison with manual counts using Fuchs‐Rosenthal cell chambers. Am J Clin Pathol 2005;123:290–296. [PubMed] [Google Scholar]
- 12. Ottiger C, Huber AR. Quantitative urine particle analysis: Integrative approach for the optimal combination of automation with UF‐100 and microscopic review with KOVA cell chamber. Clin Chem 2003;49:617–623. [DOI] [PubMed] [Google Scholar]
- 13. Park J, Kim J. Evaluation of iQ200 automated urine microscopy analyzer. Korean J Lab Med 2008;28:267–273. [DOI] [PubMed] [Google Scholar]
- 14. Lamchiagdhase P, Preechaborisutkul K, Lomsomboon P, et al. Urine sediment examination: A comparison between the manual method and the iQ200 automated urine microscopy analyzer. Clin Chim Acta 2005;358:167–174. [DOI] [PubMed] [Google Scholar]
- 15. Cao Y, Cheng M, Hu C. UrineCART, a machine learning method for establishment of review rules based on UF‐1000i flow cytometry and dipstick or reflectance photometer. Clin Chem Lab Med 2012;50:2155–2161. [DOI] [PubMed] [Google Scholar]


