Abstract
Background
Our aim was to retrospectively analyze the relationships between circulating tumor cells (CTCs) and the development of breast cancer, for elucidating the role of CTCs in breast cancer.
Methods
A total of 107 female patients with primary breast cancer and 48 matched healthy female volunteers were recruited. After blood collection, isolation of peripheral blood mononuclear cells (PBMC) was performed followed by the detection of cytokeratin 19 positive (CK19+) and CD44+/CD24−/low cells, as well as estrogen receptor (ER), progesterone, and CerbB2. Data were analyzed with the SPSS 20.0 software.
Results
None of the 48 volunteers were detected with CK19+ cells in their PBMC, while in 77 patients, 72% of 107 female patients with primary breast cancer, the CK19+ cells were detected. CK19+ could also be detected among patients in each grouping by different clinical staging and lymph node metastasis, with statistical differences (all P < 0.05). Further, among the 83 CK19+ specimens, 32 were also detected with CD44+/CD24−/low cells. Comparisons of CK19+ and CD44+/CD24−/low cells in patients with different clinical features (ER positive vs. ER negative, C‐erbB2 positive vs. C‐erbB2 negative) and molecular subtypes (triple‐negative breast cancer, ER positive, and C‐erbB2 positive) showed no obvious difference (all P > 0.05).
Conclusions
Both CTCs and tumor stem cells (TSCs) could be detected in the PBMC of breast cancer patients; besides, positive expression rate of CTCs might be obviously associated with the clinical stage and metastasis. Positive relationship of TSCs and the clinical stage of breast cancer was also proved in this study.
Keywords: circulating tumor cell, breast cancer, tumor stem cell, peripheral blood mononuclear cell
INTRODUCTION
Breast cancer results from malignant proliferation and transformation of epithelial cells lining the ducts or lobules of the breast 1, 2. Breast cancer is the second leading cause of cancer‐related deaths in women globally, especially in older women 3. More than 1 million women are diagnosed with breast cancer every year worldwide, and approximately 0.65% of these women are under 30 years of age, 2.4% are under 35 years, and 6.6% are under 40 years 4, 5. Surprisingly, the prevalence and mortality rates of breast cancer continue to be higher in majority of the developed countries, compared with developing countries 6. Breast cancer is a very heterogeneous disease; multiple risk factors, such as age at menarche or menopause, obesity, lack of physical activity, breastfeeding, tobacco smoking, heavy alcohol consumption, high body mass index, and unhealthy lifestyle, significantly contribute to individual variations in the underlying breast cancer pathophysiology among a given population 7, 8, 9. Cancer metastasis to axillary lymph nodes and histological grade are the two main prognostic determinants in breast cancer patients, and the overall 5‐year survival for breast cancer patients with lymph node metastasis is 40% lower than for patients who do not have lymph node metastasis 10, 11, 12. Besides, nearly 40% of all patients with breast cancer experience a recurrence, of which 10∼20% have locally metastatis and 60–70% have distant metastasis 13. In light of the high incidence, poor prognosis, and frequent recurrence of breast cancer, we are broadly interested in biomarkers related to metastasis and, in this study, were specifically interested in the association between circulating tumor cells (CTCs) detection and breast cancer 14, 15.
CTCs are cells that have shed into the vasculature from a primary tumor and circulate through the bloodstream accompanying tumor invasion 16. CTCs thus play vital roles for subsequent growth of additional tumors in vital distant organs, triggering a mechanism for the vast majority of cancer‐related deaths 17. It has been hypothesized that carcinoma metastasis is initiated by a subpopulation of CTCs found in the blood of patients 18, 19. Tumor metastasis is a multistep process involving disruption of intercellular adhesion and dispersal of single cells from solid tumor, invasion of blood and lymphatic vessels, immunologic escape in circulation, attachment to endothelial cells, extravasation from blood and lymph vessels, and proliferation and induction of angiogenesis 20, 21. This highlights the important role of tumor metastasis in human cancers. Coincidently, CTCs have been detected in several epithelial cancers, including prostate, lung, and colon; patients with metastatic lesions are more likely to have isolated CTCs 22, 23, 24. Besides, there have been studies that documented the presence of CTCs in peripheral blood of patients with metastatic breast cancer 25, 26. However, CTCs are extraordinarily rare and cannot be easily detected; early identification that the detection of CTCs may have predictive and prognostic implications requires careful consideration. Therefore, in this research, we performed a retrospective case–control study to evaluate the relationships between CTCs and the development of breast cancer, for elucidating the role of CTCs in breast cancer.
MATERIALS AND METHODS
Ethics Statement
Informed consent from patients and approval by the ethics committee in Second Affiliated Hospital, Qiqihar Medical University, were obtained prior to the performance of this study. All aspects of the current study complied with the Declaration of Helsinki 27.
Patient Eligibility
A total of 107 female patients with primary breast cancer, who were initially treated in the Second Affiliated Hospital, Qiqihar Medical University, from February 2010 to February 2014, were enrolled. All subjects’ ages varied from 30 to 67 years with the mean age of 53.4 ± 4.03 years and median age of 49 years. Pathologic examination results showed that, among all the eligible patients, 72 patients were diagnosed with invasive ductal carcinoma, 16 with infiltrating lobular carcinoma, 10 with medullary carcinoma, 4 with mucinous adenocarcinoma, and 2 with intraductal papilloma; 3 patients were unclassified. According to the American Joint Committee on Cancer (AJCC) staging system 28, in T (tumor) classification, 59 patients were in T1 stage, 23 were in T2, 21 were in T3, and 4 were in T4; in N (node) classification, there were 33 patients with N0, 41 with N1, 29 with N2, and 4 with N3; in M (metastasis) classification, there were 72 patients with M0 and 32 with M1. According to the clinical cancer staging (Overall Stage Grouping), there were 29 stage I patients, 44 stage II patients, and 34 stage III patients. Besides, 35 patients were premenopausal and the other 72 patients were menopausal. Further, based on the histological grades, the numbers of patients with diseases of grades 1, 2, and 3 were 21, 53, and 25, respectively; except 8 patients were unknown. Examinations showed that the ratio of estrogen receptor (ER) positive to ER‐negative patients was 80/27; ratio of progesterone (PR) positive to PR‐negative patients was 60/47; and ratio of C‐erbB2‐positive to C‐erbB2‐negative patients was 19/80, except 8 patients were unknown.
Inclusion criteria were as follows: 1 patients diagnosed with breast cancer by clinical examination and histological or cytological diagnosis—the clinical staging was applied based on the AJCC staging system for breast cancer 28; 2 patients who did not receive any anticancer therapy (chemotherapy and/or radiotherapy) before blood collection; 3 patients who were not detected with second primary cancer in the preliminary diagnosis; and 4 patients with complete case and pathological data and clear index description. Exclusion criteria were the following: 1 patients with benign breast diseases; 2 patients with severe chronic diseases, such as diseases of the respiratory, cardiovascular, and endocrine systems; 3 patients with malignant breast diseases aroused by other causes, such as lymphoma and soft‐tissue sarcoma; 4 patients who received endocrine therapy or other treatment. We also recruited 48 healthy female volunteers (mean age, 50.21 ± 3.19 years; age range, 35–72 years), general population who had healthy examination in the same hospital during the same period, as the negative control to verify the specificity of this research. The differences of ages between cases and controls were not statistically significant.
Blood Collection
A total of 155 venous blood specimens were collected, among which 107 specimens were from the patients and 48 specimens were from the healthy volunteers. Blood samples (10 ml) were collected from participants in the morning after overnight fasting. Blood samples were drawn with the negative pressure syringe and placed in a 10 ml heparin anticoagulant. Besides, to avoid the false‐positive results (venipuncture needle may bring the epidermal cells into the blood vessels), two tubes of blood samples were collected every time; the former tube was discarded and the latter (5 ml) was preserved.
Isolation of Peripheral Blood Mononuclear Cells
The 5 ml venous blood specimens, drawn from the patients or volunteers, were used for the isolation of peripheral blood mononuclear cells (PBMC). All lab operations, such as, centrifugation, immunomagnetic enrichment, and immunofluorescence were carried out at room temperature. The isolation processes were as follows: peripheral blood, normal saline solution, and the human neutrophil isolation medium (Yibaiju, Shanghai, China) were mixed in a ratio of 1:1:1. Then the mixture was centrifuged with Avant J‐25 centrifuge (Beckman, Palo Alto, CA) at 2,500 × g for 20 min to isolate the single monocytes’ cell pellet. After the centrifugation, the solution was divided into three layers; the monocytes, a milky cell layer that was between the upper and middle layers, were pipetted and transferred into a sterile tube; then the cells were diluted with phosphate buffer solution (PBS) and centrifugated at 1,000 × g for 10 min; the supernatant was removed and PBS washing step was repeated once; cells were fixed with 4% formalin (diluted with PBS) and washed with PBS twice; following that, the cell pellet was resuspended with 950 μl PBS (containing 0.1% BSA) and diluted to 1 × 106 cells/ml.
Detection of Cytokeratin 19 Positive Cells
The FITC‐conjugated cytokeratin 19 (CK‐19) monoclonal antibody (Abcam, Cambridge, UK) and Anti‐FITC MicroBeads (Miltenyi Biotec, Bergisch Gladbach, Germany) were mixed with 1 × 107 monocytes isolated previously, and then MACS buffer was added to the working concentration, which was incubated away from light for 10 min at 4–8°C. Cell suspensions were washed with 10–20 volumes of MACS buffer (PBS [pH 7.2] containing 0.5% bovine serum albumin and 2 mmol EDTA Sigma‐Aldrich (Poole, Dorset, UK)) and centrifuged at 1,000 × g for 10 min. The supernatant was removed and the washing step was repeated once. Cells were resuspended in 500 μl of MACS buffer per 1 × 108 cells for the sorting step; MS Columns (Miltenyi Biotec) were placed on the magnet (with proper strength) of a magnetic‐activated cell sorter (Miltenyi Biotec) and prepared by washing with 500 μl of MACS buffer. The cell suspension was loaded and flow‐through (unlabeled cells) was collected. Each column was washed with MACS buffer; the MACS buffer was added to MS columns, and labeled cells were pushed out with a plunger rapidly and collected with new collecting tubes.
CK19+ cells were centrifugated at 1,500 × g for 5 min, and then the supernatant was removed. Further, the samples were mixed with 10 μl PBS and centrifugated at 1,000 × g for 2 min, following which the whole field of the smears was scanned based on histopathological imaging with a fluorescence microscope (Olympus America, Melville, NY). The total number of CK19+ cells was counted and photos of the field with the most cells were taken and saved. The photographed smears were fixed with 1% methanol at room temperature and preserved at 4°C for 5 days.
Detection of the CD44+/CD24−/low Cells
CD44‐FITC antibody (rat monoclonal to CD44, Abcam) was added to the target smears and incubated at 37°C for 5 min. The smear was washed with 0.1 mol/l PBS three times (5 min each time); further, CD24‐FITC antibody was added, and samples were incubated at 37°C for 5 min. After that, smears were washed with 0.1 mol/l PBS three times (5 min each time); the whole field of the smears was scanned based on histopathological imaging with the fluorescence microscope; the total number of positive cells was counted and photos of the field with the most cells were taken and saved.
Detection of Other Indicators
ER and PR detection was based on the results of immunohistochemistry assay; and the CerbB2 detection was based on the results of immunohistochemistry assay and FISH assay. Immunohistochemistrical streptavidin‐peroxidase method was applied in strict accordance with the operating instructions 29, with pressure cooking heat‐mediated antigen retrieval method. All antibodies and immunohistochemical kit were provided by WuHan BoShiDe Biological Engineering (Wuhan, China). The positive expression of PR and ER was located in the nucleus, and C‐erbB2 was located in cytoplasm. If the proportion of positive cells, which were stained by the immunohistochemistry assay, was more than 10%, the smear was regarded as ER and PR positive. While the grade of immunohistochemistry for C‐erbB2 was 0 or +, the specimen was regarded as negative; otherwise, the “+++” grade presented positive. The specimens graded as “++” were needed to be identified further by FISH, and the final result (positive or negative) was determined by the result of FISH. Result that the examinations on ER, PR, and CerbB2 are all negative is defined as triple‐negative breast cancer (TNBC).
Statistical Analysis
Data were analyzed with the SPSS 20.0 software. The comparisons of measurement data and enumeration data (between any two groups) were conducted with the t‐test and chi‐square test, respectively. The P‐values were two tailed and P < 0.05 indicated a statistically significance.
RESULTS
Comparisons of PBMC Between Groups
None of the 48 volunteers were detected with CK19+ cells in their PBMC, while 72% of 107 female patients with primary breast cancer were detected with CK19+ cells (Fig. 1). This comparison between the two groups was statistically significant (P < 0.05).
Figure 1.
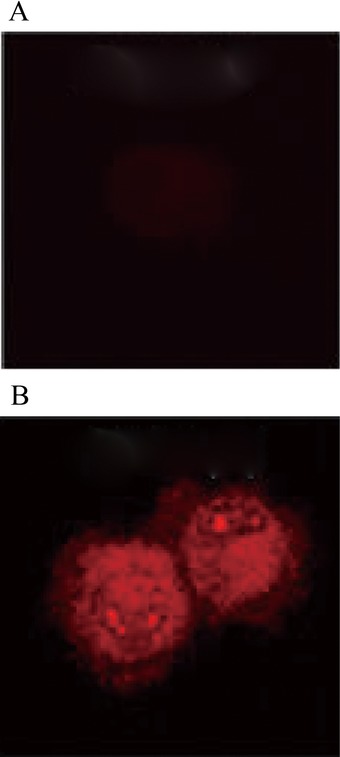
Comparisons of PBMC between groups. (A) None of the 48 volunteers were detected with CK19+ cells in their PBMC, (B) while 72% of 107 female patients with primary breast cancer were detected with CK19+ cells.
Comparisons of CK19+ and CD44+/CD24−/low in Patients With Different Clinical Staging
Among the 29 stage I patients, in 15 patients the CK19+ cells were detected in the PBMC; in 37 of 44 stage II patients and 31 of 34 stage III patients, the CK19+ cells were detected in the PBMC, respectively, showing statistical significance (all P < 0.05). Further, regarding the N stage, N0 stage patients (23/33) and N1‐3 patients (60/74) were detected with CK19+ cells, with statistical difference (P < 0.05).
Among the 83 CK19+ specimens, 32 were detected with CD44+/CD24−/low cells. We grouped them by the histological grades and found that 2 patients of stage I, 20 of stage II, and 19 of stage III were detected with CD44+/CD24−/low cells. However, the results by chi‐square test showed no statistical significance (P > 0.05).
CK19+ and CD44+/CD24−/low Positive Detection in Patients With Different Clinical Features and Molecular Subtypes
Among the 80 ER‐positive specimens, 58 were detected with CK19+ cells and 36 specimens were detected with CD44+/CD24−/low cells, while among the 27 ER‐negative specimens, 21 were detected with CK19+ cells and 14 specimens were detected with CD44+/CD24−/low cells. These results by chi‐square test displayed no apparent significance (P > 0.05). Among the 19 C‐erbB2‐positive specimens, 13 were detected with CK19+ cells, while 7 specimens were detected with CD44+/CD24−/low cells. In the 80 C‐erbB2‐negative specimens, 52 were detected with CK19+ cells and 36 were detected with CD44+/CD24−/low cells. These results by chi‐square test showed no obvious difference (all P > 0.05).
All specimens were divided into three types: TNBC (ER and PR negative, C‐erbB2 negative by FISH assay), ER positive (ER and/or PR positive), and C‐erbB2 positive (ER and PR negative, C‐erbB2 positive by FISH assay). Among the 12 TNBC specimens, all 12 were detected with CK19+ cells (100.00%) and 9 were detected with CD44+/CD24−/low cells (4/5, 80.00%); in the 46 ER‐positive specimens, 26 were detected with CK19+ cells (57.89%) and 17 were detected with CD44+/CD24−/low cells (7/11, 63.64%); of the 9 C‐erbB2‐positive specimens, 7 were detected with CK19+ cells (75.00%) and 5 were detected with CD44+/CD24−/low cells (2/3, 66.67%). These results by chi‐square test showed no statistically significance (all P > 0.05).
DISCUSSION
As an effective window on metastasis biology in malignant tumors, CTCs in human cancers, including breast cancer 31, were previously detected and hypothesized to be closely correlated with clinical stage, lymph node metastasis, and poor prognosis 24, 30. There was also abundant evidence insisting that the presence of CTCs was reported at a high frequency in human cancers; besides, their levels showed a gradually increasing trend with the increase in tumor staging 32, 33. The detection of CTCs in PBMC might hence possibly reveal an early existence of metastasis; however, it is not verified that the presence of CTCs dose necessarily implies the formation of metastasis. Further, CD44+/CD24− was observed in breast cancer cells with tumor stem cells’ (TSCs’) characteristics, which first confirmed the existence of TSCs in breast cancer 34. In fact, most CTCs do not have or only have limited ability to proliferate and differentiate, while distant metastasis can only reach when TSCs have the ability to form tumors, suggesting the valuable effect of TSCs detection in PBMC. In this study, we therefore focused on the exploration of whether CTCs detection combined with the confirmation of TSCs might have significant value in breast cancer or not.
Our results indicated that CK19+ was detected in the PBMC of most breast cancer patients, whereas none was observed in the healthy volunteers, highlighting the important role of CK19+ in predicting breast cancer development. It is worth noting that CK19 can be tested in almost all of the epithelial cells, epithelial tumor cells and some nonepithelial tumor cells, except in the PBMC, which can be regarded as an excellent marker for detecting local invasion and metastasis of tumors, and theoretically prove the existence of CTCs 35. Furthermore, this study also documented that the positive rate of CK19 might also exhibit a significantly positive association with the clinical stage and lymph node metastasis. We could hence speculate that there was the existence of CTCs during the early stage of breast cancer since CK19+ was detected in stage I patients, confirming a potential role of CTCs in hinting the metastasis of tumor cells in breast cancer progression. Meanwhile, the positive rates of CK19 in ER‐positive, C‐erbB2‐positive as well as TNBC tissues were significantly higher than those of the negative tissues, which in turn suggested a close relationship of CTCs’ positive expression with ER, C‐erbB2, and molecular typing of breast cancer. This can be explained by the reason that both ER and C‐erbB2 were previously proved to have synergetic role in breast carcinogenesis. Specifically, ERs can promote the protective effects of estrogens in humans, where estrogens are pleiotropic hormones having an effect on the reproductive system, central nervous system, as well as the cardiovascular system and skeletal system 36, 37. As for C‐erbB2 (also known as HER2), this protein largely mediates the growth of in vitro signal transduction systems, cell proliferation, and cellular transformation, while its overexpression or amplification has been reported to be related to various solid tumors such as endometrial cancer and ovarian cancer 38, 39. With respect to TNBC, evidently, patients with TNBC had significantly higher incidence of recurrence or distant metastasis associated with increased frequency of breast cancer stem cell phenotypes compared with those with non‐triple‐negative tumors 40.
Further, the importance of CD44+/CD24−/low cells (stem/progenitor cell phenotype) in breast cancer patients has been appreciated 40. Another important result of this study indicated that since there were large proportion of CD44+/CD24−/low cells detected in the CK19+ cells and micrometastasis in PBMC, and TSCs detection might thereby be closely interconnected. In breast cancer, the propagation of human breast cancer cells into the mouse mammary fat pad experiment suggested that there were different cell‐surface markers’ expression detected in breast cancer cells with tumorigenic activity as compared with those nontumorigenic cells 41. The identified cells strongly expressed the adhesion molecule CD44 with no or extremely low levels of CD24, referred to as CD44+/CD24−/low cells. In some senses, CD44+/CD24−/low cells were shown to resemble normal stem cells regarding their ability to either self‐renew, or to proliferate/differentiate into diverse cell types 42. In consistency with our results, Wei et al. also found that the prevalence of CD44+/CD24−/low cells in breast cancer may favor distant metastasis 43. Besides, with the appearance of a later clinical stage, TSCs accounted for a higher proportion of CTCs. In the present experiment, CD44+/CD24−/low cells were detected in the PBMC of stage I patients, indicating that breast cancer might be a systemic disease in its early stage and there might be a high probability of concurrent micrometastasis.
In conclusion, these findings suggest that both CTCs and TSCs could be detected in the PBMC of breast cancer patients; besides, positive expression rate of CTCs might be obviously associated with the clinical stage and metastasis. Positive relationship of TSCs and clinical stage of breast cancer was also proved in this study. All in all, the above investigation may aid the design of better tools to diagnose and treat metastatic breast cancer, as well as contribute to the development of therapeutic targets directed to breast cancer.
CONFLICT OF INTEREST
The authors have declared that no competing interests exist.
ACKNOWLEDGMENTS
The study was supported by Prescriptive research of Qigihar Municipal Science and Technology Bureau (SFGG‐201437). We thank the researcher in Second Affiliated Hospital, Qiqihar Medical University, for their helpful advice on this study. We also thank our friends and families who gave us support and assistance.
REFERENCES
- 1. Rebecca SM, Jemal A. Cancer statistics. JAMA 2013; 310: 982. [DOI] [PubMed] [Google Scholar]
- 2. DeSantis C, Siegel R, Bandi P, Jemal A. Breast cancer statistics, 2011. CA Cancer J Clin 2011; 61:409–418. [DOI] [PubMed] [Google Scholar]
- 3. Munster PN, Moore AP, Ismail‐Khan R, et al. Randomized trial using gonadotropin‐releasing hormone agonist triptorelin for the preservation of ovarian function during (neo)adjuvant chemotherapy for breast cancer. J Clin Oncol 2012; 30:533–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Assi HA, Khoury KE, Dbouk H, Khalil LE, Mouhieddine TH, El Saghir NS. Epidemiology and prognosis of breast cancer in young women. J Thorac Dis 2013; 5 Suppl 1:S2–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Youlden DR, Cramb SM, Dunn NA, Muller JM, Pyke CM, Baade PD. The descriptive epidemiology of female breast cancer: an international comparison of screening, incidence, survival and mortality. Cancer Epidemiol 2012; 36:237–248. [DOI] [PubMed] [Google Scholar]
- 6. Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin 2013; 63:11–30. [DOI] [PubMed] [Google Scholar]
- 7. Liu Y, Colditz GA, Rosner B, et al. Alcohol intake between menarche and first pregnancy: a prospective study of breast cancer risk. J Natl Cancer Inst 2013; 105:1571–1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Nelson HD, Zakher B, Cantor A, et al. Risk factors for breast cancer for women aged 40 to 49 years: a systematic review and meta‐analysis. Ann Intern Med 2012; 156:635–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Yaghjyan L, Colditz GA, Rosner B, Tamimi RM. Mammographic breast density and breast cancer risk by menopausal status, postmenopausal hormone use and a family history of breast cancer. Cancer Causes Control 2012; 23:785–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zhang Y, Ye L, Tan Y, Sun P, Ji K, Jiang WG. Expression of breast cancer metastasis suppressor‐1, BRMS‐1, in human breast cancer and the biological impact of BRMS‐1 on the migration of breast cancer cells. Anticancer Res 2014; 34:1417–1426. [PubMed] [Google Scholar]
- 11. Wu SG, He ZY, Li Q, et al. Prognostic value of metastatic axillary lymph node ratio for Chinese breast cancer patients. PLoS One 2013; 8:e61410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Rakha EA, Reis‐Filho JS, Baehner F, et al. Breast cancer prognostic classification in the molecular era: the role of histological grade. Breast Cancer Res 2010; 12:207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wang Z, Wang N, Liu P, et al. MicroRNA‐25 regulates chemoresistance‐associated autophagy in breast cancer cells, a process modulated by the natural autophagy inducer isoliquiritigenin. Oncotarget 2014; 5:7013–7026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin 2011; 61:69–90. [DOI] [PubMed] [Google Scholar]
- 15. Tutt A, Robson M, Garber JE, et al. Oral poly(ADP‐ribose) polymerase inhibitor olaparib in patients with BRCA1 or BRCA2 mutations and advanced breast cancer: a proof‐of‐concept trial. Lancet 2010; 376:235–244. [DOI] [PubMed] [Google Scholar]
- 16. Maheswaran S, Haber DA. Circulating tumor cells: a window into cancer biology and metastasis. Curr Opin Genet Dev 2010; 20:96–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gazzaniga P, Raimondi C, Nicolazzo C, et al. The rationale for liquid biopsy in colorectal cancer: a focus on circulating tumor cells. Expert Rev Mol Diagn 2015; 15:925–932. [DOI] [PubMed] [Google Scholar]
- 18. Raimondi C, Gradilone A, Naso G, et al. Epithelial‐mesenchymal transition and stemness features in circulating tumor cells from breast cancer patients. Breast Cancer Res Treat 2011; 130:449–455. [DOI] [PubMed] [Google Scholar]
- 19. Riethdorf S, Muller V, Zhang L, et al. Detection and HER2 expression of circulating tumor cells: prospective monitoring in breast cancer patients treated in the neoadjuvant GeparQuattro trial. Clin Cancer Res 2010; 16:2634–2645. [DOI] [PubMed] [Google Scholar]
- 20. Nakayama K, Nakayama N, Katagiri H, Miyazaki K. Mechanisms of ovarian cancer metastasis: biochemical pathways. Int J Mol Sci 2012; 13:11705–11717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Tekle C, Nygren MK, Chen YW, et al. B7‐H3 contributes to the metastatic capacity of melanoma cells by modulation of known metastasis‐associated genes. Int J Cancer 2012; 130:2282–2290. [DOI] [PubMed] [Google Scholar]
- 22. O'Flaherty JD, Gray S, Richard D, et al. Circulating tumour cells, their role in metastasis and their clinical utility in lung cancer. Lung Cancer 2012; 76:19–25. [DOI] [PubMed] [Google Scholar]
- 23. Danila DC, Fleisher M, Scher HI. Circulating tumor cells as biomarkers in prostate cancer. Clin Cancer Res 2011; 17:3903–3912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Iinuma H, Watanabe T, Mimori K, et al. Clinical significance of circulating tumor cells, including cancer stem‐like cells, in peripheral blood for recurrence and prognosis in patients with Dukes' stage B and C colorectal cancer. J Clin Oncol 2011; 29:1547–1555. [DOI] [PubMed] [Google Scholar]
- 25. Baccelli I, Schneeweiss A, Riethdorf S, et al. Identification of a population of blood circulating tumor cells from breast cancer patients that initiates metastasis in a xenograft assay. Nat Biotechnol 2013; 31:539–544. [DOI] [PubMed] [Google Scholar]
- 26. Cabinakova M, Tesarova P. Disseminated and circulating tumour cells and their role in breast cancer. Folia Biol (Praha) 2012; 58:87–97. [PubMed] [Google Scholar]
- 27. M PN . World Medical Association publishes the Revised Declaration of Helsinki. Natl Med J India 2014; 27:56. [PubMed] [Google Scholar]
- 28. Singletary SE, Connolly JL. Breast cancer staging: working with the sixth edition of the AJCC Cancer Staging Manual. CA Cancer J Clin 2006; 56:37–47; quiz 50–31. [DOI] [PubMed] [Google Scholar]
- 29. Nadji M, Gomez‐Fernandez C, Ganjei‐Azar P, Morales AR. Immunohistochemistry of estrogen and progesterone receptors reconsidered: experience with 5,993 breast cancers. Am J Clin Pathol 2005; 123:21–27. [DOI] [PubMed] [Google Scholar]
- 30. Hoshimoto S, Shingai T, Morton DL, et al. Association between circulating tumor cells and prognosis in patients with stage III melanoma with sentinel lymph node metastasis in a phase III international multicenter trial. J Clin Oncol 2012; 30:3819–3826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Giuliano AE, Hawes D, Ballman KV, et al. Association of occult metastases in sentinel lymph nodes and bone marrow with survival among women with early‐stage invasive breast cancer. JAMA 2011; 306:385–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Cools‐Lartigue J, Spicer J, McDonald B, et al. Neutrophil extracellular traps sequester circulating tumor cells and promote metastasis. J Clin Invest 2013; 123: 3446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hou JM, Krebs MG, Lancashire L, et al. Clinical significance and molecular characteristics of circulating tumor cells and circulating tumor microemboli in patients with small‐cell lung cancer. J Clin Oncol 2012; 30:525–532. [DOI] [PubMed] [Google Scholar]
- 34. Ponti D, Zaffaroni N, Capelli C, Daidone MG. Breast cancer stem cells: an overview. Eur J Cancer 2006; 42:1219–1224. [DOI] [PubMed] [Google Scholar]
- 35. Panabières C, Pantel K, Vendrell JP. Released cytokeratins as markers for epithelial cells. In.: Google Patents; 2013.
- 36. Bernelot Moens SJ, Schnitzler GR, Nickerson M, et al. Rapid estrogen receptor signaling is essential for the protective effects of estrogen against vascular injury. Circulation 2012; 126:1993–2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Fucic A, Gamulin M, Ferencic Z, et al. Environmental exposure to xenoestrogens and oestrogen related cancers: reproductive system, breast, lung, kidney, pancreas, and brain. Environ Health 2012; 11 Suppl 1:S8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Growdon WB, Groeneweg J, Byron V, et al. HER2 over‐expressing high grade endometrial cancer expresses high levels of p95HER2 variant. Gynecol Oncol 2015; 137:160–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Balcan E, Demirkiran F, Aydin Y, et al. Serum levels of epidermal growth factor, transforming growth factor, and c‐erbB2 in ovarian cancer. Int J Gynecol Cancer 2012; 22:1138–1142. [DOI] [PubMed] [Google Scholar]
- 40. Idowu MO, Kmieciak M, Dumur C, et al. CD44(+)/CD24(‐/low) cancer stem/progenitor cells are more abundant in triple‐negative invasive breast carcinoma phenotype and are associated with poor outcome. Hum Pathol 2012; 43:364–373. [DOI] [PubMed] [Google Scholar]
- 41. Abraham BK, Fritz P, McClellan M, Hauptvogel P, Athelogou M, Brauch H. Prevalence of CD44+/CD24‐/low cells in breast cancer may not be associated with clinical outcome but may favor distant metastasis. Clin Cancer Res 2005; 11:1154–1159. [PubMed] [Google Scholar]
- 42. Mimoto R, Kobayashi T, Imawari Y, et al. Clinical relevance and low tumor‐initiating properties of oligometastatic breast cancer in pulmonary metastasectomy. Breast Cancer Res Treat 2014; 147:317–324. [DOI] [PubMed] [Google Scholar]
- 43. Wei W, Hu H, Tan H, Chow LW, Yip AY, Loo WT. Relationship of CD44+CD24‐/low breast cancer stem cells and axillary lymph node metastasis. J Transl Med 2012; 10 Suppl 1:S6. [DOI] [PMC free article] [PubMed] [Google Scholar]


