Summary
The large-scale application of economically efficient electrocatalysts for hydrogen evolution reaction (HER) is limited in view of the high cost of polymer binders (Nafion) for immobilizing of powder catalysts. In this work, nitrogen-doped molybdenum carbide nanobelts (N-Mo2C NBs) with porous structure are synthesized through a direct pyrolysis process using the pre-prepared molybdenum oxide nanobelts (MoO3 NBs). Nanocellulose instead of Nafion-bonded N-Mo2C NBs (N-Mo2C@NCs) exhibits superior performance toward HER, because of excellent dispersibility and multiple exposed catalytically active sites. Furthermore, the conductive film composed of N-Mo2C NBs, graphene nanosheets, and nanocellulose (N-Mo2C/G@NCs) is fabricated by simple vacuum filtration, as flexible and editable electrode, which possesses excellent performance for scale HER applications. This work not only proposes the potential of nanocellulose to replace Nafion for binding powder catalysts, but also offers a facile strategy to prepare flexible and conductive films for a wide variety of nanomaterials.
Subject Areas: Catalysis, Electrochemical Energy Conversion, Nanomaterials
Graphical Abstract
Highlights
-
•
N-Mo2C nanobelts with porous structure are uniformly synthesized
-
•
Nanocellulose is proposed to replace Nafion for binding powder catalysts
-
•
A facile strategy to prepare conductive film electrode is offered with practice
-
•
The flexible editable electrode exhibits excellent performance for scalable HER
Catalysis; Electrochemical Energy Conversion; Nanomaterials
Introduction
With the depletion of energy sources (such as oil and coal) and the environmental problems caused by burning fossil fuels (global warming, air and water pollution, etc.), green renewable energy sources such as solar energy and hydrogen energy are bound to become an important energy source for future development (Gray, 2009, Turner, 2004, Hui et al., 2019). Hydrogen energy is an extremely superior new energy source with high combustion heat value, no pollution, rich resources, and wide application range, which is regarded as the ideal clean energy in the future (Dresselhaus and Thomas, 2001, Yu et al., 2018a, Yu et al., 2018b). Among various hydrogen production procedures, electrochemical water splitting is inherently considered to be an ideal eco-friendly method to produce hydrogen with high purity (Koper, 2013, Walter et al., 2010, Zeng et al., 2018, Yu et al., 2019). Efficient water splitting typically requires highly active electrocatalysts to promote the hydrogen evolution reaction (HER) (Huang et al., 2018, Fang et al., 2019). Recently, tremendous efforts have been devoted to search for non-precious metal catalysts to replace Platinum (Pt)-based catalysts in the hydrogen generation with high current densities at low overpotentials, which is thus highly desirable (Wang et al., 2016, Shi and Zhang, 2016, Voiry et al., 2016). In particular, as an alternative, molybdenum-based catalysts, such as molybdenum disulfide (MoS2) (Geng et al., 2016), molybdenum diselenide (MoSe2) (Deng et al., 2018), molybdenum phosphide (MoP) (Xiao et al., 2014), molybdenum carbide (Mo2C) (Humagain et al., 2018), and molybdenum nitride (MoN) (Zhang et al., 2016), have recently attracted great attention as potential catalysts for HER, owing to their high cost-effectiveness, abundant molybdenum resources, favorable catalytic activities, and excellent stabilities (Xing et al., 2014, Vrubel and Hu, 2012, Xie et al., 2014). Among them, Mo2C, an excellent transition metal carbide, has received increasing attention as a highly efficient HER electrocatalyst with high conductivity and optimal hydrogen absorption energy, in view of the fact that its electronic structure is almost identical to that of Pt-group elements (Wu et al., 2015, Zhao et al., 2015, Wan et al., 2014). To date, several kinds of Mo2C nanostructures have been successfully synthesized and showed the enhanced performance for HER (Wu et al., 2015, Yang et al., 2016, Xu et al., 2016). It is well known that the electrocatalytic properties of metal carbides strongly depend on their surface structure and composition, which are closely associated with the porous structure and element doping (Wu et al., 2015, Wang et al., 2015, Liu et al., 2015). Huang et al. (Xiong et al., 2018) prepared a kind of two-dimensional Mo/Mo2C heteronanosheets (Mo/Mo2C-HNS) as an efficient and stable noble metal-free electrocatalyst toward the HER in 0.5 M H2SO4. Gao et al. (Lin et al., 2016) developed cobalt-doping into Mo2C nanowires to effectively optimize the electron features around Fermi level, accomplishing the efficient HER in both acidic and basic electrolytes. Therefore, to further improve HER activity of Mo2C, the structure-activity relationship relying on controlled morphology and doping for enhancing exposure of surface active sites is demanded to be uncovered for rational catalyst design.
In addition, the agglomeration of catalysts, especially in the form of powder, can also lead to the loss of active sites (Chen et al., 2013). However, most of the as-synthesized nanoelectrocatalysts are in powder form, which need to be loaded onto glassy carbon (GC) electrodes with conductive polymer binders, such as Nafion. Unsatisfactorily, this step and the use of polymer materials will cause electrocatalysts agglomeration, which often leads to not only lower catalytic activity but also instability in the catalytic process (Asefa and Huang, 2017). Moreover, as long-term testing can cause Nafion to fall off from catalyst and electrode, resulting in poor contact between the catalyst particles and the underlying GC electrodes, the electron transfer from the electrode to electrocatalysts becomes difficult. Undoubtedly, Nafion is expensive and must undergo hazardous manufacturing processes, which is not suitable for large-scale practical applications (Liang et al., 2013, Dong et al., 2012, Kim et al., 2010). Therefore, it is urgently necessary to exploit a low-cost and readily available material that can bond the catalyst powder without reducing catalytic activity to replace the inconvenient artificial polymer, especially Nafion. Nanocelluloses (NCs), being an abundant and natural polymer with considerable physical and biological properties, including renewable resource, sustainability, excellent mechanical properties, biodegradability, and biocompatibility (Lin and Dufresne, 2014, Geyer et al., 1994), have been widely used in numerous fields such as electronics (Yan et al., 2014), medicine (Liu et al., 2007), tissue engineering (Zhang et al., 2015), and pharmacy (Zoppe et al., 2014). Moreover, nanocellulose possesses a superior hydrophilicity owing to the rich surface hydroxyl group, as well as high specific surface area and thermal stability, making it an advantageous carrier and binder for nanomaterials, especially in the powder form (Mo et al., 2009, Kaushik and Moores, 2016, Nyström et al., 2009).
In this work, we report nitrogen-doped molybdenum carbide nanobelts (N-Mo2C NBs) with porous structure synthesized through a direct pyrolysis process using the pre-prepared molybdenum oxide nanobelts (MoO3 NBs). Subsequently, nanocellulose instead of Nafion was employed in the bonding and fixing of N-Mo2C NBs. Surprisingly, nanocellulose bonded N-Mo2C NBs (N-Mo2C@NCs) exhibited superior performance for HER, which possessed a lower overpotential (achieved 10 mA cm−2) of 163 mV than Nafion bonded N-Mo2C NBs (N-Mo2C@Nafion) (180 mV) in an acidic electrolyte. To develop a simple and inexpensive methodology for practical applications, the flexible film electrode composed of N-Mo2C NBs, graphene nanosheets, and nanocelluloses (N-Mo2C/G@NCs) was fabricated by vacuum filtration. The obtained N-Mo2C/G@NCs possessed a small onset potential of −41 mV versus RHE to reach 1 mA cm−2, low Tafel slope (58.83 mA dec−1), and superior long-term stability (remaining the current densities of 100 mA cm−2 for 100 h, 200 mA cm−2 for 77 h, and 300 mA cm−2 for 60 h with negligible degradation of 0.92%, 3.69%, and 4.04%, respectively) toward HER. This work not only proposed the potential of nanocellulose to replace Nafion for binding powder catalysts, but also offered a facile strategy to prepare flexible conductive film electrode for a wide variety of nanomaterials.
Results and Discussion
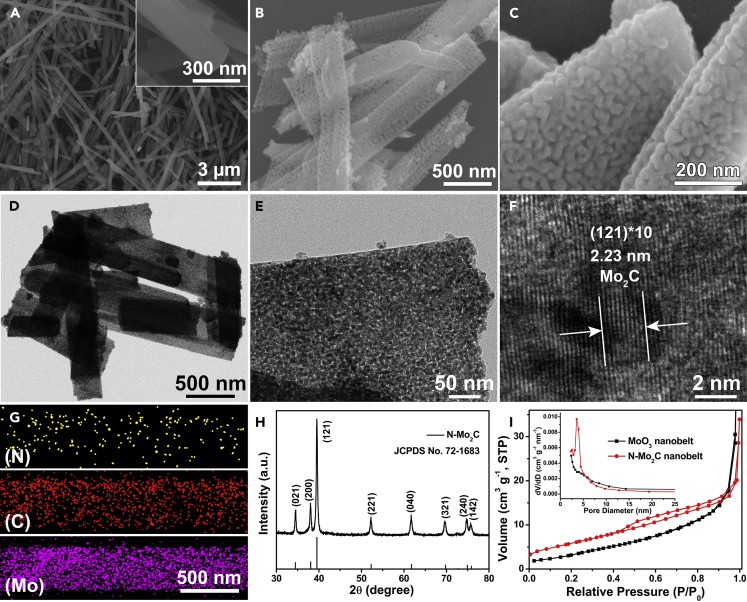
Herein, we performed a detailed characterization of MoO3 NBs and N-Mo2C NBs. The MoO3 NBs with smooth surface were successfully synthesized by hydrothermal reaction, which was confirmed by scanning electron microscopy (SEM) image (Figure 1A), transmission electron microscopy (TEM) images (Figure S1), and X-ray powder diffraction (XRD, Figure S2). After calcining MoO3 NBs with dicyandiamide at 800°C for 2 h under Ar gas atmosphere, the prepared porous N-Mo2C NBs were confirmed by the XRD pattern (Figure 1H, JCPDS No. 72–1683), in which the characteristic peaks at 34.47° (021), 38.07° (200), 39.53° (121), 52.29° (221), 61.76° (040), 69.77° (321), 74.90° (240), and 75.85° (142) were detected (Wang et al., 2017). Moreover, the SEM (Figures 1B and 1C) and TEM (Figures 1D and 1E) images indicated the nanobelt morphology of N-Mo2C with the width of ∼500 nm and length of 2–8 μm inherited from the pristine MoO3 NBs. However, the rough and porous surface of N-Mo2C NBs was observed. High-resolution transmission electron microscopy (HRTEM) image in Figure 1F revealed well-resolved lattice fringes with the interval distance of 0.223 nm that could be indexed to the (121) plane of Mo2C phase. The element mapping result exhibited a homogeneous distribution of the N, C, and Mo elements in the resultant N-Mo2C NBs (Figure 1G). Furthermore, the Brunauer-Emmett-Teller specific surface area of the porous N-Mo2C NBs was determined by N2 adsorption-desorption isotherm as 21.60 m2 g−1, which was larger than that of MoO3 NBs (11.03 m2 g−1), causing the penetration of electrolyte to be promoted and rich active sites to be exposed (Figure 1I) (Gao et al., 2016). Simultaneously, what we could get was that the pore size distribution of N-Mo2C was below 10 nm (inset of Figure 1I). Subsequently, the thicknesses of MoO3 NBs and N-Mo2C NBs were measured by atomic force microscope, as demonstrated in Figure S3. It could be observed that the thickness of the N-Mo2C NBs (∼120 nm) was greater than that of MoO3 NBs (∼58 nm), which should be derived from the rough porous structure formed on the surface of the N-Mo2C NBs, resulting in that N-Mo2C NBs possessed a larger specific surface area and exposed more catalytically active sites.
Figure 1.
Morphology and Structural Characterization of MoO3 NBs and N-Mo2C NBs
(A–C) SEM images of (A) MoO3 NBs and (B and C) N-Mo2C NBs.
(D–H) (D and E) TEM images, (F) HRTEM image, (G) the elemental mapping, and (H) XRD pattern of N-Mo2C NBs.
(I) The nitrogen adsorption-desorption isotherms and pore size distributions of MoO3 NBs and N-Mo2C NBs.
See also Figures S1–S3.
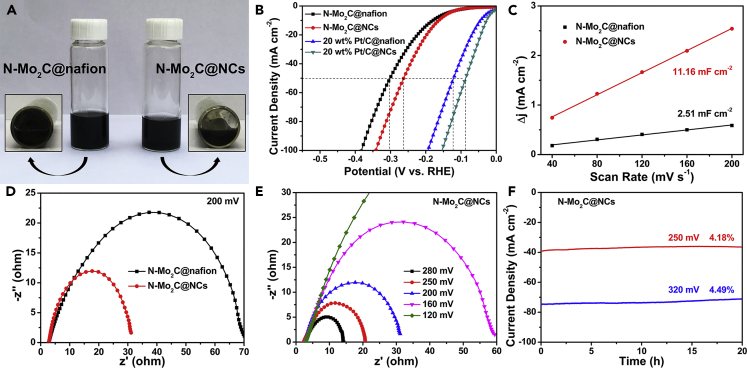
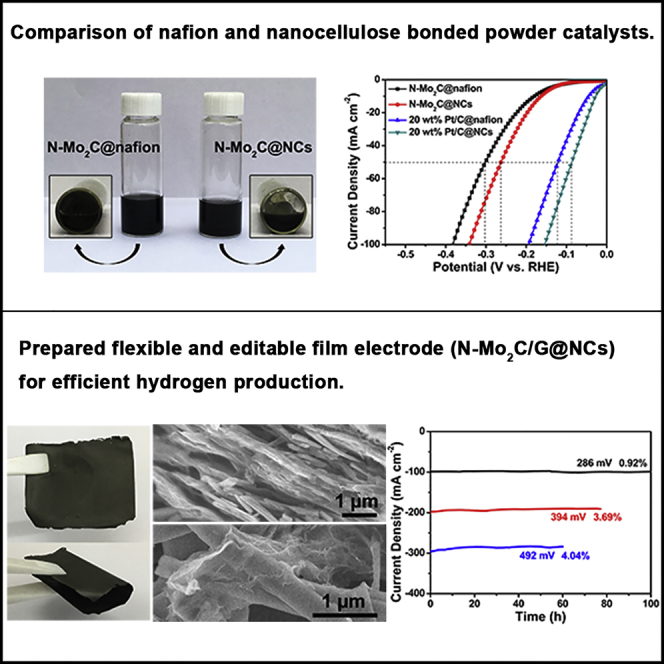
Pleasantly, the N-Mo2C was steadily dispersed in nanocellulose aqueous solution, which maintained 24 h with negligible precipitation, as shown in Figure 2A. However, the N-Mo2C dispersed in both Nafion solution (Figure 2A) and aqueous solution (Figure S4) did not show satisfactory results. SEM was implemented to examine the morphologies and structures of N-Mo2C@Nafion and N-Mo2C@NCs in Figures S5 and S6. As displayed in Figure S5, through the Nafion link, the N-Mo2C NBs were concentrated and buried, indicating that the use of Nafion would cause catalysts to aggregate and tightly cover catalysts, so that their active sites could not be fully exposed. On the contrary, in Figure S6, the morphology of the N-Mo2C NBs was maintained, and the addition of nanocelluloses caused the nanobelts to be bonded together and well fixed. Simultaneously, the N-Mo2C NBs could be fully released, which was beneficial to the exposure of active sites. To further confirm this point of view, the commercial 20 wt% Pt/C was taken the same dispersion in Nafion and nanocellulose solution. SEM in Figures S7 and S8 indicated that 20 wt% Pt/C@NCs possessed a higher exposure rate than that of 20 wt% Pt/C@Nafion.
Figure 2.
Comparison of Properties of Nafion- and Nanocellulose-Bonded Powder Catalysts
(A) Photos of N-Mo2C aqueous dispersion with nanocelluloses and Nafion solutions, respectively.
(B) The LSV curves (without iR corrected) of N-Mo2C@Nafion, N-Mo2C@NCs, 20 wt% Pt/C@Nafion, and 20 wt% Pt/C@NCs.
(C and D) (C) Plot of positive and negative current density differences at a given potential (0.15 V versus the RHE) against the CV scan rates, and (D) EIS Nyquist plots at −200 mV versus RHE recorded of porous N-Mo2C@Nafion and N-Mo2C@NCs.
(E and F) (E) EIS Nyquist plots with different overpotentials and (F) the chronoamperometry curves recorded at the overpotentials of 250 and 320 mV driven from N-Mo2C@NCs. All electrochemical tests were performed in 0.5 M H2SO4 solution.
See also Figures S4–S16.
Next, we investigated the HER electrocatalytic activity of N-Mo2C@NCs modified GC electrode in an acidic electrolyte (0.5 M H2SO4), where the potential of the reference electrode was calibrated with respect to a reversible hydrogen electrode (RHE) performed in a high-purity H2 (99.999%)-saturated electrolyte (Figure S9). For comparison, the electrocatalytic actives of N-Mo2C@Nafion, 20 wt% Pt/C@Nafion, and 20 wt% Pt/C@NCs were also investigated as benchmarks under the same conditions, as displayed in Figure 2B. As expected, 20 wt% Pt/C@NCs behaved the best performance for HER among our catalysts, which only required an overpotential of 28 mV to reach a current density of 10 mA cm−2, which was lower than that of 20 wt% Pt/C@Nafion catalyst (44 mV). Moreover, N-Mo2C@NCs possessed an overpotential (achieved 10 mA cm−2) of 163 mV lower than that of N-Mo2C@Nafion catalyst (180 mV). When at a current density of 50 mA cm−2 or even higher, both 20 wt% Pt/C@NCs and N-Mo2C@NCs exhibited significant potential reductions of ∼38 mV compared with 20 wt% Pt/C@Nafion and N-Mo2C@Nafion, respectively, indicating the potential of nanocellulose to replace Nafion for bonding powder catalysts. In addition, Tafel plots derived from Figure 2B indicated that the nanocellulose-bonded powder catalyst possessed a faster HER kinetics (Figure S10), further revealing the potential of nanocellulose to replace Nafion. The double-layer capacitance of N-Mo2C@NCs and N-Mo2C@Nafion was measured by cyclic voltammograms (CVs), which was a pivotal parameter for estimating the electrochemical area at the solid-liquid interface. The double-layer capacitance of N-Mo2C@CNC (11.16 mF cm−2) was extremely larger than that of N-Mo2C@Nafion (2.51 mF cm−2), as exhibited in Figures 2C and S11. The larger electrochemical area was associated with more active sites on the surface of N-Mo2C@NCs at the solid-liquid interface, which was driven from the double-layer capacitance. The HER kinetics of N-Mo2C@NCs and N-Mo2C@Nafion at the electrode/electrolyte interface were further investigated in detail. In Figure 2D, the charge-transfer resistance (Rct) of N-Mo2C@NCs (31.8 Ω) was much lower than that of N-Mo2C@Nafion (71.2 Ω), suggesting the favorable kinetics of N-Mo2C@NCs during the HER process. Figure 2E showed the representative Nyquist plots of N-Mo2C@NCs at various overpotentials. It could be seen that, as the overpotential increased, the diameter of the semicircle in the low frequency region decreased accordingly (60 Ω at 160 mV to 15 Ω at 280 mV), indicating a diminishment of Rct. In addition, to study the durability of N-Mo2C@NCs, long-term stability tests at the constant potentials (250 and 320 mV) have been operated. As shown in Figure 2F, N-Mo2C@NCs displayed the current densities of 38 and 75 mA cm−2, which remained stable for 20 h with minimal degradation. Compared with N-Mo2C@NCs, it could be observed from Figure S12 that N-Mo2C@Nafion exhibited the obvious attenuation of 15.8% after i-t testing for 20 h. Therefore, the nanocellulose that replaced Nafion as binding agent was found to enhance not only the HER activity, but also the stability of the electrocatalyst. To reflect the practicality of film formation, loading on the surface of the substrate (carbon fiber cloth, abbreviated as CFC) was also achieved, which effectively reduced the amount of catalyst slurry. As manifested in Figures S13 and S14, N-Mo2C@NCs and 20 wt% Pt/C@NCs hybrid components were evenly distributed on the surface of carbon nanofibers.
In addition, to highlight the performance advantages of nanocellulose in bonding powder catalysts compared with Nafion as a binder, additional five powder catalysts were further obtained, which were cobalt nanoparticles encapsulated in nitrogen-doped carbon nanotubes (Co@N-C) obtained via an uncomplicated pyrolysis of Co-MOF (ZIF-67) (Yu et al., 2018a, Yu et al., 2018b), nitrogen-doped carbon nanotube-coated cobalt nanoparticles (Co@NC) by pyrolysis of cobalt salt with dicyandiamide, nitrogen-doped carbon nanotube-coated nickel nanoparticles (Ni@NC) by pyrolysis of nickel salt with dicyandiamide (Zou et al., 2014, Zhou et al., 2016a, Zhou et al., 2016b), the Co2P nanoparticles embedded into N, P co-doped carbon shells (Co2P@NPC) derived from cobalt ions-adsorbed saccharomycete cells (Li et al., 2018), MoSe2 nanosheet/MoO2 nanobelt/carbon nanotube (MoSe2/MoO2/CNTs) composed of highly conductive CNTs, and hierarchical MoSe2 nanosheets on MoO2 nanobelts (Yang et al., 2018), respectively. The phase composition of the as-prepared Co@N-C, Co@NC, Ni@NC, Co2P@NPC, and MoSe2/MoO2/CNTs catalysts could be confirmed by XRD results in Figure S15, which illustrated the successful preparation of the target samples. It could be seen from Figure S16 that the nanocellulose-linked catalysts exhibited better HER performance than the corresponding catalysts bonded by Nafion, further embodying the advantages of nanocellulose as a binder.
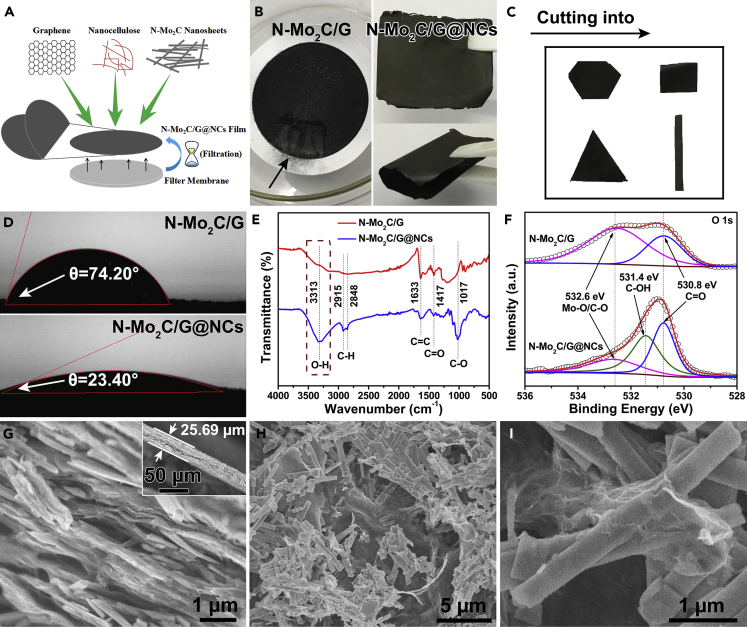
Conversely, flexible nanocellulose-based N-Mo2C electrically conductive films were obtained by simple suction filtration on the resulted aqueous mixtures with nanocellulose linkages, as shown in Figure 3A. It is well known that the pure nanocellulose film exhibited network structure owing to the strong hydrogen bonding among the whiskers (Figure S17) (Nekouei et al., 2018, Liu et al., 2014). The nanocellulose could bind graphene nanosheets and N-Mo2C nanobelts with hydrogen bonds of hydroxys, contributing to outstanding flexibility, which indicated that nanocellulose possessed the excellent bonding between cellulose nanowhiskers and N-Mo2C nanobelts. Adding a small amount of graphene nanosheets was favored to enhance the conductivity. Particularly, nanocellulose played the key role in forming a flexible film. As shown on the right side of Figure 3B, a photo of an integrated N-Mo2C film was presented, which possessed high flexibility so that it could be bent by 180° without breaking. Without nanocellulose, the N-Mo2C/G could not form a film and was easy to powder, as shown in the left photo of Figure 3B. A physical film of N-Mo2C@NCs was also demonstrated in Figure S18, formed by suction filtration only using N-Mo2C nanobelts and nanocellulose, from which it could be observed that the effect of flexible film formation is due to the film-forming properties of nanocellulose, whereas enhanced conductive graphene nanosheets did not contribute to a film-forming effect. In addition, the obtained N-Mo2C/G@NCs films could be easily tailored to pieces with appropriate size for practical applications. As shown in Figure 3C, hexagons, rectangles, triangles, and ribbon electrodes were rendered through the obtained films being cut, indicating that the film electrodes possessed unparalleled simplicity and convenience for the actual operation process.
Figure 3.
Characterization of Structural Properties of N-Mo2C/G@NCs Film
(A) Schematic diagram of forming N-Mo2C/G@NCs film.
(B) Photos of the nanocellulose-free N-Mo2C/G filter membrane with a scraping operation (left) and free-standing flexible N-Mo2C film (right).
(C) Physical images with N-Mo2C/G@NCs electrodes cut into different shapes.
(D–I) (D) Contact angle measurement, (E) FTIR spectra, and (F) High-resolution XPS spectra for O 1s of N-Mo2C/G and N-Mo2C/G@NCs. SEM images with (G) cross-sectional view and (H and I) plane view of N-Mo2C/G@NCs.
See also Figures S17–S24.
To disclose a better performance of the N-Mo2C/G@NCs than N-Mo2C/G, the wettability was checked by measuring the contact angles toward sample surfaces. It could be seen from Figure 3D that the contact angles with water for N-Mo2C/G and N-Mo2C/G@NCs were measured as 74.20° and 23.40°, respectively, which meant that N-Mo2C/G@NCs could display a more hydrophilic behavior than N-Mo2C/G. Figure 3E manifested the Fourier transform infrared (FTIR) spectra of N-Mo2C/G and N-Mo2C/G@NCs. Compared with N-Mo2C/G, the evident enhancement in the intensity of H-O bond at around 3,313 cm−1 indicated the formation of more hydroxyl groups on the N-Mo2C/G@NCs surfaces caused by the addition of nanocellulose (Du et al., 2019, Hsu et al., 2019). In addition, N-Mo2C/G@NCs showed peaks from 2,915 to 2,848 cm−1, corresponding to the stretching vibrations of C-H (Du et al., 2019, Hsu et al., 2019), which demonstrated that N-Mo2C/G has been successfully connected by nanocellulose in N-Mo2C/G@NCs. X-ray photoelectron (XPS) spectra for O 1s of both samples could be deconvoluted to Mo-O or C-O (532.6 eV), and C=O (530.8 eV) bonds, except that there was a C-OH (531.4 eV) bond in N-Mo2C/G@NCs (Lee et al., 2018), as shown in Figure 3F. Among them, Mo-O bond attributed to the surface oxidation of N-Mo2C NBs exposed to air (Huang et al., 2016), and C-OH bond owed to the introduction of hydroxyl groups by adding nanocellulose (Figure S19), as well as that C-O and C=O bonds arised from partial oxidation of carbon in N-Mo2C/G@NCs and functional groups in nanocellulose.
Subsequently, the structure of N-Mo2C/G@NCs was strictly characterized. It could be presented from the inset of Figure 3G that the N-Mo2C/G@NCs film possessed a thickness of ∼25.69 μm, which could be controlled by adding aqueous mixtures (Figure S20). And a wavy layered structure could be clearly observed from the cross section in Figure 3G, which possessed an open architecture for rapid diffusion of ion/electron, maximizing the exposure of the active sites of N-Mo2C/G@NCs (Yang et al., 2018). As for the surface of N-Mo2C/G@NCs film, the N-Mo2C nanobelts and graphene nanosheets were observed and fully exposed, which provided a large number of catalytic active sites, as shown in Figures 3H and 3I. In contrast, the pure graphene nanosheets possessed a smooth surface (Figure S21), which limited the amount of supported electrocatalysts. At last, XRD (Figure S22), Raman (Figure S23), and XPS (Figure S24) results also confirmed the successful synthesis of N-Mo2C/G@NCs.
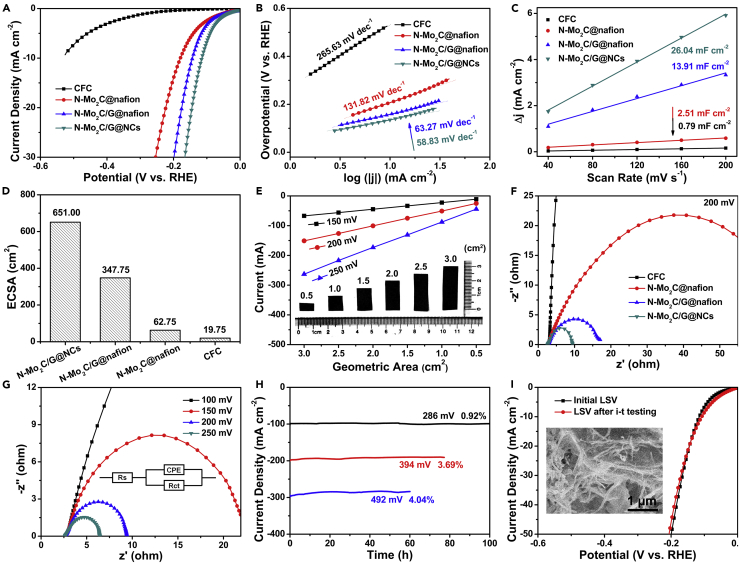
The prepared N-Mo2C/G@NCs could be directly used as 3D electrodes for HER. For comparison, N-Mo2C@Nafion and N-Mo2C/G@Nafion were loaded on CFC to construct 3D electrodes, which were investigated as electrocatalysts for HER. Conversely, bare CFC as supporter was nearly electrochemically inert for HER. The N-Mo2C/G@NCs electrode exhibited excellent catalytic activity with an onset potential of −41 mV versus RHE, as displayed in Figure 4A. To drive a current density of 10 mA cm−2, the N-Mo2C/G@NCs electrode required an overpotential of 115 mV, which was much lower than those of N-Mo2C@Nafion/CFC (178 mV) and N-Mo2C/G@Nafion/CFC (143 mV). The above-mentioned comparison implied that the addition of nanocellulose instead of Nafion was beneficial to improve the HER performance of the N-Mo2C catalyst. Furthermore, the HER kinetics of different samples were further evaluated by using the corresponding Tafel plots. As shown in Figure 4B, the Tafel slope of N-Mo2C/G@NCs catalyst was calculated to be 58.83 mA dec−1, which was much lower than those of bare CFC (265.63 mA dec−1), N-Mo2C@Nafion/CFC (131.82 mA dec−1), and N-Mo2C/G@Nafion/CFC (63.27 mA dec−1), revealing the favorable HER kinetics over N-Mo2C/G@NCs. According to three principal steps (Volmer, Heyrovsky, and Tafel steps) in the mechanism of hydrogen generation in acidic electrolytes (Huang et al., 2018, Jaramillo et al., 2007), the as-prepared N-Mo2C/G@NCs electrocatalyst might proceed via a Volmer-Heyrovsky mechanism, where electrochemical desorption was the rate-limiting step (Xue et al., 2018, Cheng et al., 2017).
Figure 4.
HER Performance of Bare CFC, N-Mo2C@Nafion/CFC, N-Mo2C/G@Nafion/CFC, and N-Mo2C/G@NCs
(A and B) (A) The LSV curves (iR uncorrected) and (B) the corresponding Tafel plots of bare CFC, N-Mo2C@Nafion/CFC, N-Mo2C/G@Nafion/CFC, and N-Mo2C/G@NCs.
(C and D) (C) Plot of positive and negative current density differences at a given potential (0.15 V versus RHE) against the CV scan rates, and (D) electrochemical active surface area (ECSA) of bare CFC, N-Mo2C@Nafion/CFC, N-Mo2C/G@Nafion/CFC, and N-Mo2C/G@NCs electrodes.
(E) A plot of current for HER as a function of the geometric area from N-Mo2C/G@NCs film under different overpotentials. The inset of (E) was the photos of N-Mo2C/G@NCs with different geometric area.
(F) EIS Nyquist plots at −200 mV versus RHE recorded of bare CFC, N-Mo2C@Nafion/CFC, N-Mo2C/G@Nafion/CFC, and N-Mo2C/G@NCs electrodes.
(G) EIS Nyquist plots of N-Mo2C/G@NCs with different overpotentials.
(H) The chronoamperometry curves of the N-Mo2C/G@NCs electrode recorded at the overpotentials of 286, 394, and 492 mV.
(I) The LSV curves of N-Mo2C/G@NCs electrode after i-t testing. The inset of (I) displayed the corresponding SEM image after i-t testing. All tests were performed in 0.5 M H2SO4 solution.
See also Figures S25–S30.
The electrochemical surface area (ECSA) of different samples was evaluated, using the electrical double-layer capacitance tested by CVs. From Figures 4C and S25, the double-layer capacitance value of N-Mo2C/G@NCs was 26.04 mF cm−2, which was 26.84, 10.37, and 1.87 times larger than those of bare CFC (0.97 mF cm−2), N-Mo2C@Nafion/CFC (2.51 mF cm−2), and N-Mo2C/G@Nafion/CFC (13.91 mF cm−2), respectively. It was clearly found that the porous N-Mo2C nanobelts added nanocellulose and graphene nanosheets could result in a high ECSA, which might be associated with the greatly enhanced electrocatalytic HER activity (Figure 4D) (Suen et al., 2017). To explore the exposure of the active sites of the catalysts when using hydroxy nanocellulose instead of Nafion, the turnover frequency (TOF) per active site was estimated (Zhang et al., 2019), which was calculated and plotted against potential in Figure S26. What could be observed was that the TOF value of N-Mo2C/G@NCs (e.g., 0.37 s−1@100 mV) was larger than that of N-Mo2C/G@Nafion (e.g., 0.16 s−1@100 mV) under the same potential, which well supported the result that nanocellulose used as a binder could be exposed more to catalytically active sites than Nafion. In addition, the geometric areas of electrode could also be easily manipulated owing to the editability of N-Mo2C/G@NCs. It could be observed from Figure 4E that the current increased linearly with increasing geometric area of N-Mo2C/G@NCs.
To explore the interfacial properties of obtained catalysts, electrochemical impedance spectroscopy (EIS) measurements at the overpotential of 200 mV were conducted. The Nyquist plots given in Figure 4F revealed that the charge-transfer resistance (Rct) of N-Mo2C/G@NCs (9.5 Ω) was obviously lower than those of bare CFC (>1500 Ω), N-Mo2C@Nafion/CFC (72.9 Ω), and N-Mo2C/G@Nafion/CFC (17.5 Ω), thus achieving the lowest charge-transfer resistance among all the as-synthesized electrocatalysts, which implied the fastest and most efficient charge transport of N-Mo2C/G@NCs during the electrocatalytic HER process (Li et al., 2018). Furthermore, the typical Nyquist plots of N-Mo2C/G@NCs electrode at various overpotentials are displayed in Figure 4G, where the fitting parameters of Rs and CPE were 2.57 and 0.84 Ω, respectively. It could be seen that the Rct values of N-Mo2C/G@NCs significantly decreased with increasing overpotentials, from 83.67 Ω at 100 mV to 6.44 Ω at 250 mV, which indicated the fast reaction rate and favorable HER kinetics at the electrode/electrolyte interface (Zhou et al., 2016a, Zhou et al., 2016b).
In addition to catalytic activity, electrochemical stability was another crucial factor in evaluating the properties of synthetic materials. The long-term stability of N-Mo2C/G@NCs electrode was performed by continuous electrolysis at three fixed overpotentials of 286, 394, and 492 mV in 0.5 M H2SO4. Obviously, the current densities of 100 mA cm−2 for 100 h, 200 mA cm−2 for 77 h, 300 mA cm−2 for 60 h were rather stable, possessing negligible degradation of 0.92%, 3.69%, and 4.04%, respectively, as demonstrated in the time-dependent current density curves of Figure 4H. Furthermore, no obvious changes were observed in the LSV curves after i-t testing investigated by the above-mentioned continuous electrolysis (Figure 4I), revealing the excellent electrocatalytic stability of the N-Mo2C/G@NCs electrode for HER. At the same time, the corresponding electrode maintained a complete morphology in the inset of Figures 4I and S27, indicating its superior structural stability. By comparing XPS survey spectrum after prolonged i-t testing with the initial survey spectrum in Figure S28, the stability of the chemical elements of N-Mo2C/G@NCs could be observed. Therefore, the excellent performance of the N-Mo2C/G@NCs electrode could be attributed to the following. First, nitrogen doping could increase electrocatalytic activity of Mo2C. Second, the porous nanobelt structure exposed the N-Mo2C catalyst to more catalytically active sites. Third, graphite nanosheet enhanced conductivity of N-Mo2C/G@NCs. Fourth, nanocellulose with more hydroxyl groups increased the hydrophilicity of the electrode. Fifth, film formation could avoid the shedding of Nafion and powder catalyst, making its performance and structure stable.
In addition, to highlight the universality of this supported film method, we selected the above-mentioned five powder catalysts for surface filming operations, which were further characterized and tested. After the film formation of the powder catalysts on the surface of CFC, the corresponding SEM images of the synthesized Co@N-C/G@NCs, Co@NC/G@NCs, Ni@NC/G@NCs, Co2P@NPC/G@NCs, and MoSe2/MoO2/CNTs/G@NCs were presented in Figure S29. On the one hand, under the film forming using graphene and nanocellulose, the morphology of the powder catalysts was maintained. On the other hand, the catalysts were uniformly dispersed on the surface of the carbon fiber. Moreover, the detailed electrochemical data of these flexible electrodes were shown in Figure S30. Therefore, the above-mentioned results indicated that the obtained powder catalyst could be efficiently loaded and filmed to form a flexible material through cross-linking with the one-dimensional structure of nanocelluloses and two-dimensional structure of graphene nanosheets.
Conclusion
In conclusion, we have proposed the nanocellulose to replace Nafion for binding powder catalysts and offered a facile strategy to prepare flexible conductive film electrode with porous N-Mo2C nanobelts, graphene nanosheets, and nanocellulose. When at a current density of 50 mA cm−2 or even higher, both 20 wt% Pt/C@NCs and N-Mo2C@NCs exhibited significant potential reduction (approximately 38 mV) compared with 20 wt% Pt/C@Nafion and N-Mo2C@Nafion, respectively, indicating the potential of nanocellulose to replace Nafion for bonding powder catalysts. In addition, the flexible editable N-Mo2C/G@NCs film was fabricated by vacuum filtration, possessing a wavy layered structure, which exhibited a small onset potential of −41 mV versus RHE, low Tafel slope of 58.83 mA dec−1, and superior long-term stability (remaining the current densities of 100 mA cm−2 for 100 h, 200 mA cm−2 for 77 h, 300 mA cm−2 for 60 h without negligible degradation of 0.92%, 3.69%, and 4.04%, respectively) toward HER in 0.5 M H2SO4. It was determined that the present methodology developed paved the way for practical applications of that the powder catalyst was bonded with nanocellulose to replace Nafion and further formed a flexible film for energy storage and conversion.
Limitations of the Study
This work proposes the potential that nanocellulose can replace Nafion for binding powder catalysts. Although the experimental conclusions are further illustrated by different catalysts, the different catalysts for nanocellulose bonding demonstrate different degrees of improvement of electrocatalytic activity for HER, which may be limited by the properties of the applied catalysts. In addition, it can be observed from the work that functional group modification of nanocellulose or other organic reagents can be performed in subsequent studies to obtain a highly efficient alternative to Nafion-like binders.
Methods
All methods can be found in the accompanying Transparent Methods supplemental file.
Acknowledgments
This work was supported by Guangdong Natural Science Funds for Distinguished Young Scholar (2017B030306001), Taishan Scholars Project Special Funds, Natural Science Foundation of Shandong Province (ZR2019YQ20), and Guangdong Innovative and Entrepreneurial Research Team Program (2014ZT05N200).
Author Contributions
G.L. analyzed the data and wrote the manuscript. G.L., J.Y., Z.Z., and Z.X. conducted the synthetic experiments. G.L., J.Y., and R.L. performed material characterization. G.L., Q.C., Z.Z., and L.Z. conducted electrochemical performance for hydrogen evolution reaction. J.Y., Z.Z., Q.C., R.L., and L.Z. executed the paper modification. X.W. and X.P. served as a guide for flexible film preparation. W.Z. and H.L., as supervisors, designed the project and provided guidance, as well as financial support.
Declaration of Interests
The authors declare no competing interests.
Published: September 27, 2019
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.isci.2019.08.055.
Contributor Information
Hong Liu, Email: hongliu@sdu.edu.cn.
Weijia Zhou, Email: ifc_zhouwj@ujn.edu.cn.
Supplemental Information
References
- Asefa T., Huang X. Heteroatom-doped carbon materials for electrocatalysis. Chem. Eur. J. 2017;23:10703–10713. doi: 10.1002/chem.201700439. [DOI] [PubMed] [Google Scholar]
- Chen W.F., Wang C.H., Sasaki K., Marinkovic N., Xu W., Muckerman J.T., Zhu Y., Adzic R.R. Highly active and durable nanostructured molybdenum carbide electrocatalysts for hydrogen production. Energy Environ. Sci. 2013;6:943–951. [Google Scholar]
- Cheng Y., Lu S., Liao F., Liu L., Li Y., Shao M. Rh-MoS2 nanocomposite catalysts with Pt-like activity for hydrogen evolution reaction. Adv. Funct. Mater. 2017;27:1700359. [Google Scholar]
- Deng S., Yang F., Zhang Q., Zhong Y., Zeng Y., Lin S., Wang X., Lu X., Wang C.-Z., Gu L. Phase modulation of (1T-2H)-MoSe2/TiC-C shell/core arrays via nitrogen doping for highly efficient hydrogen evolution reaction. Adv. Mater. 2018;30:1802223. doi: 10.1002/adma.201802223. [DOI] [PubMed] [Google Scholar]
- Dong H., Yu H., Wang X., Zhou Q., Feng J. A novel structure of scalable air-cathode without Nafion and Pt by rolling activated carbon and PTFE as catalyst layer in microbial fuel cells. Water Res. 2012;46:5777–5787. doi: 10.1016/j.watres.2012.08.005. [DOI] [PubMed] [Google Scholar]
- Dresselhaus M.S., Thomas I.L. Alternative energy technologies. Nature. 2001;414:332. doi: 10.1038/35104599. [DOI] [PubMed] [Google Scholar]
- Du X., Du W., Wang Z., Liu K., Li S. Defects in graphene nanoplatelets and their interface behavior to reinforce magnesium alloys. Appl. Surf. Sci. 2019;484:414–423. [Google Scholar]
- Fang Y., Xue Y., Hui L., Yu H., Liu Y., Xing C., Lu F., He F., Liu H., Li Y. In situ growth of graphdiyne based heterostructure: toward efficient overall water splitting. Nano Energy. 2019;59:591–597. [Google Scholar]
- Gao X., Zhang H., Li Q., Yu X., Hong Z., Zhang X., Liang C., Lin Z. Hierarchical NiCo2O4 hollow microcuboids as bifunctional electrocatalysts for overall water-splitting. Angew. Chem. Int. Ed. 2016;128:6398–6402. doi: 10.1002/anie.201600525. [DOI] [PubMed] [Google Scholar]
- Geng X., Sun W., Wu W., Chen B., Al-Hilo A., Benamara M., Zhu H., Watanabe F., Cui J., Chen T.-P. Pure and stable metallic phase molybdenum disulfide nanosheets for hydrogen evolution reaction. Nat. Commun. 2016;7:10672. doi: 10.1038/ncomms10672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geyer U., Heinze T., Stein A., Klemm D., Marsch S., Schumann D., Schmauder H.P. Formation, derivatization and applications of bacterial cellulose. Int. J. Biol. Macromol. 1994;16:343–347. doi: 10.1016/0141-8130(94)90067-1. [DOI] [PubMed] [Google Scholar]
- Gray H.B. Powering the planet with solar fuel. Nat. Chem. 2009;1:7. doi: 10.1038/nchem.141. [DOI] [PubMed] [Google Scholar]
- Hsu H.H., Khosrozadeh A., Li B., Luo G., Xing M., Zhong W. An eco-friendly, nanocellulose/RGO/in situ formed polyaniline for flexible and free-standing supercapacitors. ACS Sustain. Chem. Eng. 2019;7:4766–4776. [Google Scholar]
- Huang Y., Gong Q., Song X., Feng K., Nie K., Zhao F., Wang Y., Zeng M., Zhong J., Li Y. Mo2C nanoparticles dispersed on hierarchical carbon microflowers for efficient electrocatalytic hydrogen evolution. ACS Nano. 2016;10:11337–11343. doi: 10.1021/acsnano.6b06580. [DOI] [PubMed] [Google Scholar]
- Huang Y., Ge J., Hu J., Zhang J., Hao J., Wei Y. Nitrogen-doped porous molybdenum carbide and phosphide hybrids on a carbon matrix as highly effective electrocatalysts for the hydrogen evolution reaction. Adv. Energy Mater. 2018;8:1701601. [Google Scholar]
- Hui L., Xue Y., Yu H., Liu Y., Fang Y., Xing C., Huang B., Li Y. Highly efficient and selective generation of ammonia and hydrogen on a graphdiyne-based catalyst. J. Am. Chem. Soc. 2019 doi: 10.1021/jacs.9b03004. [DOI] [PubMed] [Google Scholar]
- Humagain G., MacDougal K., MacInnis J., Lowe J.M., Coridan R.H., MacQuarrie S., Dasog M. Highly efficient, biochar-derived molybdenum carbide hydrogen evolution electrocatalyst. Adv. Energy Mater. 2018;8:1801461. [Google Scholar]
- Jaramillo T.F., Jørgensen K.P., Bonde J., Nielsen J.H., Horch S., Chorkendorff I. Identification of active edge sites for electrochemical H2 evolution from MoS2 nanocatalysts. Science. 2007;317:100. doi: 10.1126/science.1141483. [DOI] [PubMed] [Google Scholar]
- Kaushik M., Moores A. Review: nanocelluloses as versatile supports for metal nanoparticles and their applications in catalysis. Green. Chem. 2016;18:622–637. [Google Scholar]
- Kim S., Yan J., Schwenzer B., Zhang J., Li L., Liu J., Yang Z., Hickner M.A. Cycling performance and efficiency of sulfonated poly (sulfone) membranes in vanadium redox flow batteries. Electrochem. Commun. 2010;12:1650–1653. [Google Scholar]
- Koper M.T.M. A basic solution. Nat. Chem. 2013;5:255. doi: 10.1038/nchem.1600. [DOI] [PubMed] [Google Scholar]
- Lee J., Oh J., Jeon Y., Piao Y. Multi-heteroatom-doped hollow carbon attached on graphene using LiFePO4 nanoparticles as hard templates for high-performance lithium–sulfur batteries. ACS Appl. Mater. Interfaces. 2018;10:26485–26493. doi: 10.1021/acsami.8b00925. [DOI] [PubMed] [Google Scholar]
- Li G., Yu J., Jia J., Yang L., Zhao L., Zhou W., Liu H. Cobalt–cobalt phosphide Nanoparticles@Nitrogen-phosphorus doped carbon/graphene derived from cobalt ions adsorbed saccharomycete yeasts as an efficient, stable, and large-current-density electrode for hydrogen evolution reactions. Adv. Funct. Mater. 2018;28:1801332. [Google Scholar]
- Liang X., Zhang F., Feng W., Zou X., Zhao C., Na H., Liu C., Sun F., Zhu G. From metal–organic framework (MOF) to MOF–polymer composite membrane: enhancement of low-humidity proton conductivity. Chem. Sci. 2013;4:983–992. [Google Scholar]
- Lin N., Dufresne A. Nanocellulose in biomedicine: current status and future prospect. Eur. Polym. J. 2014;59:302–325. [Google Scholar]
- Lin H., Liu N., Shi Z., Guo Y., Tang Y., Gao Q. Cobalt-doping in molybdenum-carbide nanowires toward efficient electrocatalytic hydrogen evolution. Adv. Funct. Mater. 2016;26:5590–5598. [Google Scholar]
- Liu Z., Sharma B.K., Erhan S.Z. From oligomers to molecular giants of soybean oil in supercritical carbon dioxide medium: 1. Preparation of polymers with lower molecular weight from soybean oil. Biomacromolecules. 2007;8:233–239. doi: 10.1021/bm060496y. [DOI] [PubMed] [Google Scholar]
- Liu D.Y., Sui G.X., Bhattacharyya D. Synthesis and characterisation of nanocellulose-based polyaniline conducting films. Compos. Sci. Technol. 2014;99:31–36. [Google Scholar]
- Liu Y., Yu G., Li G.-D., Sun Y., Asefa T., Chen W., Zou X. Coupling Mo2C with nitrogen-rich nanocarbon leads to efficient hydrogen-evolution electrocatalytic sites. Angew. Chem. Int. Ed. 2015;127:10902–10907. doi: 10.1002/anie.201504376. [DOI] [PubMed] [Google Scholar]
- Mo Z.-l., Zhao Z.-l., Chen H., Niu G.-p., Shi H.-f. Heterogeneous preparation of cellulose–polyaniline conductive composites with cellulose activated by acids and its electrical properties. Carbohydr. Polym. 2009;75:660–664. [Google Scholar]
- Nekouei F., Nekouei S., Kargarzadeh H. Enhanced adsorption and catalytic oxidation of ciprofloxacin on hierarchical CuS hollow nanospheres@N-doped cellulose nanocrystals hybrid composites: kinetic and radical generation mechanism studies. Chem. Eng. J. 2018;335:567–578. [Google Scholar]
- Nyström G., Razaq A., Strømme M., Nyholm L., Mihranyan A. Ultrafast all-polymer paper-based batteries. Nano Lett. 2009;9:3635–3639. doi: 10.1021/nl901852h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y., Zhang B. Recent advances in transition metal phosphide nanomaterials: synthesis and applications in hydrogen evolution reaction. Chem. Soc. Rev. 2016;45:1529–1541. doi: 10.1039/c5cs00434a. [DOI] [PubMed] [Google Scholar]
- Suen N.-T., Hung S.-F., Quan Q., Zhang N., Xu Y.-J., Chen H.M. Electrocatalysis for the oxygen evolution reaction: recent development and future perspectives. Chem. Soc. Rev. 2017;46:337–365. doi: 10.1039/c6cs00328a. [DOI] [PubMed] [Google Scholar]
- Turner J.A. Sustainable hydrogen production. Science. 2004;305:972. doi: 10.1126/science.1103197. [DOI] [PubMed] [Google Scholar]
- Voiry D., Fullon R., Yang J., de Carvalho Castro e Silva C., Kappera R., Bozkurt I., Kaplan D., Lagos M.J., Batson P.E., Gupta G. The role of electronic coupling between substrate and 2D MoS2 nanosheets in electrocatalytic production of hydrogen. Nat. Mater. 2016;15:1003. doi: 10.1038/nmat4660. [DOI] [PubMed] [Google Scholar]
- Vrubel H., Hu X. Molybdenum boride and carbide catalyze hydrogen evolution in both acidic and basic solutions. Angew. Chem. Int. Ed. 2012;124:12875–12878. doi: 10.1002/anie.201207111. [DOI] [PubMed] [Google Scholar]
- Walter M.G., Warren E.L., McKone J.R., Boettcher S.W., Mi Q., Santori E.A., Lewis N.S. Solar water splitting cells. Chem. Rev. 2010;110:6446–6473. doi: 10.1021/cr1002326. [DOI] [PubMed] [Google Scholar]
- Wan C., Regmi Y.N., Leonard B.M. Multiple phases of molybdenum carbide as electrocatalysts for the hydrogen evolution reaction. Angew. Chem. Int. Ed. 2014;126:6525–6528. doi: 10.1002/anie.201402998. [DOI] [PubMed] [Google Scholar]
- Wang S., Wang J., Zhu M., Bao X., Xiao B., Su D., Li H., Wang Y. Molybdenum-carbide-modified nitrogen-doped carbon vesicle encapsulating nickel nanoparticles: a highly efficient, low-cost catalyst for hydrogen evolution reaction. J. Am. Chem. Soc. 2015;137:15753–15759. doi: 10.1021/jacs.5b07924. [DOI] [PubMed] [Google Scholar]
- Wang J., Cui W., Liu Q., Xing Z., Asiri A.M., Sun X. Recent progress in cobalt-based heterogeneous catalysts for electrochemical water splitting. Adv. Mater. 2016;28:215–230. doi: 10.1002/adma.201502696. [DOI] [PubMed] [Google Scholar]
- Wang H., Sun C., Cao Y., Zhu J., Chen Y., Guo J., Zhao J., Sun Y., Zou G. Molybdenum carbide nanoparticles embedded in nitrogen-doped porous carbon nanofibers as a dual catalyst for hydrogen evolution and oxygen reduction reactions. Carbon. 2017;114:628–634. [Google Scholar]
- Wu H.B., Xia B.Y., Yu L., Yu X.-Y., Lou X.W. Porous molybdenum carbide nano-octahedrons synthesized via confined carburization in metal-organic frameworks for efficient hydrogen production. Nat. Commun. 2015;6:6512. doi: 10.1038/ncomms7512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao P., Sk M.A., Thia L., Ge X., Lim R.J., Wang J.-Y., Lim K.H., Wang X. Molybdenum phosphide as an efficient electrocatalyst for the hydrogen evolution reaction. Energy Environ. Sci. 2014;7:2624–2629. [Google Scholar]
- Xie J., Zhang J., Li S., Grote F., Zhang X., Zhang H., Wang R., Lei Y., Pan B., Xie Y. Correction to controllable disorder engineering in oxygen-incorporated MoS2 ultrathin nanosheets for efficient hydrogen evolution. J. Am. Chem. Soc. 2014;136:1680. doi: 10.1021/ja408329q. [DOI] [PubMed] [Google Scholar]
- Xing Z., Liu Q., Asiri A.M., Sun X. Closely interconnected network of molybdenum phosphide nanoparticles: a highly efficient electrocatalyst for generating hydrogen from water. Adv. Mater. 2014;26:5702–5707. doi: 10.1002/adma.201401692. [DOI] [PubMed] [Google Scholar]
- Xiong J., Li J., Shi J., Zhang X., Suen N.-T., Liu Z., Huang Y., Xu G., Cai W., Lei X. In situ engineering of double-phase interface in Mo/Mo2C heteronanosheets for boosted hydrogen evolution reaction. ACS Energy Lett. 2018;3:341–348. [Google Scholar]
- Xu X., Nosheen F., Wang X. Ni-decorated molybdenum carbide hollow structure derived from carbon-coated metal–organic framework for electrocatalytic hydrogen evolution reaction. Chem. Mater. 2016;28:6313–6320. [Google Scholar]
- Xue Y., Huang B., Yi Y., Guo Y., Zuo Z., Li Y., Jia Z., Liu H., Li Y. Anchoring zero valence single atoms of nickel and iron on graphdiyne for hydrogen evolution. Nat. Commun. 2018;9:1460. doi: 10.1038/s41467-018-03896-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan C., Wang J., Kang W., Cui M., Wang X., Foo C.Y., Chee K.J., Lee P.S. Highly stretchable piezoresistive graphene–nanocellulose nanopaper for strain sensors. Adv. Mater. 2014;26:2022–2027. doi: 10.1002/adma.201304742. [DOI] [PubMed] [Google Scholar]
- Yang X., Feng X., Tan H., Zang H., Wang X., Wang Y., Wang E., Li Y. N-Doped graphene-coated molybdenum carbide nanoparticles as highly efficient electrocatalysts for the hydrogen evolution reaction. J. Mater. Chem. A. 2016;4:3947–3954. [Google Scholar]
- Yang L.J., Deng Y.Q., Zhang X.F., Liu H., Zhou W.J. MoSe2 nanosheet/MoO2 nanobelt/carbon nanotube membrane as flexible and multifunctional electrodes for full water splitting in acidic electrolyte. Nanoscale. 2018;10:9268–9275. doi: 10.1039/c8nr01572d. [DOI] [PubMed] [Google Scholar]
- Yu J., Li G., Liu H., Wang A., Yang L., Zhou W., Hu Y., Chu B. Simultaneous water recovery and hydrogen production by bifunctional electrocatalyst of nitrogen-doped carbon nanotubes protected cobalt nanoparticles. Int. J. Hydrogen Energy. 2018;43:12110–12118. [Google Scholar]
- Yu H., Xue Y., Hui L., Zhang C., Li Y., Zuo Z., Zhao Y., Li Z., Li Y. Efficient hydrogen production on a 3D flexible heterojunction material. Adv. Mater. 2018;30:1707082. doi: 10.1002/adma.201707082. [DOI] [PubMed] [Google Scholar]
- Yu H., Xue Y., Huang B., Hui L., Zhang C., Fang Y., Liu Y., Zhao Y., Li Y., Liu H., Li Y. Ultrathin nanosheet of graphdiyne-supported palladium atom catalyst for efficient hydrogen production. iScience. 2019;11:31–41. doi: 10.1016/j.isci.2018.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng L., Yang L., Lu J., Jia J., Yu J., Deng Y., Shao M., Zhou W. One-step synthesis of Fe-Ni hydroxide nanosheets derived from bimetallic foam for efficient electrocatalytic oxygen evolution and overall water splitting. Chin. Chem. Lett. 2018;29:1875–1878. [Google Scholar]
- Zhang Y., Sargent J.L., Boudouris B.W., Phillip W.A. Nanoporous membranes generated from self-assembled block polymer precursors: Quo Vadis? J. Appl. Polym. Sci. 2015;132:21. [Google Scholar]
- Zhang Y., Ouyang B., Xu J., Chen S., Rawat R.S., Fan H.J. 3D porous hierarchical nickel–molybdenum nitrides synthesized by RF plasma as highly active and stable hydrogen-evolution-reaction electrocatalysts. Adv. Energy Mater. 2016;6:1600221. [Google Scholar]
- Zhang L., Jia Y., Liu H., Zhuang L., Yan X., Lang C., Wang Xi., Yang D., Huang K., Feng S., Yao X. Charge polarization from atomic metals on adjacent graphitic layers for enhancing the hydrogen evolution reaction. Angew. Chem. Int. Ed. 2019;58:9404–9408. doi: 10.1002/anie.201902107. [DOI] [PubMed] [Google Scholar]
- Zhao Y., Kamiya K., Hashimoto K., Nakanishi S. In situ CO2-emission assisted synthesis of molybdenum carbonitride nanomaterial as hydrogen evolution electrocatalyst. J. Am. Chem. Soc. 2015;137:110–113. doi: 10.1021/ja5114529. [DOI] [PubMed] [Google Scholar]
- Zhou W., Lu J., Zhou K., Yang L., Ke Y., Tang Z., Chen S. CoSe2 nanoparticles embedded defective carbon nanotubes derived from MOFs as efficient electrocatalyst for hydrogen evolution reaction. Nano Energy. 2016;28:143–150. [Google Scholar]
- Zhou W., Xiong T., Shi C., Zhou J., Zhou K., Zhu N., Li L., Tang Z., Chen S. Bioreduction of precious metals by microorganism: efficient gold@N-doped carbon electrocatalysts for the hydrogen evolution reaction. Angew. Chem. Int. Ed. 2016;55:8416–8420. doi: 10.1002/anie.201602627. [DOI] [PubMed] [Google Scholar]
- Zoppe J.O., Ruottinen V., Ruotsalainen J., Rönkkö S., Johansson L.-S., Hinkkanen A., Järvinen K., Seppälä J. Synthesis of cellulose nanocrystals carrying tyrosine sulfate mimetic ligands and inhibition of alphavirus infection. Biomacromolecules. 2014;15:1534–1542. doi: 10.1021/bm500229d. [DOI] [PubMed] [Google Scholar]
- Zou X., Huang X., Goswami A., Silva R., Sathe B.R., Mikmeková E., Asefa T. Cobalt-embedded nitrogen-rich carbon nanotubes efficiently catalyze hydrogen evolution reaction at all pH values. Angew. Chem. Int. Ed. 2014;53:4372–4376. doi: 10.1002/anie.201311111. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.