Abstract
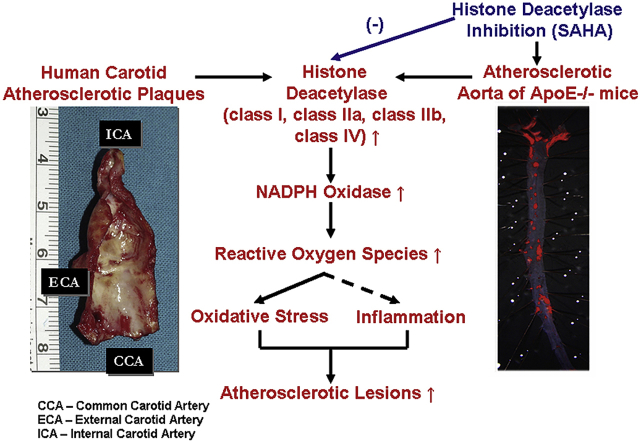
NADPH oxidase (Nox)-derived reactive oxygen species (ROS) are instrumental in all inflammatory phases of atherosclerosis. Dysregulated histone deacetylase (HDAC)-related epigenetic pathways have been mechanistically linked to alterations in gene expression in experimental models of cardiovascular disorders. Hitherto, the relation between HDAC and Nox in atherosclerosis is not known. We aimed at uncovering whether HDAC plays a role in mediating Nox up-regulation, oxidative stress, inflammation, and atherosclerotic lesion progression. Human non-atherosclerotic and atherosclerotic arterial samples, ApoE−/− mice, and in vitro polarized monocyte-derived M1/M2-macrophages (Mac) were examined. Male ApoE−/− mice, maintained on normal or high-fat, cholesterol-rich diet, were randomized to receive 10 mg/kg suberoylanilide hydroxamic acid (SAHA), a pan-HDAC inhibitor, or its vehicle, for 4 weeks. In the human/animal studies, real-time PCR, Western blot, lipid staining, lucigenin-enhanced chemiluminescence assay, and enzyme-linked immunosorbent assay were employed. The protein levels of class I, class IIa, class IIb, and class IV HDAC isoenzymes were significantly elevated both in human atherosclerotic tissue samples and in atherosclerotic aorta of ApoE−/− mice. Treatment of ApoE−/− mice with SAHA reduced significantly the extent of atherosclerotic lesions, and the aortic expression of Nox subtypes, NADPH-stimulated ROS production, oxidative stress and pro-inflammatory markers. Significantly up-regulated HDAC and Nox subtypes were detected in inflammatory M1-Mac. In these cells, SAHA reduced the Nox1/2/4 transcript levels. Collectively, HDAC inhibition reduced atherosclerotic lesion progression in ApoE−/− mice, possibly by intertwined mechanisms involving negative regulation of Nox expression and inflammation. The data propose that HDAC-oriented pharmacological interventions could represent an effective therapeutic strategy in atherosclerosis.
Keywords: NADPH oxidase, Oxidative stress, Epigenetics, Histone deacetylase, Atherosclerosis
Abbreviations: ROS, Reactive oxygen species; NADPH, Nicotinamide adenine dinucleotide phosphate (reduced form); Nox, NADPH oxidase; HDAC, Histone deacetylase; CVD, Cardiovascular disorders; LPS, Lipopolizaharide; IFNγ, Interferon gamma; IL-4, Interleukin -4; IL-10, Interleukin -10; TNFα, tumor necrosis factor α; TLR2/4, Toll-like receptor 2/4; CD206, Cluster of differentiation 206/manose receptor; CD45/68, Cluster of differentiation 45/68; MMP9, Matrix metalloproteinase 9; NOS2, Nitric oxide synthase 2; SOD, Superoxide dismutase; LDL, Low-density lipoprotein; HDL, High-density lipoprotein; EDTA, Ethylenediaminetetraacetic acid disodium salt dehydrate; SDS-PAGE, Sodium dodecyl sulfate–polyacrylamide gel electrophoresis; DMSO, Dimethyl sulfoxide; PBS, Phosphate-buffered saline; RIPA, Radioimmunoprecipitation assay buffer
Graphical abstract
Highlights
-
•
Class I, II, and IV HDAC are induced in human and in experimental atherosclerosis.
-
•
HDAC inhibition reduces the progression of atherosclerotic lesions in mice.
-
•
HDAC subtypes mediate Nox upregulation and oxidative stress in atherosclerotic mice.
-
•
Class I, II, and IV HDAC are up-regulated in proinflammatory M1 macrophages in vitro.
-
•
HDAC inhibition reduces mRNA levels of Nox in cultured M1 macrophages.
1. Introduction
Despite the major improvements in primary and secondary prevention strategies, atherosclerosis-related cardiovascular diseases (CVD) remain the main cause of morbidity and mortality worldwide [1,2]. In the recent years, complex genetic and epigenetic interactions converging to changes in gene function and cell phenotype that occurs independently of DNA sequence have been increasingly implicated in CVD [3,4]. Three major epigenetic mechanisms have been characterized: DNA methylation, post-translational modification of nucleosomal histones, and non-coding RNA. In this context, unveiling the patient epigenetic background represents an important goal of precision medicine in CVD patients [5]. Notably, unlike non-modifiable genetic variants, the plasticity of epigenetic modifications offers the advantage of gene expression reprogramming by means of pharmacological interventions.
Among other epigenetic systems, it has become increasingly evident that alterations in histone acetylation may play a role in CVD, particularly in atherosclerosis. Histone acetylation is controlled by two enzyme families namely, histone acetyltransferase (HAT) and histone deacetylase (HDAC). According to the canonical theory, acetylation of nucleosomal histones by HAT induces chromatin relaxation, a conformational state that promotes genes transcription by enabling the interactions among transcription factors and their consensus DNA binding elements in the genome. Other than the effect on histones, HAT and HDAC also act on non-histone proteins such as transcription factors and transcriptional co-activators to induce or repress the gene expression [6,7].
Experimental results in mice suggest that HDAC is a promising novel target for the treatment of a number of human cardiometabolic diseases including heart failure, hypertension, aortic aneurism, diabetes, and atherosclerosis [[8], [9], [10], [11], [12], [13], [14], [15]]. HDAC family comprises four different classes, namely class I (HDAC1, 2, 3, 8), class IIa (HDAC4, 5, 7, 9), class IIb (HDAC6, 10), class III (sirtuin, Sirt1-7), and class IV (HDAC11). HDAC subtypes feature distinct enzymatic activities and intracellular compartmentalization that dictate their biological functions. While HDAC1, 2, 8 are mainly localized in the cell nucleus, the other HDAC subtypes can shuttle between cytoplasm and nucleus [16,17].
Despite the important reported data, the precise role of the HDAC system in atherosclerosis is debatable [[18], [19], [20]]. NADPH oxidase (Nox)-derived reactive oxygen species (ROS) underlay early and advanced inflammatory reactions in atherosclerosis [21]. Yet, the implication of HDAC in mediating Nox up-regulation and oxidative stress in atherosclerosis is largely unknown. We hypothesized that HDAC isoenzymes may act as up-stream modulators of Nox up-regulation and the ensuing oxidative stress and inflammation in the process of atheroma formation. To test this hypothesis, we designed experiments on human non-, and atherosclerotic arterial specimens, apolipoprotein E deficient (ApoE−/−) mice, and in vitro polarized mouse monocyte (Mon)-derived macrophages (Mac).
We provide evidence that class I, IIa, IIb, and IV HDAC isoenzymes are up-regulated in human carotid artery-derived atherosclerotic lesions, atherosclerotic aorta of ApoE−/− mice, and in inflammatory M1-Mac. In addition, we demonstrated that pharmacological inhibition of HDAC (SAHA) reduces the progression of atherosclerotic lesions in the aorta of hypercholesterolemic ApoE−/− mice, a condition that was correlated with reduced immune cell infiltration, Nox expression, NADPH-stimulated ROS production, and down-regulated levels of markers of oxidative stress and inflammation. Moreover, in cultured M1-Mac, exposure to SAHA reduces significantly the augmented expression levels of Nox and tumor necrosis factor α (TNFα).
The results of this study strengthen and extend the rationale of using HDAC inhibitors in order to counteract oxidative stress and inflammation in atherosclerosis, possibly connected to up-regulated Nox-derived ROS production.
2. Materials and methods
2.1. Materials
If not specified, standard chemicals, reagents, kits, and disposables were obtained from Sigma-Aldrich, Thermo Fisher Scientific/Invitrogen, R&D Systems, Roche, Bio-Rad, TPP, and Eppendorf. Primary and secondary antibodies were from Santa Cruz Biotechnology, Sigma-Aldrich, and R&D Systems.
2.2. Collection of human non-atherosclerotic and atherosclerotic tissue samples
Non-atherosclerotic tissue specimens resulting from superior thyroid arteries (control) and carotid artery-derived atherosclerotic plaques were obtained as discarded biological materials from patients undertaking extended carotid endarterectomy (at University Hospital, Bucharest). Ultrasound imaging interrogation and angiography were used to demonstrate the severe stenosis of the atherosclerotic carotid arteries in each patient. Clinical characteristics of the patients are shown in Table 1 (Supplemental file). The study was conducted in agreement with the ethical directives for medical research involving human subjects (The Code of Ethics of the World Medical Association, Declaration of Helsinki). Written informed consent was obtained from all patients. The study protocols were approved by the ethical committee of the Institute of Cellular Biology and Pathology (ICBP) “Nicolae Simionescu”.
2.3. In vivo set-up of experimental atherosclerosis model and treatment strategy
Male ApoE−/− and C57BL/6J mice derived from The Jackson Laboratory were used in this study. The mice were exposed to 12-h cycles of light/dark and had access to diet and water ad libitum. At 8 weeks of age, ApoE−/− mice (n = 24) were fed a high-fat, cholesterol-rich diet (HD) for 10 weeks in order to develop intermediate atherosclerotic lesions along the aorta [22]. Age-matched ApoE−/− mice (n = 12), fed a normal diet (ND) were used as controls. After 10 weeks, ApoE−/− mice maintained on either normal or atherogenic diet were randomized to receive via intraperitoneal injection 10 mg/kg suberoylanilide hydroxamic acid (SAHA), a pan-HDAC inhibitor or its vehicle (DMSO) every other day for 4 weeks. There were three experimental groups (n = 12/group): (i) ApoE−/− (ND) + vehicle, (ii) ApoE−/− (HD) + vehicle, and (iii) ApoE−/− (HD) + SAHA. The concentration and the route of SAHA administration to mice were established in agreement with the preceding in vitro and in vivo experiments [15,23]. The extent of atherosclerotic lesions along the aorta was assessed by en face Oil Red O (ORO) staining. ImageJ™ software (NIH Image, USA) was employed to quantify the ORO positive staining area. The animal studies were done in accordance with the guidelines of EU Directive 2010/63/EU and the experimental protocols were approved by the ethical committee of the Institute of Cellular Biology and Pathology “Nicolae Simionescu”.
2.4. Cell culture experimental design
In vitro studies were done on resting (M0) and polarized (M1/M2) mouse Mon-derived Mac. Mon were separated by negative selection from the spleen of C57BL6J mice (n = 80) fed a normal diet employing EasySep™ mouse monocyte isolation kit (Stemcell™ Technologies). To induce Mon-to-Mac differentiation, freshly isolated Mon were seeded at a density of 1.5 × 105 cells per well into 12-well tissue culture plates, and cultured for 7 days in RPMI-1640 medium containing 10% fetal bovine serum (FBS), 10% L929 cell line (Sigma)-derived conditioned medium, and 2% penicillin-streptomycin-neomycin solution (Sigma). To promote pro-inflammatory (M1) or anti-inflammatory (M2) Mac phenotype, the adherent cells (Mac) were cultured for another 3 days in RPMI-1640 medium containing 10% FBS and 2% antibiotics supplemented with 100 ng/ml LPS + 20 ng IFNγ to induce the M1-like Mac or with 20 ng/ml IL-4 to induce the M2-like Mac [24]. The cells exposed to the culture medium alone were taken as resting Mac (M0). In some experiments, the M1-and M2-Mac were further challenged for 24 h with polarization factors (e.g., LPS + IFNγ/M1-Mac; IL-4/M2-Mac) in the absence or presence of 5 μM SAHA. The optimal concentration of SAHA was employed as previously published [15].
2.5. Assessment of plasma levels of total cholesterol, LDL-cholesterol, HDL-cholesterol, and triglycerides in mice
Blood samples were taken from each animal via cardiac puncture at the time of sacrifice on BD Vacutainer® spray-coated EDTA tubes (Becton Dickinson) to prepare the plasma. The levels of total cholesterol, LDL-cholesterol, HDL-cholesterol, and triglycerides were measured spectrophotometrically in the plasma of mice using standard kits (Dialab).
2.6. Real-time polymerase chain reaction assay (real time-PCR)
Total cellular RNA was isolated from cultured Mac employing a silica-based column purification kit (Sigma). M-MLV reverse-transcriptase was used to synthesize the complementary DNA strand (cDNA) from single-stranded RNA in accordance to manufacturer's instructions (Thermo Fisher Scientific). SYBR™ Green I probe was used to monitor cDNA amplification (LightCycler™ 480 II thermocycler, Roche). The mRNA expression levels were quantified employing the comparative CT method [25] using the β-Actin mRNA level for internal normalization [26]. The sequences of the oligonucleotide primers [[27], [28], [29]] and the GeneBank® accession numbers are included in Table 2 (Supplemental file).
2.7. Western blot assay
Total protein extracts derived from human and mouse non-atherosclerotic and atherosclerotic arterial tissues and cultured Mac were prepared as previously described [30]. Briefly, tissue samples were washed in PBS (pH 7.4, 4 °C), resuspended in RIPA buffer containing a protease inhibitor cocktail (Sigma), and subjected to mechanical disruption employing a glass bead (1.0 mm diameter) homogenizer (BioSpec). The tissue homogenates and cultured cells were resuspended in 2 x Laemmli's electrophoresis sample buffer and incubated for 20 min at 95 °C. Protein samples (tissue: 30 μg protein/lane, cells: 50 μg protein/lane) were run on SDS-PAGE and transferred onto nitrocellulose membranes (Bio-Rad). The following primary antibodies were used: HDAC1 (rabbit polyclonal, sc-7872, dilution 1:200), HDAC2 (rabbit polyclonal, sc-7899, dilution 1:200), HDAC3 (mouse monoclonal, sc-17795, dilution 1:200), HDAC4 (mouse monoclonal, sc-46672, dilution 1:200), HDAC6 (mouse monoclonal, sc-28386, dilution 1:200 and rabbit polyclonal, SAB4500011, dilution 1:500), HDAC11 (mouse monoclonal, sc-390737, dilution 1:200), Nox1 (rabbit polyclonal, sc-25545, dilution 1:200), Nox2/gp91phox (mouse monoclonal, sc-130543, dilution 1:200), Nox4 (rabbit polyclonal, sc-30141, dilution 1:200), 4-hydroxynonenal (mouse monoclonal, MAB3249, dilution 1 μg/mL) or β-Actin (mouse monoclonal, sc-47778, dilution 1:500). Anti-rabbit IgG-HRP (sc-2370, dilution 1:2000) and anti-mouse IgG-HRP (sc-2031, dilution 1:2000) secondary antibodies were used. Chemiluminescence imaging of protein bands was done using a digital detection system (ImageQuant LAS 4000, Fujifilm). The expression level of β-Actin protein was used for internal normalization. TotalLab™ software was employed for densitometric analysis of protein expression levels.
2.8. Measurement of ROS production
Lucigenin-enhanced chemiluminescence assay was employed to determine the NADPH-stimulated ROS production in intact aortic segments as previously described [31]. Briefly, the samples were exposed to 50 mM phosphate buffer (pH 7.0) containing EDTA-free protease inhibitor cocktail, 5 μM lucigenin, and 100 μM NADPH. The light emission was recorded in a tube luminometer (Berthold) and the ROS production was calculated from the ratio of mean light units (MLU) to vessel dry weight.
2.9. Enzyme-linked immunosorbent assay (ELISA)
TNFα level was determined in Mac culture supernatants employing an ELISA kit in accordance to the manufacturer's protocol (R&D Systems).
2.10. Statistical analysis
Data derived from minimum three independent experiments were expressed as mean ± standard deviation (SD). Statistical analysis was done by t-test and one-way analysis of variance (ANOVA™) followed by Tukey's post hoc test; P < 0.05 was considered as statistically significant.
3. Results
3.1. Class I, IIa, IIb, and IV HDAC subtypes are up-regulated in atherosclerotic human carotid arteries
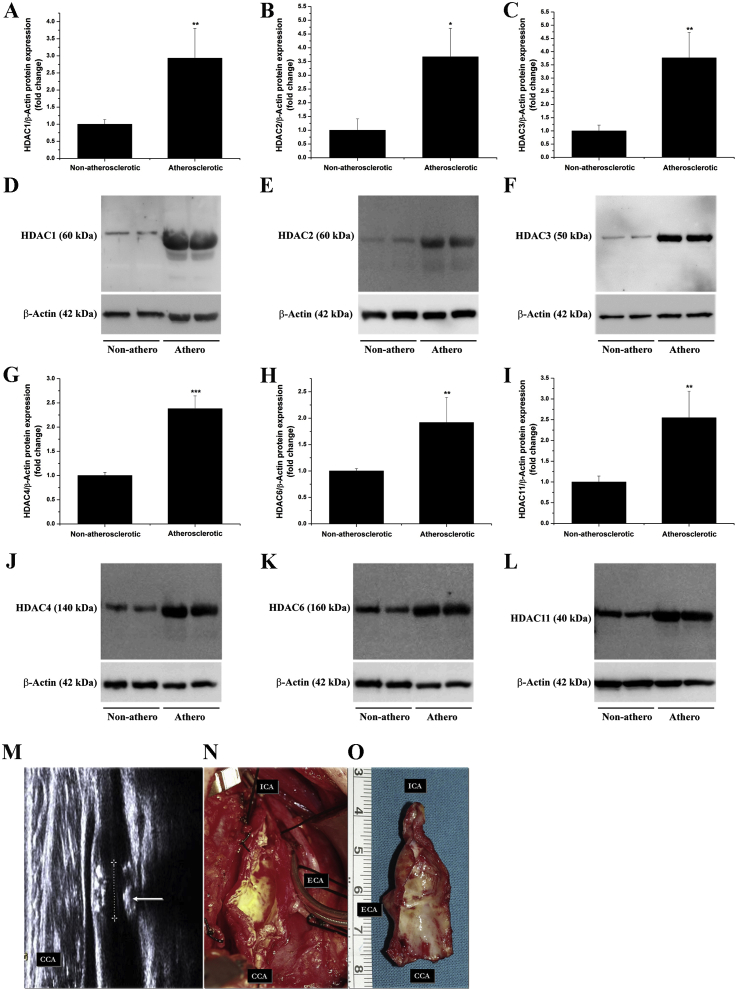
The rationale of using HDAC inhibitors in various experimental models of CVD is generally based on the up-regulated HDAC expression, a condition that promotes epigenetic instability and alterations in gene expression. Previous studies have provided persuasive evidence indicating that HDAC3 and HDAC9 isoforms are up-regulated in human atherosclerotic lesions and they are mechanistically implicated in the process of atheroma formation in mice [[32], [33], [34]]. To further explore the potential implication of other HDAC isoforms in human atherosclerosis, the protein expression levels of additional HDAC subtypes were investigated in atherosclerotic tissue specimens derived from human carotid arteries and in non-atherosclerotic tissue samples obtained from superior thyroid arteries. Significant increases in HDAC1 (≈2.9-fold), HDAC2 (≈3.7-fold), HDAC3 (≈3.8-fold), HDAC4 (≈2.4-fold), HDAC6 (≈1.9-fold), and HDAC11 (≈2.5-fold) protein levels were revealed by Western blot analysis in atherosclerotic tissues compared to values obtained for non-atherosclerotic control samples (Fig. 1A-L). Representative ultrasound imaging interrogation showing the extensive atherosclerotic disease beyond the carotid bifurcation and an extended endarterectomy-derived atherosclerotic tissue sample employed in the study are depicted in Fig. 1M–O. These results provide further support for the existence of a positive correlation between up-regulated HDAC protein levels and severe atherosclerotic lesions in humans. Moreover, additional HDAC subtypes that fall into class I (HDAC1, HDAC2, and previously highlighted HDAC3), class IIa (HDAC4), class IIb (HDAC6), and class IV (HDAC11) categories were found significantly elevated in human atherosclerotic tissues.
Fig. 1.
The protein levels of class I, IIa, IIb, and IV HDAC subtypes are significantly up-regulated in atherosclerotic human carotid arteries. (A-C, G-I) Densitometric analysis showing fold change relative protein expression levels of HDAC1, HDAC2, HDAC3, HDAC4, HDAC6, and HDAC11 subtypes in human non-, and atherosclerotic arterial specimens. (D-F, J-L) Representative immunoblots depicting the up-regulation of HDAC protein levels in atherosclerotic tissue derived from human carotid arteries as compared to non-atherosclerotic arterial samples. (M) Representative ultrasound interrogation image in a study patient with severe stenosis of the atherosclerotic carotid artery. ICA: internal carotid artery, ECA: external carotid artery. CCA: common carotid artery. (N, O) Representative images of an atherosclerotic tissue specimen derived from a patient undergoing carotid endarterectomy. n = 4, *P < 0.05, **P < 0.01, ***P < 0.001. P-values were taken in relation to non-atherosclerotic condition.
3.2. HDAC inhibition has no effect on body weight and plasma levels of total cholesterol, LDL-cholesterol, HDL-cholesterol, and triglycerides in hypercholesterolemic ApoE−/− mice
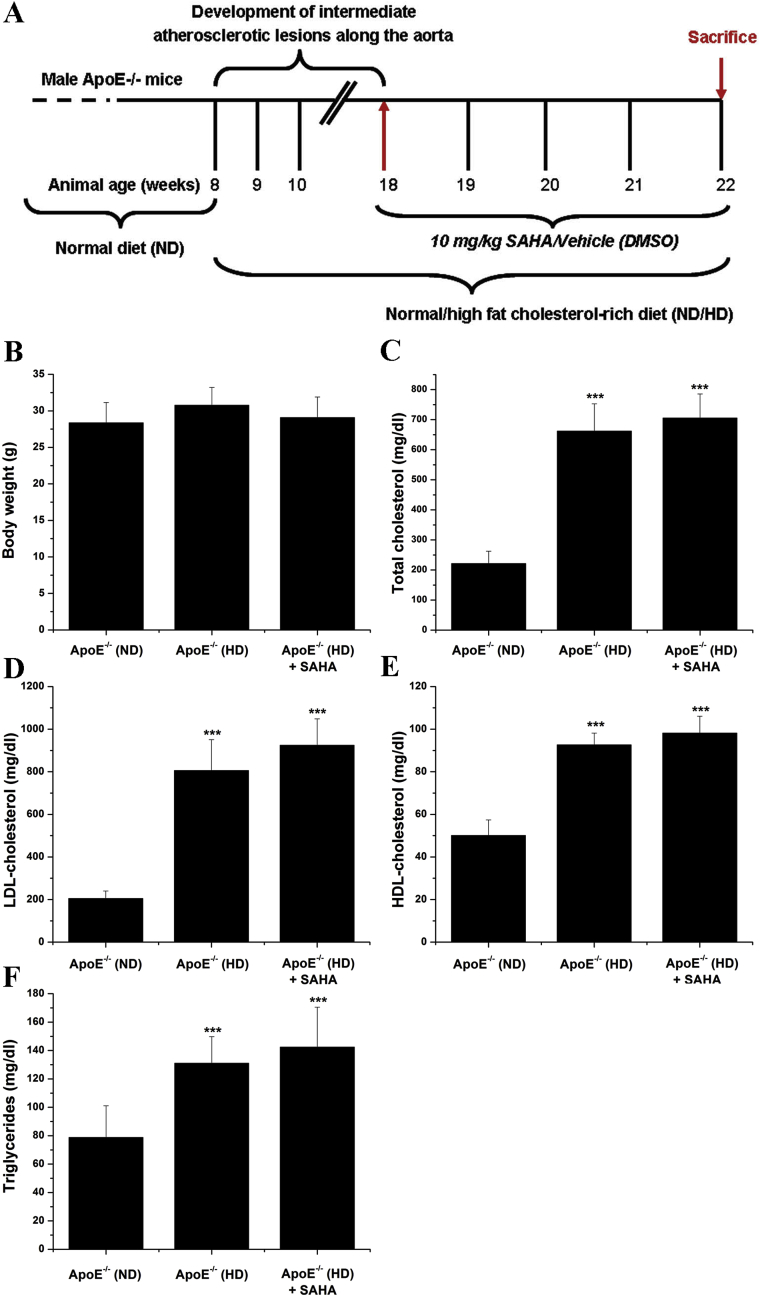
For further mechanistic determinations of our findings obtained on human atherosclerotic specimens we employed ApoE−/− mice as an in vivo model of experimental atherosclerosis. Noteworthy, the expression of HDAC and Nox subtypes and the levels of oxidative stress and inflammatory markers were determined in ApoE−/− mice with established aortic atherosclerosis, an experimental set-up that is considered potentially relevant for human atherosclerotic disease [22,35]. Thus, after 10 weeks on high-fat, cholesterol-rich diet, ApoE−/− mice featuring intermediate atherosclerotic lesions throughout the aorta [22], were further divided in two experimental groups to receive SAHA or its vehicle (DMSO) for 4 weeks. Te results showed that at the end of the treatment procedure the plasma levels of total cholesterol, LDL-cholesterol, HDL-cholesterol, and triglycerides were significantly higher in ApoE−/− (HD) mice compared with ApoE−/− (ND) animals. Notably, no significant changes in body weight and plasma levels of total cholesterol, LDL, HDL, and triglycerides were determined in response to SAHA administration (Fig. 2).
Fig. 2.
(A) Schematic representation of the experimental setup to induce atherosclerotic lesion formation in the aorta of ApoE−/− mice and the time periods of SAHA/vehicle administration to mice. (B–F) Assessment of body weight and plasma levels of total cholesterol, LDL-cholesterol, HDL-cholesterol, and triglycerides for each animal group at the end of the treatment procedure. n = 8–12, ***P < 0.001. P-values were taken in relation to ApoE−/− (ND) condition.
3.3. Class I, IIa, IIb, and IV HDAC subtypes are up-regulated in the atherosclerotic aorta of hypercholesterolemic ApoE−/− mice
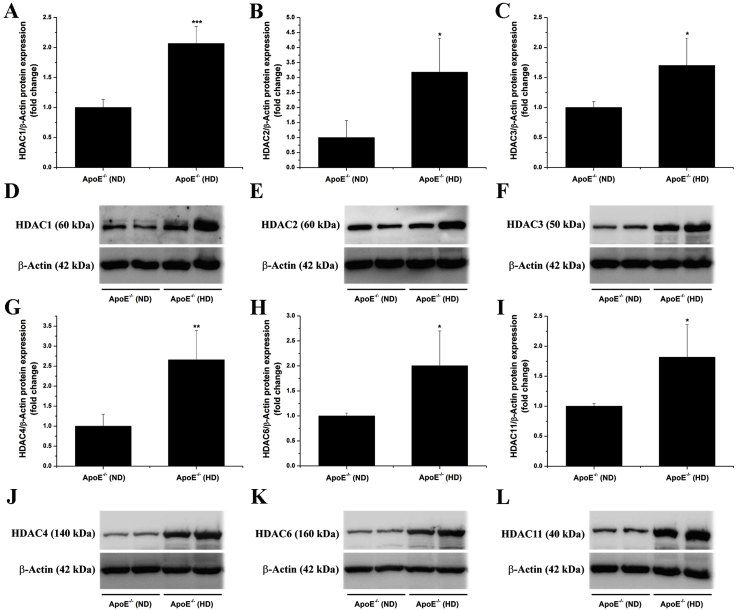
Based on the fact that several HDAC subtypes are induced in human atherosclerotic tissues, we next questioned whether a comparable expression profile exists in atherosclerotic ApoE−/− mice. To this purpose, the aorta derived from ApoE−/− mice fed a normal or a high-fat, cholesterol-rich diet were dissected out, processed for protein extraction, and subjected to Western blot analysis. After 14 weeks of hypercholesterolemia, significant elevations in HDAC1 (≈2.1-fold), HDAC2 (≈3.2-fold), HDAC3 (≈1.7-fold), HDAC4 (≈2.7-fold), HDAC6 (≈2-fold), and HDAC11 (≈1.8-fold) protein levels were determined in the atherosclerotic aorta of ApoE−/− (HD) mice compared with ApoE−/− (ND) animals (Fig. 3).
Fig. 3.
The protein levels of class I, IIa, IIb, and IV HDAC subtypes are up-regulated in the atherosclerotic aorta of hypercholesterolemic ApoE−/− mice. (A-C, G-I) Densitometric analysis showing the increased expression of the HDAC1, HDAC2, HDAC3, HDAC4, HDAC6, and HDAC11 proteins in the atherosclerotic aorta of ApoE−/− mice after 14 weeks on high-fat, cholesterol-rich diet. (D-F, J-L) Representative immunoblots depicting the up-regulation of HDAC subtype in the aorta of ApoE−/− (HD) as compared with ApoE−/− (ND) mice. n = 4–5, *P < 0.05, **P < 0.01, ***P < 0.001. P-values were taken in relation to ApoE−/− (ND) condition.
3.4. SAHA reduces significantly the progression of atherosclerotic lesions in the aorta of hypercholesterolemic ApoE−/− mice
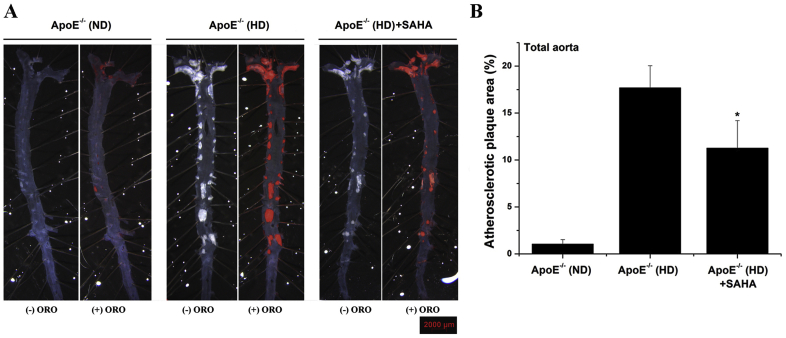
Since multiple Zn2+-containing HDAC isoforms are induced in the atherosclerotic aorta of ApoE−/− (HD) mice, a clinically approved pan-HDAC inhibitor was employed in order to assess the implication of HDAC-related signalling pathways in the process of atheroma formation. As revealed by Oil Red O staining, the formation of atherosclerotic lesions was markedly increased (≈17-fold) throughout the aorta of ApoE−/− (HD) compared with ApoE−/− (ND) at the moment of animal sacrifice. Notably, compared to vehicle-treated ApoE−/− (HD) mice, SAHA administration induced a significant reduction (≈37%) of the atherosclerotic plaque area along the aorta of ApoE−/− (HD) mice (Fig. 4).
Fig. 4.
The progression of atherosclerotic lesions is significantly reduced by SAHA in the aorta of hypercholesterolemic ApoE−/− mice. (A) Representative en face Oil Red O (ORO) stained atherosclerotic lesions displayed along the aorta of ApoE−/− mice. Images were taken in the absence (-ORO) and presence (+ORO) of the staining solution. Note the staining of the lesional lipid deposits/atherosclerotic lesions at different aortic territories (i.e., aortic arch, thoracic aorta, abdominal aorta). (B) Quantification of atherosclerotic lesion area. n = 5–6, *P < 0.05. P-value was taken in relation to ApoE−/− (HD) condition. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
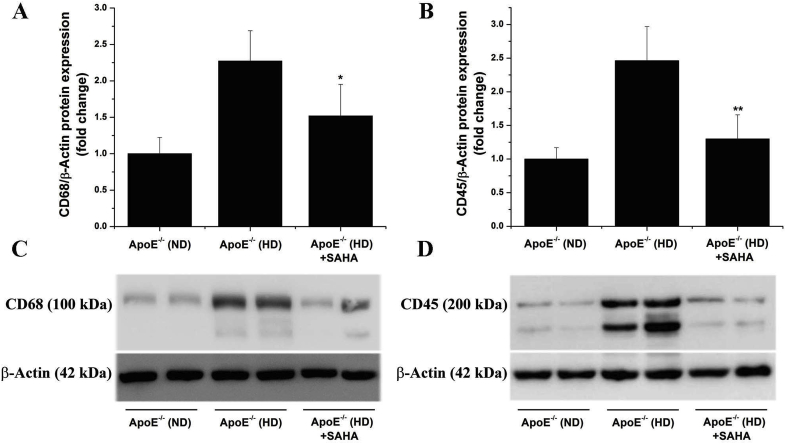
3.5. SAHA decreases CD68 and CD45 protein levels in the atherosclerotic aorta of ApoE−/− mice
To elucidate the potential mechanisms of action of SAHA that may partially explain the reduction in atherosclerotic lesion area, the expression of CD68 and CD45, reliable markers of immune cell infiltration (especially Mac infiltration that is typical for ApoE−/− model), were assessed by Western blot using aortic protein extracts from each experimental group. As predicted, robust increases in CD68 (≈2.3-fold) and CD45 (≈2.5-fold) protein levels were detected in the aorta of ApoE−/− (HD) mice compared with ApoE−/− (ND) mice. Noteworthy, administration of SAHA resulted in a significant reduction in aortic CD68 and CD45 protein levels in ApoE−/− (HD) mice compared with vehicle-treated ApoE−/− (HD) mice (Fig. 5). These data indicate that reduced aortic Mon recruitment and subsequent Mac-derived foam cell formation in response to pan-HDAC pharmacological inhibition may explain the impact of SAHA treatment on atherosclerotic lesion progression in ApoE−/− (HD) mice.
Fig. 5.
SAHA decreases the levels of markers of immune cell infiltration in the atherosclerotic aorta of hypercholesterolemic ApoE−/− mice. (A, B) Western blot assay indicating the significant reduction in CD68 and CD45 protein levels in the aorta of SAHA-treated ApoE−/− (HD) mice. (C, D) Representative immunoblots showing the regulation of CD68 and CD45 protein levels in the aortic protein extracts derived from each animal group. n = 4–5, *P < 0.05, **P < 0.01. P-values were taken in relation to ApoE−/− (HD) condition.
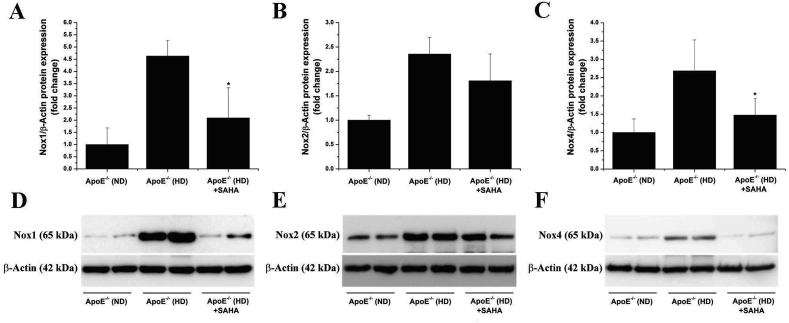
3.6. SAHA down-regulates Nox1 and Nox4 protein levels in the atherosclerotic aorta of ApoE−/− mice
Overproduction of ROS triggered by up-regulated Nox is acknowledged as master regulator of inflammatory signalling pathways in atherosclerosis [21,36]. To explore whether mechanistic links exist between HDAC and Nox in atherosclerosis, we next determined the aortic protein expression of the catalytic subunits of the Nox complex, namely Nox1, Nox2, and Nox4. The results showed that the protein level of each Nox subunit was significantly up-regulated (Nox1: ≈4.6-fold, Nox2: ≈2.5-fold, Nox4: ≈2.7-fold) in the atherosclerotic aorta of ApoE−/− (HD) mice compared with ApoE−/− (ND) mice. Interestingly, the up-regulated protein expression levels of Nox1 and Nox4 subtypes, but not Nox2 were significantly reduced in response to SAHA treatment in ApoE−/− (HD) mice (Fig. 6). These results indicate that various Zn2+-containing HDAC isoforms function as up-stream regulators of Nox expression and potentially Nox overactivity in experimental atherosclerosis.
Fig. 6.
Activation of HDAC-dependent signalling pathways augments the protein expression levels of Nox subtypes in the atherosclerotic aorta of ApoE−/− mice. (A–C) Quantification of Western blot data showing the fold changes in Nox1, Nox2, and Nox4 protein expression levels in the aorta of vehicle- or SAHA-treated ApoE−/− mice maintained on normal (ND) or high-fat, cholesterol-rich diet (HD). (D–F) Representative immunoblots depicting the regulation of Nox1, Nox2, and Nox4 expression in the aortic protein extracts derived from each animal group. n = 4–5, *P < 0.05. P-values were taken in relation to ApoE−/− (HD) condition.
3.7. SAHA down-regulates NADPH-stimulated ROS production in the atherosclerotic aorta of ApoE−/− mice
To determine the implication of HDAC signalling in the regulation of NADPH-induced ROS production in the aorta of ApoE−/− mice, lucigenin-enhanced chemiluminescence assay was employed. The results showed a significant up-regulation (≈1.8-fold) of the NADPH-stimulated chemiluminescence signal in the aorta of ApoE−/− (HD) mice as compared with ApoE−/− (ND) mice. Interestingly, treatment of ApoE−/− (HD) mice with SAHA reduced the augmented ROS production to a level that was comparable to the value obtained for ApoE−/− (ND) mice (Fig. 1 in Supplemental file).
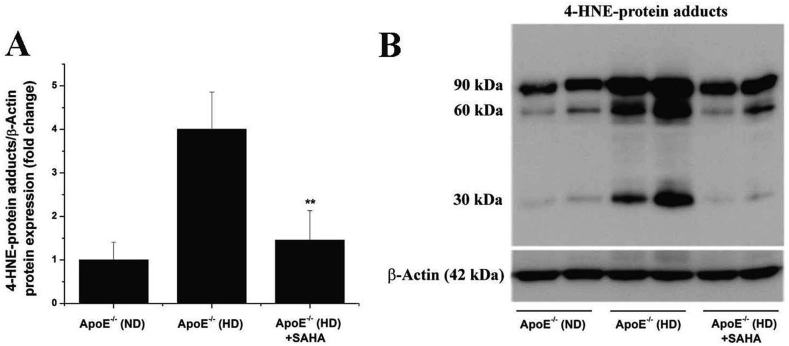
3.8. SAHA reduces the formation of 4-HNE-protein adducts in the atherosclerotic aorta of ApoE−/− mice
To explore whether SAHA has a potential anti-oxidative stress effect in atherosclerotic mice, we determined the formation of 4-hydroxy-2E-nonenal (4-HNE), a highly reactive lipid peroxidation product [37]. The aortic production of 4-HNE was indirectly assessed by Western blot assay employing a mouse monoclonal antibody raised against 4-HNE-(histidine) protein adducts. The antibody labelled protein bands of ≈90 kDa, ≈ 60 kDa, and ≈30 kDa in the aortic protein extracts derived from ApoE−/− (ND) and ApoE−/− (HD) animal groups. Significant increases in 4-HNE-protein adducts (≈4-fold) were detected in the aorta of ApoE−/− (HD) mice compared to ApoE−/− (ND) mice. Treatment of ApoE−/− (HD) mice with SAHA resulted in a marked decrease in aortic 4-HNE-protein adducts formation compared to untreated ApoE−/− (HD) animals (Fig. 7). Based on previous data demonstrating that Nox-derived ROS induce 4-HNE formation in diabetic conditions [38], we may assume that up-regulated Nox-derived ROS may partially contribute to 4-HNE overproduction in the aorta of hypercholesterolemic ApoE−/− mice.
Fig. 7.
SAHA significantly reduces the level of lipid peroxidation in the atherosclerotic aorta of ApoE−/− mice. (A) Densitometric analysis depicting the down-regulation of 4-HNE-protein adducts formation in SAHA-treated ApoE−/− (HD) mice. (B) Representative immunoblot demonstrating the negative impact of SAHA on 4-HNE-protein adducts accumulation in aorta of ApoE−/− mice under hypercholesterolemic conditions. n = 4, **P < 0.01. P-value was taken in relation to the values obtain for vehicle-treated ApoE−/− (HD) mice.
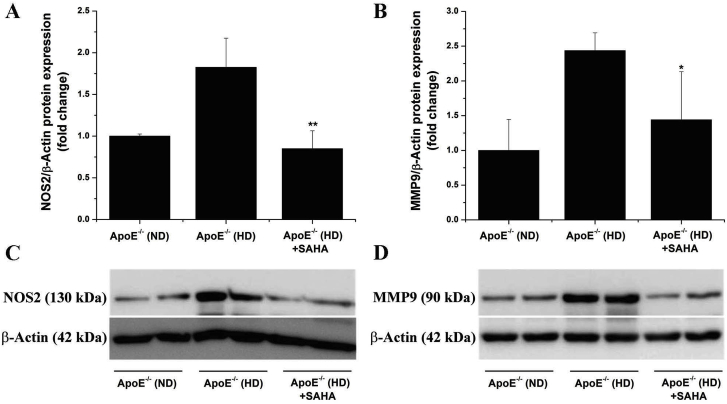
3.9. SAHA-induced pharmacological inhibition of HDAC down-regulates markers of inflammation in the atherosclerotic aorta of ApoE−/− mice
To examine the impact of HDAC-activated signalling pathways on vascular inflammation in experimental atherosclerosis, we determined the protein expression levels of NOS2 and MMP9, two pro-atherogenic enzymes whose expression is tightly regulated by several pro-inflammatory transcription factors including nuclear factor kB (NF-kB) and activator protein 1 (AP-1) [39,40]. Significant increases in NOS2 (≈1.8-fold) and MMP9 (≈2.4-fold) protein levels were detected in the atherosclerotic aorta of ApoE−/− (HD) mice compared with ApoE−/− (ND) animals. Notably, SAHA treatment prevented the up-regulation of NOS2 and MMP9 proteins in the aorta of ApoE−/− (HD) mice (Fig. 8). The data indicate that SAHA was very effective not only at reducing Nox expression and oxidative stress but also on down-regulation of markers of aortic inflammation, namely NOS2 and MMP9. Based on the fact that oxidative stress and inflammation are interrelated in atherosclerosis [41], we may safely assume that SAHA alters the function of common transcription factors regulating oxidative stress-, and pro-inflammatory genes.
Fig. 8.
Pharmacological inhibition of HDAC down-regulates the expression of proinflammatory markers in the aorta of atherosclerotic ApoE−/− mice. (A, B) Down-regulation of important biomarkers of vascular inflammation and remodelling, the NOS2 and MMP9 protein levels, following SAHA treatment, in the aorta of ApoE−/− (HD) mice. (C, D) Representative immunoblots depicting the fold changes in NOS2 and MMP9 expression levels in the aortic protein extracts derived from each animal group. n = 4–5, *P < 0.05, **P < 0.01. P-values were taken in relation to ApoE−/− (HD) condition.
3.10. Up-regulated HDAC induces significant increases in TNFα mRNA and secreted protein levels in cultured M1-like Mac
Our in vivo studies were complemented by in vitro experiments on cultured mouse Mon-derived Mac. Based on the fact that two major Mac subsets (i.e., M1 and M2) have been detected within atherosclerotic lesions [42], cultured Mac were polarized toward pro-inflammatory M1-like and anti-inflammatory M2-like phenotypes. As expected, higher mRNA levels of MCP-1 (≈9.8-fold), TNFα (≈4.2-fold), TLR2 (≈5.2-fold), and TLR4 (≈3.6-fold) were detected in M1-like Mac whilst the M2-like phenotype was defined by increased mRNA levels of CD206 (≈2.7-fold) and IL-10 (≈4.2-fold) (Fig. 2 in Supplemental file). Moreover, the gene expression profiling indicated that similar to human and mouse atherosclerotic tissues, the transcript levels of HDAC1 (≈4.9-fold), HDAC2 (≈3.6-fold), HDAC3 (≈3.2-fold), HDAC4 (≈2.3-fold), HDAC6 (≈3.1-fold), and HDAC11 (≈2.5-fold) were significantly augmented in M1-like Mac compared with resting (M0-like) Mac. Particularly, significant increases in HDAC1 (≈3.2-fold) and HDAC11 (≈1.7-fold) mRNA expression were detected in M2-like Mac, but their gene expression levels remained under the values obtained in M1-like Mac (Fig. 3 in Supplemental file). The data suggested that up-regulated HDAC subtypes may play an important role in the modulation of M1-like Mac pro-inflammatory functions. To answer to this issue, the gene and protein expression levels of the pro-inflammatory cytokine TNFα were assessed in resting and polarized M1/M2 Mac in the absence/presence of the HDAC inhibitor, SAHA. Significant elevations in TNFα mRNA (≈5.1-fold) and secreted protein (≈30-fold) levels were determined in M1-like Mac compared to resting Mac. Noteworthy, the induction of TNFα mRNA and protein levels were significantly reduced in SAHA-exposed M1-like Mac compared with non-treated M1-like cells (Fig. 4 in Supplemental file). These results may provide the rationale and extended support for the previous demonstration of the potential anti-inflammatory effects of HDAC inhibitors in cultured Mac [24].
3.11. SAHA-induced pharmacological inhibition of HDAC dampens the gene expression of Nox subtypes in cultured M1-like Mac
Evidence exists that ROS production is enhanced in M1-like Mac compared to either resting or M2-like Mac [43]. Yet, the precise role and regulation of Nox expression in different Mac subsets is not entirely understood. The gene expression analysis demonstrated significant elevations in Nox1 (≈2.2-fold), Nox2 (≈5.6-fold), and Nox4 (≈2.3-fold) transcript levels in M1-like Mac compared with resting Mac. Since the expression of various Nox isoforms is tightly regulated by pro-inflammatory transcription factors such as NF-kB and STAT1 [[44], [45], [46]], we may safely assume that the activation of these transcriptional regulators by combined actions of LPS and IFNγ potentially mediate the up-regulation of the catalytic Nox subunits in cultured M1-like Mac. Moreover, pharmacological inhibition of HDAC by SAHA blunted the up-regulation of Nox isoforms in M1-like Mac (Fig. 5 in Supplemental file). Collectively, the data further confirm the expression pattern of the Nox subtypes and the pharmacological effects of SAHA in cultured M1-like murine Mac as determined in the aorta of atherosclerotic ApoE−/− mice.
To provide additional support for the implication of HDAC in the regulation of redox-sensitive pro-inflammatory signaling pathways, the mRNA expression analysis of genes known to be up-regulated in response to oxidative stress and inflammation, namely, SOD1 and SOD2 [45] were determined. The gene expression analysis revealed significant increases in SOD1 (≈2-fold) and SOD2 (≈2.8-fold) mRNA levels in M1-Mac as compared to resting Mac. SAHA-induced pharmacological inhibition of HDAC down-regulated SOD1 and SOD2 mRNA expression levels in M1-Mac. Notably, a significant decrease in SOD2 transcript level was detected in M2-Mac in response to SAHA treatment (Fig. 6 in Supplemental file).
4. Discussion
Accumulating evidence indicates that alterations in the gene expression linked to atheroma formation are typically driven by dysregulated epigenetic-based mechanisms acting in conjunction with specific transcription factors [47]. Thus, personalized treatment of atherosclerosis based on epigenetic understanding of patient disease has emerged as an important therapeutic strategy in reducing the burden of CVD [48].
Within the epigenetic landscape, HDAC-based mechanisms have been increasingly connected to atherosclerosis-associated pathological processes including endothelial cell (EC) dysfunction, inflammation, vascular smooth muscle cell (SMC) proliferation and migration, and extracellular matrix synthesis. In cultured Mac, HDAC blockade negatively regulates M1-Mac activation and reduces Mac-derived foam cell formation via histone acetylation-based mechanisms that up-regulate ATP-binding cassette transporters ABCA1 and ABCG1 to induce cholesterol efflux in these cells [24,33,[49], [50], [51]]. Yet, the precise role of HDAC and the rationale of using HDAC inhibitors in atherosclerosis continue to represent an arguable issue [18]. In addition, the role of HDAC in mediating Nox up-regulation and oxidative stress in atherosclerosis is not known.
In this study we focused on HDAC as a potential crossing point epigenetic system that integrate and transduce pro-atherogenic signals to generate vascular oxidative stress. We hypothesized that the atherosclerotic plaque micro-environment drives HDAC-dependent epigenetic changes in vascular cells and infiltrated immune cells leading to abnormal signal transduction that ultimately induce the up-regulation of Nox subtypes and the ensuing oxidative stress, a pathological condition that is acknowledged as an important trigger of inflammatory reactions and metabolic abnormalities in atherogenesis [21].
To tackle this issue we employed human non-, and atherosclerotic tissue samples, ApoE−/− mice, and cultured primary mouse Mon-derived Mac. The main findings of this study are: (1) class I (HDAC1, HDAC2, HDAC3), class IIa (HDAC4), class IIb (HDAC6), and class IV (HDAC11) HDAC are upregulated in atherosclerotic human carotid arteries and in the atherosclerotic aorta of hypercholesterolemic ApoE−/− mice; (2) pharmacological inhibition of HDAC by SAHA significantly reduces the progression of atherosclerosis and of the markers of immune cells infiltration in the aorta of ApoE−/− mice; (3) HDAC-dependent pathways mediate the induction of Nox1 and Nox4 expression, NADPH-stimulated ROS production, and the formation of 4-HNE-protein adducts (marker of oxidative stress) in the aorta of atherosclerotic ApoE−/− mice; (4) SAHA treatment down-regulates the aortic level of NOS2 and MMP9 proteins, major contributors to vascular inflammation and remodelling in atherosclerosis; (5) class I, IIa, IIb, and IV HDAC subtypes along with Nox1, Nox2, and Nox4 isoforms are significantly elevated in mouse pro-inflammatory M1-Mac in vitro; (6) pharmacological blockade of HDAC (by SAHA) reduces the expression levels of Nox1, Nox2, Nox4, and TNFα in cultured mouse M1-like Mac.
The augmented expression of various HDAC subtypes has been found significantly elevated in different experimental models of CVD [12,14,15]. Therefore, several HDAC pharmacological inhibitors have been systematically employed in order to counteract the alterations in gene expression due to epigenetic instability in CVD. Evidence exists that HDAC3 and HDAC9 isoforms are mechanistically connected to atheroma formation [32,33]. To further explore the potential association of other members of the HDAC family with atherosclerosis, we analyzed the expression pattern of key HDAC isoforms in human non-atherosclerotic and atherosclerotic arterial samples. Our data showed that additional HDAC subtypes were significantly up-regulated in human atherosclerotic carotid artery, namely members of class I (HDAC1, HDAC2, HDAC3), class IIa (HDAC4), class IIb (HDAC6), and class IV (HDAC11) HDAC. Notably, within the HDAC family, HDAC1 and HDAC2 display unique biological functions that can not be substituted by other HDAC subtypes [52]. To the best of our knowledge, the present study provides the first evidence that HDAC1, HDAC2, and HDAC11 (less explored in CVD) proteins are induced in advanced human atherosclerotic lesions.
In order to partially recapitulate key pathobiological processes associated with human atherosclerotic disease (i.e., oxidative stress and inflammation), ApoE−/− mice were employed as an in vivo model of atherosclerosis. Our data demonstrated that all HDAC proteins analyzed in human specimens were also found significantly augmented in the atherosclerotic aorta of hypercholesterolemic ApoE−/− mice. Collectively, these results suggest that various HDAC subtypes are similarly regulated in both human and experimental atherosclerosis, and presumably, they direct analogous pathophysiological effects.
Our data are in good agreement and extend a compelling study demonstrating the elevated expression levels of HDAC1, HDAC2, HDAC4, and HDAC7 in human abdominal aortic aneurysm (AAA) and in the aorta of angiotensin II-infused ApoE−/− mice [14]. Based on the fact that both atherosclerosis and AAA are characterized by severe oxidative stress and robust inflammation, we may safely assume that up-regulated HDAC isoforms are important tissue-specific biomarkers and also molecular triggers of key pathological pathways in these vascular diseases. Furthermore, our in vivo findings are consistent with a previous report demonstrating that HDAC1, HDAC2 and HDAC3 proteins are induced by mitogenic stimulation in cultured rat aortic SMC, an experimental set-up that recapitulate atherosclerosis-associated SMC hypertrophy and hyperplasia, a feature of neointima formation, in vitro [19].
Based on the fact that several Zn2+-dependent HDAC isoforms are induced in both human and experimental atherosclerosis, we used a pan-HDAC pharmacological inhibitor, SAHA, to further explore the potential implication of HDAC-dependent pathways in atherogenesis. The results showed that atherosclerotic lesion progression and the protein levels of CD68 and CD45 molecules, indicators of Mac infiltration, were significantly reduced in response to SAHA in the aorta of ApoE−/− mice whereas plasma levels of total cholesterol, LDL-cholesterol, HDL-cholesterol, and triglycerides remained unaffected compared with vehicle-treated ApoE−/− mice fed a high-fat, cholesterol-rich diet.
To uncover the potential down-stream molecular effectors of HDAC, we focused on Nox enzymes, major contributors to ROS overproduction and oxidative stress in atherosclerosis [21]. We found that the protein levels of the Nox catalytic subunits, Nox1 and Nox4, but not Nox2, were significantly reduced following SAHA treatment in the aorta of hypercholesterolemic ApoE−/− mice. Moreover, employing a method that partially detects Nox-derived ROS [53], namely, lucigenin-enhanced chemiluminescence assay, we have determined that SAHA treatment blunted the induction of NADPH-dependent ROS generation in the atherosclerotic aorta ApoE−/− (HD) mice. Interestingly, previous studies demonstrated that the Nox1/4 dual pharmacological inhibitor, GKT137831, reduced aortic inflammation and the development of atherosclerotic lesions in diabetic ApoE−/− mice, suggesting that Nox-derived ROS are essential mediators of diabetes-accelerated atherogenesis [35,36]. Consistent with these findings, we may safely assume that Nox1 and Nox4 down-regulation in response to HDAC blockade in ApoE−/− mice may partially explain the anti-atherosclerotic effects of SAHA, i.e., a reduced production of Nox1/4-derived ROS. Evidence from experimental pulmonary hypertension showed that pan-pharmacological inhibition of HDAC reduced Nox expression and mitigated structural alterations of the pulmonary arteries in rats [12]. Other than that, in a recent study we have demonstrated that HDAC-dependent pathways play an important role in mediating Nox up-regulation, ROS overproduction, and oxidative stress in experimental diabetes in vivo and in vitro [15]. Together, the data of present study extend to experimental atherosclerosis the role of HDAC subtypes as up-stream regulators of Nox expression.
The formation of lipid peroxidation products is acknowledged as an important biomarker of oxidative stress. Therefore, in this study we further assessed the occurrence of oxidative stress in the aorta of ApoE−/− mice by means of 4-HNE production, a major lipid peroxidation product of poly-unsaturated fatty acids. Particularly, we have previously reported that Nox-derived ROS enhanced the formation of 4-HNE in high glucose-exposed human aortic SMCs [38]. The current data demonstrated that treatment of hypercholesterolemic ApoE−/− mice with SAHA led to a significant reduction in the formation of 4-HNE-protein adducts, possibly generated by Nox-induced ROS overproduction in the atherosclerotic aorta of these mice. Yet, the implication of other non-enzymatic or enzymatic pathways of 4-HNE formation should not be excluded.
Most of our current understanding of HDAC expression and function originate from cancer biology studies. HDAC isoforms are up-regulated in most cancer cells, a condition that induce chromatin condensation and consequently, the transcriptional repression of genes that function in tumor suppression, growth arrest, differentiation and apoptosis [54]. Yet, in our study the HDAC inhibitor SAHA, at relatively low concentration, significantly reduced the expression of Nox, oxidative stress, inflammation, and atherosclerotic lesion progression in mice. Notably, the activity of some HDAC inhibitors seems to be very peculiar, featuring pro-inflammatory effects at high concentrations and anti-inflammatory effects at low concentrations [55]. Mechanistically, others and we have shown that various HDAC subtypes control the activities of several pro-inflammatory transcription factors including NF-kB, AP-1, and STAT by molecular means that remain largely unclear [8,15,56]. Based on the fact that these transcription factors are instrumental for Nox up-regulation under inflammatory conditions [[44], [45], [46],57], we may assume that SAHA negatively interferes with the function of specific transcription factors regulating Nox expression. In addition, possible mechanisms of HDAC inhibitor-induced down-regulation of gene expression may involve degradation of p300/CBP, an essential transcriptional co-activator [58].
To further ascertain the involvement of HDAC-dependent pathways in vascular inflammation, the protein expression levels of NOS2 and MMP9 were determined. We found that the expression of both molecules contributing to vascular inflammation and remodelling was reduced in SAHA-treated mice. Moreover, the mRNA expression levels of SOD1 and SOD2, two antioxidant enzymes whose expression is tightly regulated by redox-sensitive pro-inflammatory transcription factors [45], were significantly reduced following SAHA treatment in cultured M1-Mac. This evidence further supports the anti-inflammatory potential of SAHA in experimental atherosclerosis.
Evidence exists that various pharmacological inhibitors of HDAC reduce the pro-inflammatory responses while enhancing the anti-inflammatory functions of M2-polarized Mac in vitro [24]. Yet, the underlying mechanisms and the rationale of using HDAC inhibitors on these cells are scantily elucidated. Thus, we performed experiments on M1-like pro-inflammatory and M2-like anti-inflammatory Mac owing to their important role in atherogenesis [42]. Our data indicated a robust up-regulation of the HDAC transcripts in M1-Mac, suggesting that various HDAC subtypes may play a role in mediating inflammatory Mac functions. Consistent with the findings in mice, augmented Nox1, Nox2, and Nox4 mRNA expression levels were determined in M1-Mac compared with M0-or M2-Mac. Moreover, HDAC blockade by SAHA reduced mRNA levels of Nox isoforms and the gene and protein expression of TNFα in cultured M1-Mac. Collectively, the gene expression profile of the HDAC isoforms provides additional support for the rationale of using HDAC inhibitors on cultured Mac and may fairly explain the potential anti-atherosclerotic effects of HDAC inhibitors.
As mention above, several line of evidence implicates various HDAC subtypes in atherosclerosis either directly or indirectly. Silencing of HDAC1, HDAC2 or HDAC3 subtypes prevented serum-induced vascular SMC proliferation. Notably, similar findings were demonstrated employing scriptaid, a broad-spectrum HDAC inhibitor, both in vitro and in a mouse model of neointima formation [19]. In contrast, other study showed that knockdown of HDAC7 promotes SMC proliferation in vitro [49]. Up-regulation of HDAC3 has been demonstrated in human atherosclerotic lesions, especially in Mac-rich areas. In addition, genetic ablation of HDAC3 in myeloid cells promoted anti-inflammatory Mac function, reduced lipid depositions, and accelerated the production of collagen by vascular SMC via Mac-derived TGF-β secretion, a condition that contributes to the atherosclerotic plaque stabilization in low-density lipoprotein receptor-deficient (LDLr−/− mice) [32]. Consistent with these findings, it was reported that HDAC3 subtype plays a major role in the regulation of pro-inflammatory gene expression in Mac [59]. On the same line, overexpression of HDAC6 induced Nox-derived ROS-dependent inflammatory responses in cultured mouse Mac [50]. Interestingly, the formation of atherosclerotic lesions was reduced in LDLr−/− HDAC9−/− double knockout mice, whereas genetic ablation of HDAC9 in bone morrow cells reduced atherosclerosis and promoted anti-inflammatory Mac polarization in vitro [33].
Considering these independent reports, one can predict that several HDAC subtypes could have overlapping biological functions or are interrelated in the process of atheroma formation. Interestingly, our data provide evidence that in the context of multiple HDAC isoform induction, the pan-HDAC inhibitor SAHA displays similar anti-atherosclerotic effects in mice, comparable to previous reports highlighting that systemic or cell-specific genetic ablation of a single HDAC isoform impedes atherosclerosis. Thus, the existence of compensatory or redundant regulatory mechanisms within HDAC system, as well as the complex networking among HDAC subtypes and cell/gene-specific transcription factors remains open issues. In this context, the rationale of using pan- or isoform specific HDAC inhibitors in atherosclerosis is still questionable and requires further and closer attention.
Collectively, the main findings of this study indicate that in atherosclerosis, pharmacological inhibition of up-regulated HDAC reduces the augmented aortic expression of Nox subtypes and the ensuing ROS overproduction and oxidative stress, decreases the markers of inflammation, and the formation of atherosclerotic lesions in mice. Since multiple HDAC subtypes are also up-regulated in human atherosclerosis, HDAC-oriented pharmacological interventions could be an effective novel or additional therapeutic strategy in atherosclerosis.
Declaration of competing interest
The authors declare no conflict of interest.
Acknowledgements
Work supported by grants from the Romanian National Authority for Scientific Research and Innovation, UEFISCDI [PN–III–P4-ID-PCE-2016-0665, PN–III–P1-1.1-TE-2016-0851, PN-IIIP2-2.1-PED-2016-1308, PN–III–P4-ID-PCCF-2016-0172] and from the Romanian Academy.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.redox.2019.101338.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Collins D.R., Tompson A.C., Onakpoya I.J., Roberts N., Ward A.M., Heneghan C.J. Global cardiovascular risk assessment in the primary prevention of cardiovascular disease in adults: systematic review of systematic reviews. BMJ Open. 2017;7(3) doi: 10.1136/bmjopen-2016-013650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pretorius E. Precision medicine and a patient-orientated approach: is this the future for tracking cardiovascular disorders? Curr. Pharmaceut. Des. 2017;23(6):889–893. doi: 10.2174/1381612822666161006124400. [DOI] [PubMed] [Google Scholar]
- 3.Khyzha N., Alizada A., Wilson M.D., Fish J.E. Epigenetics of atherosclerosis: emerging mechanisms and methods. Trends Mol. Med. 2017;23(4):332–347. doi: 10.1016/j.molmed.2017.02.004. [DOI] [PubMed] [Google Scholar]
- 4.Zhang W., Song M., Qu J., Liu G.H. Epigenetic modifications in cardiovascular aging and diseases. Circ. Res. 2018;123(7):773–786. doi: 10.1161/CIRCRESAHA.118.312497. [DOI] [PubMed] [Google Scholar]
- 5.Costantino S., Libby P., Kishore R., Tardif J.C., El-Osta A., Paneni F. Epigenetics and precision medicine in cardiovascular patients: from basic concepts to the clinical arena. Eur. Heart J. 2018;39(47):4150–4158. doi: 10.1093/eurheartj/ehx568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pons D., de Vries F.R., van den Elsen P.J., Heijmans B.T., Quax P.H., Jukema J.W. Epigenetic histone acetylation modifiers in vascular remodelling: new targets for therapy in cardiovascular disease. Eur. Heart J. 2009;30(3):266–277. doi: 10.1093/eurheartj/ehn603. [DOI] [PubMed] [Google Scholar]
- 7.Wang Y., Miao X., Liu Y., Li F., Liu Q., Sun J., Cai L. Dysregulation of histone acetyltransferases and deacetylases in cardiovascular diseases. Oxidative Med. Cell. Longev. 2014;2014 doi: 10.1155/2014/641979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhou B., Margariti A., Zeng L., Xu Q. Role of histone deacetylases in vascular cell homeostasis and arteriosclerosis. Cardiovasc. Res. 2011;90(3):413–420. doi: 10.1093/cvr/cvr003. [DOI] [PubMed] [Google Scholar]
- 9.Xu S.S., Alam S., Margariti A. Epigenetics in vascular disease - therapeutic potential of new agents. Curr. Vasc. Pharmacol. 2014;12(1):77–86. doi: 10.2174/157016111201140327155551. [DOI] [PubMed] [Google Scholar]
- 10.Kee H.J., Kook H. Roles and targets of class I and IIa histone deacetylases in cardiac hypertrophy. J. Biomed. Biotechnol. 2011;2011 doi: 10.1155/2011/928326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McKinsey T.A. Targeting inflammation in heart failure with histone deacetylase inhibitors. Mol. Med. 2011;17(5–6):434–441. doi: 10.2119/molmed.2011.00022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen F., Li X., Aquadro E., Haigh S., Zhou J., Stepp D.W., Weintraub N.L., Barman S.A., Fulton D.J.R. Inhibition of histone deacetylase reduces transcription of NADPH oxidases and ROS production and ameliorates pulmonary arterial hypertension. Free Radic. Biol. Med. 2016;99:167–178. doi: 10.1016/j.freeradbiomed.2016.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Reddy M.A., Zhang E., Natarajan R. Epigenetic mechanisms in diabetic complications and metabolic memory. Diabetologia. 2015;58(3):443–455. doi: 10.1007/s00125-014-3462-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Galán M., Varona S., Orriols M., Rodríguez J.A., Aguiló S., Dilmé J., Camacho M., Martínez-González J., Rodriguez C. Induction of histone deacetylases (HDACs) in human abdominal aortic aneurysm: therapeutic potential of HDAC inhibitors. Dis. Model. Mech. 2016;9(5):541–552. doi: 10.1242/dmm.024513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Manea S.A., Antonescu M.L., Fenyo I.M., Raicu M., Simionescu M., Manea A. Epigenetic regulation of vascular NADPH oxidase expression and reactive oxygen species production by histone deacetylase-dependent mechanisms in experimental diabetes. Redox Biol. 2018;16:332–343. doi: 10.1016/j.redox.2018.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dokmanovic M., Clarke C., Marks P.A. Histone deacetylase inhibitors: overview and perspectives. Mol. Cancer Res. 2007;5(10):981–989. doi: 10.1158/1541-7786.MCR-07-0324. [DOI] [PubMed] [Google Scholar]
- 17.Wang Z.Y., Qin W., Yi F. Targeting histone deacetylases: perspectives for epigenetic-based therapy in cardio-cerebrovascular disease. J. Geriatr. Cardiol. 2015;12(2):153–164. doi: 10.11909/j.issn.1671-5411.2015.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Choi J.H., Nam K.H., Kim J., Baek M.W., Park J.E., Park H.Y., Kwon H.J., Kwon O.S., Kim D.Y., Oh G.T. Trichostatin A exacerbates atherosclerosis in low density lipoprotein receptor-deficient mice. Arterioscler. Thromb. Vasc. Biol. 2005;25(11):2404–2409. doi: 10.1161/01.ATV.0000184758.07257.88. [DOI] [PubMed] [Google Scholar]
- 19.Findeisen H.M., Gizard F., Zhao Y., Qing H., Heywood E.B., Jones K.L., Cohn D., Bruemmer D. Epigenetic regulation of vascular smooth muscle cell proliferation and neointima formation by histone deacetylase inhibition. Arterioscler. Thromb. Vasc. Biol. 2011;31(4):851–860. doi: 10.1161/ATVBAHA.110.221952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zheng X.X., Zhou T., Wang X.A., Tong X.H., Ding J.W. Histone deacetylases and atherosclerosis. Atherosclerosis. 2015;240(2):355–366. doi: 10.1016/j.atherosclerosis.2014.12.048. [DOI] [PubMed] [Google Scholar]
- 21.Vendrov A.E., Sumida A., Canugovi C., Lozhkin A., Hayami T., Madamanchi N.R., Runge M.S. NOXA1-dependent NADPH oxidase regulates redox signaling and phenotype of vascular smooth muscle cell during atherogenesis. Redox Biol. 2019;21 doi: 10.1016/j.redox.2018.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nakashima Y., Plump A.S., Raines E.W., Breslow J.L., Ross R. ApoE-deficient mice develop lesions of all phases of atherosclerosis throughout the arterial tree. Arterioscler. Thromb. 1994;14(1):133–140. doi: 10.1161/01.atv.14.1.133. [DOI] [PubMed] [Google Scholar]
- 23.Dalgard C.L., Van Quill K.R., O'Brien J.M. Evaluation of the in vitro and in vivo antitumor activity of histone deacetylase inhibitors for the therapy of retinoblastoma. Clin. Cancer Res. 2008;14(10):3113–3123. doi: 10.1158/1078-0432.CCR-07-4836. [DOI] [PubMed] [Google Scholar]
- 24.Van den Bossche J., Neele A.E., Hoeksema M.A., de Heij F., Boshuizen M.C., van der Velden S., de Boer V.C., Reedquist K.A., de Winther M.P. Inhibiting epigenetic enzymes to improve atherogenic macrophage functions. Biochem. Biophys. Res. Commun. 2014;455(3–4):396–402. doi: 10.1016/j.bbrc.2014.11.029. [DOI] [PubMed] [Google Scholar]
- 25.Pfaffl M.W. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29(9):e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stephens A.S., Stephens S.R., Morrison N.A. Internal control genes for quantitative RT-PCR expression analysis in mouse osteoblasts, osteoclasts and macrophages. BMC Res. Notes. 2011;4:410. doi: 10.1186/1756-0500-4-410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shaul M.E., Bennett G., Strissel K.J., Greenberg A.S., Obin M.S. Dynamic, M2-like remodeling phenotypes of CD11c+ adipose tissue macrophages during high-fat diet--induced obesity in mice. Diabetes. 2010;59(5):1171–1181. doi: 10.2337/db09-1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li X., Wu X.Q., Xu T., Li X.F., Yang Y., Li W.X., Huang C., Meng X.M., Li J. Role of histone deacetylases(HDACs) in progression and reversal of liver fibrosis. Toxicol. Appl. Pharmacol. 2016;306:58–68. doi: 10.1016/j.taap.2016.07.003. [DOI] [PubMed] [Google Scholar]
- 29.Zhong Y., Li J., Wang J.J., Chen C., Tran J.T., Saadi A., Yu Q., Le Y.Z., Mandal M.N., Anderson R.E., Zhang S.X. X-box binding protein 1 is essential for the anti-oxidant defense and cell survival in the retinal pigment epithelium. PLoS One. 2012;7(6) doi: 10.1371/journal.pone.0038616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Manea S.A., Fenyo I.M., Manea A. c-Src tyrosine kinase mediates high glucose-induced endothelin-1 expression. Int. J. Biochem. Cell Biol. 2016;75:123–130. doi: 10.1016/j.biocel.2016.04.008. [DOI] [PubMed] [Google Scholar]
- 31.Guzik T.J., Mussa S., Gastaldi D., Sadowski J., Ratnatunga C., Pillai R., Channon K.M. Mechanisms of increased vascular superoxide production in human diabetes mellitus: role of NAD(P)H oxidase and endothelial nitric oxide synthase. Circulation. 2002;105(14):1656–1662. doi: 10.1161/01.cir.0000012748.58444.08. [DOI] [PubMed] [Google Scholar]
- 32.Hoeksema M.A., Gijbels M.J., Van den Bossche J., van der Velden S., Sijm A., Neele A.E., Seijkens T., Stöger J.L., Meiler S., Boshuizen M.C., Dallinga-Thie G.M., Levels J.H., Boon L., Mullican S.E., Spann N.J., Cleutjens J.P., Glass C.K., Lazar M.A., de Vries C.J., Biessen E.A., Daemen M.J., Lutgens E., de Winther M.P. Targeting macrophage histone deacetylase 3 stabilizes atherosclerotic lesions. EMBO Mol. Med. 2014;6(9):1124–1132. doi: 10.15252/emmm.201404170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cao Q., Rong S., Repa J.J., St Clair R., Parks J.S., Mishra N. Histone deacetylase 9 represses cholesterol efflux and alternatively activated macrophages in atherosclerosis development. Arterioscler. Thromb. Vasc. Biol. 2014;34(9):1871–1879. doi: 10.1161/ATVBAHA.114.303393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Azghandi S., Prell C., van der Laan S.W., Schneider M., Malik R., Berer K., Gerdes N., Pasterkamp G., Weber C., Haffner C., Dichgans M. Deficiency of the stroke relevant HDAC9 gene attenuates atherosclerosis in accord with allele-specific effects at 7p21.1. Stroke. 2015;46(1):197–202. doi: 10.1161/STROKEAHA.114.007213. [DOI] [PubMed] [Google Scholar]
- 35.Gray S.P., Jha J.C., Kennedy K., van Bommel E., Chew P., Szyndralewiez C., Touyz R.M., Schmidt H.H.H.W., Cooper M.E., Jandeleit-Dahm K.A.M. Combined NOX1/4 inhibition with GKT137831 in mice provides dose-dependent reno- and atheroprotection even in established micro- and macrovascular disease. Diabetologia. 2017;60(5):927–937. doi: 10.1007/s00125-017-4215-5. [DOI] [PubMed] [Google Scholar]
- 36.Gray S.P., Di Marco E., Okabe J., Szyndralewiez C., Heitz F., Montezano A.C., de Haan J.B., Koulis C., El-Osta A., Andrews K.L., Chin-Dusting J.P., Touyz R.M., Wingler K., Cooper M.E., Schmidt H.H., Jandeleit-Dahm K.A. NADPH oxidase 1 plays a key role in diabetes mellitus-accelerated atherosclerosis. Circulation. 2013;127(18):1888–1902. doi: 10.1161/CIRCULATIONAHA.112.132159. [DOI] [PubMed] [Google Scholar]
- 37.Uchida K., Shibata T., Toyokuni S., Daniel B., Zarkovic K., Zarkovic N., Sasson S. Development of a novel monoclonal antibody against 4-hydroxy-2E,6Z-dodecadienal (4-HDDE)-protein adducts: immunochemical application in quantitative and qualitative analyses of lipid peroxidation in vitro and ex vivo. Free Radic. Biol. Med. 2018;124:12–20. doi: 10.1016/j.freeradbiomed.2018.05.079. [DOI] [PubMed] [Google Scholar]
- 38.Manea A., Manea S.A., Todirita A., Albulescu I.C., Raicu M., Sasson S., Simionescu M. High-glucose-increased expression and activation of NADPH oxidase in human vascular smooth muscle cells is mediated by 4-hydroxynonenal-activated PPARα and PPARβ/δ. Cell Tissue Res. 2015;361(2):593–604. doi: 10.1007/s00441-015-2120-0. [DOI] [PubMed] [Google Scholar]
- 39.Vacek T.P., Rehman S., Neamtu D., Yu S., Givimani Sr, Tyagi S.C. Matrix metalloproteinases in atherosclerosis: role of nitric oxide, hydrogen sulfide, homocysteine, and polymorphisms. Vasc. Health Risk Manag. 2015;11:173–183. doi: 10.2147/VHRM.S68415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gliozzi M., Scicchitano M., Bosco F., Musolino V., Carresi C., Scarano F., Maiuolo J., Nucera S., Maretta A., Paone S., Mollace R., Ruga S., Zito M.C., Macrì R., Oppedisano F., Palma E., Salvemini D., Muscoli C., Mollace V. Modulation of nitric oxide synthases by oxidized LDLs: role in vascular inflammation and atherosclerosis development. Int. J. Mol. Sci. 2019;20(13) doi: 10.3390/ijms20133294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Manea A., Simionescu M. Nox enzymes and oxidative stress in atherosclerosis. Front. Biosci. (Sch. Ed.) 2012;4:651–670. doi: 10.2741/s291. [DOI] [PubMed] [Google Scholar]
- 42.Chinetti-Gbaguidi G., Colin S., Staels B. Macrophage subsets in atherosclerosis. Nat. Rev. Cardiol. 2015;12(1):10–17. doi: 10.1038/nrcardio.2014.173. [DOI] [PubMed] [Google Scholar]
- 43.Helfinger V., Palfi K., Weigert A., Schröder K. The NADPH oxidase Nox4 controls macrophage polarization in an NFκB-dependent manner. Oxidative Med. Cell. Longev. 2019;2019 doi: 10.1155/2019/3264858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Manea A., Tanase L.I., Raicu M., Simionescu M. Jak/STAT signaling pathway regulates nox1 and nox4-based NADPH oxidase in human aortic smooth muscle cells. Arterioscler. Thromb. Vasc. Biol. 2010;30(1):105–112. doi: 10.1161/ATVBAHA.109.193896. [DOI] [PubMed] [Google Scholar]
- 45.Manea A., Tanase L.I., Raicu M., Simionescu M. Transcriptional regulation of NADPH oxidase isoforms, Nox1 and Nox4, by nuclear factor-kappaB in human aortic smooth muscle cells. Biochem. Biophys. Res. Commun. 2010;396(4):901–907. doi: 10.1016/j.bbrc.2010.05.019. [DOI] [PubMed] [Google Scholar]
- 46.Manea A., Manea S.A., Florea I.C., Luca C.M., Raicu M. Positive regulation of NADPH oxidase 5 by proinflammatory-related mechanisms in human aortic smooth muscle cells. Free Radic. Biol. Med. 2012;52(9):1497–1507. doi: 10.1016/j.freeradbiomed.2012.02.018. [DOI] [PubMed] [Google Scholar]
- 47.Grimaldi V., Vietri M.T., Schiano C., Picascia A., De Pascale M.R., Fiorito C., Casamassimi A., Napoli C. Epigenetic reprogramming in atherosclerosis. Curr. Atheroscler. Rep. 2015;17(2):476. doi: 10.1007/s11883-014-0476-3. [DOI] [PubMed] [Google Scholar]
- 48.Jain K.K. Personalized management of cardiovascular disorders. Med. Princ. Pract. 2017;26(5):399–414. doi: 10.1159/000481403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhou B., Margariti A., Zeng L., Habi O., Xiao Q., Martin D., Wang G., Hu Y., Wang X., Xu Q. Splicing of histone deacetylase 7 modulates smooth muscle cell proliferation and neointima formation through nuclear β-catenin translocation. Arterioscler. Thromb. Vasc. Biol. 2011;31(11):2676–2684. doi: 10.1161/ATVBAHA.111.230888. [DOI] [PubMed] [Google Scholar]
- 50.Youn G.S., Lee K.W., Choi S.Y., Park J. Overexpression of HDAC6 induces pro-inflammatory responses by regulating ROS-MAPK-NF-κB/AP-1 signaling pathways in macrophages. Free Radic. Biol. Med. 2016;97:14–23. doi: 10.1016/j.freeradbiomed.2016.05.014. [DOI] [PubMed] [Google Scholar]
- 51.Kietzmann T., Petry A., Shvetsova A., Gerhold J.M., Görlach A. The epigenetic landscape related to reactive oxygen species formation in the cardiovascular system. Br. J. Pharmacol. 2017;174(12):1533–1554. doi: 10.1111/bph.13792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jamaladdin S., Kelly R.D., O'Regan L., Dovey O.M., Hodson G.E., Millard C.J., Portolano N., Fry A.M., Schwabe J.W., Cowley S.M. Histone deacetylase (HDAC) 1 and 2 are essential for accurate cell division and the pluripotency of embryonic stem cells. Proc. Natl. Acad. Sci. U. S. A. 2014;111(27):9840–9845. doi: 10.1073/pnas.1321330111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rezende F., Löwe O., Helfinger V., Prior K.K., Walter M., Zukunft S., Fleming I., Weissmann N., Brandes R.P., Schröder K. Unchanged NADPH oxidase activity in Nox1-Nox2-Nox4 triple knockout mice: what do NADPH-stimulated chemiluminescence assays really detect? Antioxidants Redox Signal. 2016;24(7):392–399. doi: 10.1089/ars.2015.6314. [DOI] [PubMed] [Google Scholar]
- 54.Li Y., Seto E. HDACs and HDAC Inhibitors in cancer development and therapy. Cold Spring Harb. Persp. Med. 2016;6(10) doi: 10.1101/cshperspect.a026831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Halili M.A., Andrews M.R., Labzin L.I., Schroder K., Matthias G., Cao C., Lovelace E., Reid R.C., Le G.T., Hume D.A., Irvine K.M., Matthias P., Fairlie D.P., Sweet M.J. Differential effects of selective HDAC inhibitors on macrophage inflammatory responses to the Toll-like receptor 4 agonist LPS. J. Leukoc. Biol. 2010;87(6):1103–1114. doi: 10.1189/jlb.0509363. [DOI] [PubMed] [Google Scholar]
- 56.Chen L., Fischle W., Verdin E., Greene W.C. Duration of nuclear NF-kappaB action regulated by reversible acetylation. Science. 2001;293(5535):1653–1657. doi: 10.1126/science.1062374. [DOI] [PubMed] [Google Scholar]
- 57.Manea A., Manea S.A., Gafencu A.V., Raicu M., Simionescu M. AP-1-dependent transcriptional regulation of NADPH oxidase in human aortic smooth muscle cells: role of p22phox subunit. Arterioscler. Thromb. Vasc. Biol. 2008;28(5):878–885. doi: 10.1161/ATVBAHA.108.163592. [DOI] [PubMed] [Google Scholar]
- 58.Hakami N.Y., Dusting G.J., Peshavariya H.M. Trichostatin A, a histone deacetylase inhibitor suppresses NADPH oxidase 4-derived redox signalling and angiogenesis. J. Cell Mol. Med. 2016;20(10):1932–1944. doi: 10.1111/jcmm.12885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chen X., Barozzi I., Termanini A., Prosperini E., Recchiuti A., Dalli J., Mietton F., Matteoli G., Hiebert S., Natoli G. Requirement for the histone deacetylase Hdac3 for the inflammatory gene expression program in macrophages. Proc. Natl. Acad. Sci. U. S. A. 2012;109(42):E2865–E2874. doi: 10.1073/pnas.1121131109. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.