Abstract
Background
There is a growing need to develop rapid laboratory research methods to counter the menace of drug resistant tuberculosis (MDR‐TB) cases worldwide especially in developing countries. The present study was undertaken to investigate the type and frequency of rpoB and katG mutations in rifampicin (RIF) and isoniazid (INH) resistant strains respectively of Mycobacterium tuberculosis (MTB) circulating in Northern India and to explore the utility of multiplex‐allele‐specific (MAS)‐PCR assay for detection of drug‐resistant MTB isolates in low resource set up. J. Clin. Lab. Anal. 27:31–37, 2013. © 2012 Wiley Periodicals, Inc.
Methods
Phenotypic and genotypic drug susceptibility testing (DST) was performed on 354 MTB isolates.
Results
Mutation in rpoB gene was found most frequently at codons 531, 526 and 516 (59.83%, 45.29% and 22.22%, respectively). Further, combinations of 2–3 point mutations were also observed in 19.66% of RIF‐resistant MTB strains. The frequency of mutations in katG gene was found at codon 315 among 82.95% of the INH‐resistant MTB isolates. MAS‐PCR detected rpoB and katG mutations in phenotypically resistant isolates with sensitivities of 93% and 83% respectively.
Conclusion
MAS‐PCR assays can be used for rapid detection of drug‐resistant TB strains in routine diagnostic practice, enabling early administration of appropriate treatment regimens to the affected patients.
Keywords: Drug‐resistant TB, rifamipicin, isoniazid, point mutation, sensitivity
INTRODUCTION
India has the highest number of tuberculosis (TB) cases in the world with the situation worsening by the appearance of drug resistant especially multidrug‐resistant (MDR) and extensively drug‐resistant (XDR) strains of Mycobacterium tuberculosis (MTB). A large scale population based survey in several states of India indicated multidrug resistance levels of 3% among new TB cases and 12–17% among previously treated TB patients. Further, the annual incidence of MDR‐TB is estimated to be 99,000 cases 1. MDR‐TB is a form of TB that is difficult and expensive to treat because it fails to respond to two important first‐line drugs rifampicin (RIF) and isoniazid (INH) 2. The development of drug resistance may be a tragedy not only for the patients himself but for others as they can infect other people with their drug‐resistant organisms. To meet the targets set in the global plan, diagnosis and treatment of MDR‐TB needs to be rapidly scaled up in five highly affected countries including India 3.
RIF together with INH is the most important cocktail of drugs used to treat TB. Many studies have reported that mutations in three codons (516, 526, or 531) of the rpoB gene explained the majority (70–95%) of RIF resistant strains 4. Further, approximately 50 to 95% of INH‐resistant strains have been found to contain mutations in codon 315 of the katG gene 5, 6.
Presently, only 2% of MDR‐TB cases worldwide are being diagnosed, mainly because of inadequate laboratory services 7. Conventional drug‐susceptibility testing (DST), still considered as gold standard method for determination of drug resistance, has limitations of long turnaround time of about 4–6 weeks. Modern broth‐based culture systems, such as Mycobacteria Growth Indicator Tube MGIT 960 (Becton Dickinson, Cockeysville, MD) or BacT/ALERT 3D (bioMerieux, Durham, NC), are somewhat quicker but are expensive and need sophisticated equipments. To address this growing crisis, WHO is repeatedly suggesting that developing countries have to use rapid molecular‐based assays for the diagnosis of drug‐resistant TB bacilli 8, 9. Molecular tests can provide results within a day or two and thus enable us for early and appropriate treatment of the drug‐resistant cases. This not only reduces morbidity and mortality of MDR TB cases but also interrupt transmission of drug‐resistant TB strains.
Multiplex allele‐specific PCR (MAS‐PCR) assay was first described by Mokrousov et al. 10 for the detection of embB306 mutations in ethambutol (EMB) resistant MTB strains. Although a number of studies have been carried out to evaluate the performance of MAS‐PCR assay 11, 12, to the best of our knowledge its utility is still awaiting to be explored in Indian set up.
The aims of the present study were twofold: first, to observe the type and frequency of rpoB and katG mutations in RIF and INH resistant isolates circulating in Northern India and second, to appraise clinical efficacy of MAS‐PCR assay in comparison with conventional proportion method (PM) and DNA sequencing for the detection of RIF‐resistant and INH‐resistant TB strains in a low resource setting.
PATIENTS AND METHODS
Settings
Study was conducted in Department of Microbiology, Institute of Medical Sciences, Banaras Hindu University (BHU). Sir Sundar Lal (SS) Hospital, a tertiary‐care hospital of BHU, has a vast catchments area, being the only tertiary care hospital in North Eastern Uttar Pradesh (UP) providing medical cover to more than 15 crore of population of Eastern UP, Western Bihar, adjoining areas of Madhya Pradesh, and Nepal. MTB isolates (n = 354), included in the present study were isolated, during January 2008 to January 2010. It included samples from both inpatients and outpatients.
Specimens/Mycobacterial Strains/Phenotypic DST
Pulmonary (85.72%) and extrapulmonary (14.28%) specimens from 3,746 clinically suspected TB patients were collected. Extrapulmonary specimens included pus, endometrial tissue, pleural fluid, lymph node aspirates, urine, and cerebrospinal fluid. Three hundred fifty‐four MTB strains were isolated, identified, and subjected to indirect DST according to the gold standard PM with the recommended critical concentrations of 40 μg/ml for RIF, 0.2 μg/ml for INH, 2 μg/ml for EMB, and 4 μg/ml for streptomycin (STR; 13, 14. H37Rv (ATCC 27294) and a known MDR strain were used as negative and positive controls, respectively.
MAS‐PCR Assays for Detection of RIF and INH Resistance Determinants
DNA was isolated as described by van Embden et al. 15 with minor modifications. A two‐step MAS‐PCR assay was performed to detect mutations at rpoB codons (516, 526, and 531) and katG codon 315 using 354 purified MTB DNA. Two outer (forward and reverse) and three inner forward primers were used for three MAS‐PCRs targeting three different codons of the rpoB gene while two outer and one inner reverse primers were used for katG315 MAS PCR (Table 1). The compositions of reaction mixtures and PCR conditions for each of three different rpoB codons and katG315 codon were as described by Mokrousov et al. 16, 17 with few modifications. Briefly, for rpoBMAS‐PCR each 25‐μl reaction mixture contained 0.5‐μl purified DNA sample, MgCl2 (2.5 mM for rpoB526‐ and rpoB531‐PCR or 1.5 mM for rpoB516‐PCR), 1 U of recombinant Taq DNA polymerase (Bangalore Genei, Bangalore), 200 μM concentrations of each of the deoxynucleoside triphosphates (dNTPs), outer forward (5 pmol), and outer reverse primer (50 pmol for rpoB526‐ and rpoB531‐PCR, or 10 pmol for rpoB516‐PCR) and one of allele‐specific inner forward primers R4 (50 pmol) or R5 (50 pmol), or R3 (15 pmol). The reactions of rpoB526‐PCR and rpoB531‐PCR were performed under the following conditions: initial denaturation at 96°C for 3 min; five cycles of 95°C for 45 sec, 62°C for 50sec, and 72°C for 20 sec; five cycles of 95°C for 40 sec, 60°C for 50 sec, and 72°C for 20 sec; 20 cycles 94°C for 50 sec, 58°C for 40 sec, and 70°C for 20 sec; and final elongation at 72°C for 3 min. The conditions of rpoB516‐PCR were as follows: 96°C for 3 min; 30 cycles of 95°C for 50 sec, 65°C for 45 sec, and 72°C for 20 sec; and 72°C for 3 min. For katG 315 MAS‐PCR, each 30‐μl reaction mixture contained 0.5 μl purified DNA sample, 1.5 mM MgCl2, 1 U of recombinant Taq DNA polymerase (Bangalore Genei), 200 μM concentrations of each of the dNTPs, outer forward (30 pmol) and outer reverse primer (40 pmol, and one of allele‐specific inner reverse primer (30 pmol). The PCR conditions are as follows: initial denaturation at 96°C for 3 min; five cycles of 95°C for 1 min, 68°C for 1 min, and 72°C for 30 sec; five cycles of 95°C for 1 min, 66°C for 40 sec, and 72°C for 30 sec; 20 cycles 94°C for 1 min, 64°C for 40 sec, and 72°C for 30 sec; and final elongation at 72°C for 3 min.
Table 1.
Description of Primers for the Amplification of rpoB and katG Gene Segments and Nucleotide Changes Responsible for RIF and INH Resistance
| Codons/ | Nucleotide | ||||||
|---|---|---|---|---|---|---|---|
| H37Rv | change (amino | Sequence | Size | Position | Reference | ||
| Genes | Primer | sequence | acid change) | (5′‐3′) | (bp) | (H37Rv) | (Primers) |
| rpoB | Outer F (R1) | GTC GCC GCG ATC AAG GA | 249 | 1252–1500 | Mokrousov et al., 2003 | ||
| Outer R (R2) | TGA CCC GCG CGT ACA C | ||||||
| Inner F (R3) | 516/GAC | GAC → GTC or TAC (Ser → Leu or Trp) | GCT GAG CCA ATT CAT GGA | 214 | 1287–1500 | ||
| Inner F (R4) | 526/CAC | CAC → CGC or GAC or TAC or AAC or GGC (His → Arg or Asp or Tyr or Asn or Gly) | GTC GGG GTT GAC CCA | 181 | 1320–1500 | ||
| Inner F (R5) | 531/TCG | TCG → TTG or TGG (Ser → Leu or Trp) | ACA AGC GCC GAC TGT C | 167 | 1334–1500 | ||
| katG | Outer F (K1) | GCA GAT GGG GCT GAT CTA CG | 435 | 669–1103 | Mokrousov et al., 2002 | ||
| Outer R (K2) | AAC GGG TCC GGG ATG GTG | ||||||
| Inner R (K3) | 315/AGC | AGC → ACC & ACA (Ser → Thr) | ATA CGA CCT CGA TGC CGC | 293 | 669–961 | ||
| Inner R (K4) Inner R (K5) | 315 aACC | AGC → ACC (Ser → Thr) | ATA CGA CCT CGA TGC CGG | 293 | _ | Lavender et al., 2005 | |
| 315 aACA | AGC → ACA (Ser → Thr) | ATA CGA CCT CGA TGC CTG | 293 | _ |
For mutant allele; F, forward; R, reverse.
To check the specificity and reproducibility of katG315 MAS‐PCR assay, a modified MAS‐PCR assay 12, which detected mutated alleles instead of wild‐type alleles, was performed. Those isolates recognized as not having a wild‐type katG codon 315 (AGC) were then screened using K4 and K5 mutant allele‐specific primers (Table 1) to detect the presence of katG315 ACC or ACA mutations, respectively.
After amplification, the amplicon were analyzed by 1.5% agarose gel electrophoresis. Isolate with wild‐type codon (either rpoB 531, 526, 516, or katG 315) produced two PCR products while those having mutation at respective codon produced only one PCR product (Supporting information Fig. 2 file 1).
DNA Sequence Analysis
To verify point mutations (single, double, and triple) detected by MAS‐PCR assays and to analyze the samples having discrepancy between the results of PM and MAS‐PCR assays, 34 (14 susceptible and 20 resistant), each of 249 bp rpoB and 435 bp katG preamplified fragments were randomly selected for DNA sequence analysis. The amplified DNA fragment was purified using a HiYield™ Gel/PCR DNA Extraction Kit (Real Biotech Co., Taipei County, Taiwan) and DNA sequencing was performed by using a BigDye Terminator v3.1 cycle sequencing reaction kit (Applied Biosystems, Foster City, CA) in an ABI prism 3130 Genetic Analyzer Machine. DNA sequence analysis and comparisons of rpoB and katG were carried out with Chromas V.2.3 and ClustalV programs.
Ethical Issues
The study was approved by the ethical committee of the Institution.
RESULTS
Clinical Characteristics
In our study, of 3,746 specimens, 366 were culture positive among which 354 (96.72%) were tubercle and 12 (3.39%) were nontubercle bacilli. Of 354 tubercle bacilli, 13 (3.67%) were from extrapulmonary and 341 (96.33%) were from pulmonary TB (PTB) cases. Further, new and retreated cases were 230 (64.97%) and 124 (35.03%), respectively. It was observed that 66% cases were males and 34% females among culture positive subjects. Most cases of PTB were in the age group of 21–40 years. Extrapulmonary TB was documented mostly in the age group of <10 years. DST for all four first‐line anti‐TB drugs was performed, i.e., RIF, INH, STR, and EMB. Among all MTB isolates included in this study, 117, 129, 123, and 113 were detected resistant to RIF, INH, STR, and EMB, respectively, by PM while 104 strains (29.38%) were detected MDR. Further, among 171 any drug‐resistant cases, 72 (42.10%) and 99 (57.89%) were detected primary and acquired drug‐resistant cases, respectively.
While performing MAS‐PCR assay for detecting mutations in RIF resistance determining region (RRDR) of rpoB gene sequence (codons 516, 526, and 531), all 237 RIF‐susceptible and 109 of 117 RIF‐resistant strains produced two and one PCR products for wild type and mutant allele, respectively (Fig. 1A–C). Single‐point mutations resulting in amino‐acid change were found in 86 (73.54%) isolates, while 19 (16.24%) and four (3.42%) isolates had double and triple mutations, respectively (Supporting information Table 2). Amino‐acid change at codon 531 occurred with the highest frequency (59.83%), followed by those at codon 526 (45.29%) and codon 516 (22.22%).
Figure 1.
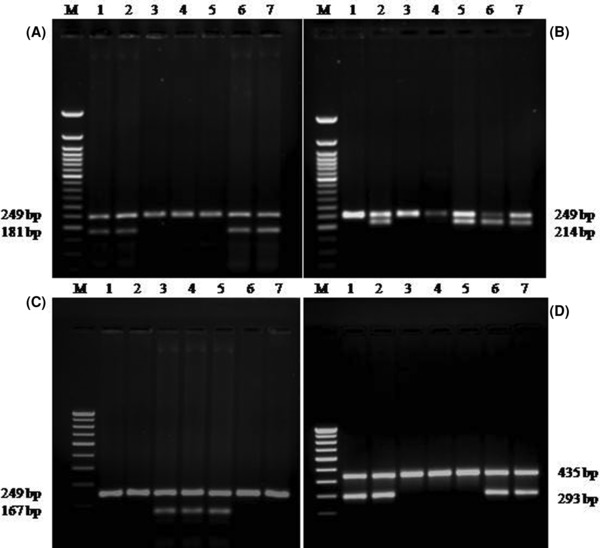
Agarose gel electrophoresis patterns of MAS–PCR assays: (A) rpoB526 analysis: Lanes: 3‐5, and 1,2,6 strains with rpoB526 mutant and susceptible alleles respectively; Lane 7: H37Rv. (B) rpoB516 analysis: Lanes: 1, 3, 4, and 2,5,6 strains with rpoB516 mutant and susceptible alleles respectively; Lane 7: H37 Rv. (C) rpoB531 analysis: Lanes: 1, 2, 6,7, and 3,4 strains with rpoB531 mutant and susceptible alleles respectively; Lane 5: H37Rv. (D) katG315 analysis: Lanes 3,4,5, and 1,2,6 strains with katG315 mutant and susceptible alleles respectively; Lane 7: H37Rv. M (Molecular marker): (A & B) 50‐bp, (C & D) 100‐bp DNA ladder (Bangalore Gene, Bangalore).
All 225 INH susceptible strains produced two PCR products, one of which was 293 bp allele specific (Fig. 1D, lanes 1, 2, 6, and 7), implying no mutation in the katG315 second base and another 435 bp, amplification product of entire region under study. A single MAS‐PCR 435‐bp fragment was amplified in 107/129 INH‐resistant strains (Fig. 1D, lanes 3–5). Of 129 INH‐resistant isolates, 107 (82.94%) had mutations at katG codon 315. The wild‐type codon, AGC (Ser), was altered to ACC (Thr) in 98 (91.59%) strains and ACA (Thr) in nine (8.4%) strains.
The results obtained by MAS‐PCR assays were compared with respect to internationally accepted gold standard PM. Of 117 MTB isolates, which were found to be resistant to RIF by PM, 109 isolates were found to have mutations in RRDR region of rpoB gene sequence. None of the strains, which were RIF susceptible (n = 237), had shown mutation in the above mentioned gene sequence by MAS‐PCR assay. Further, of 129 INH resistant isolates of MTB, 107 isolates were found to have mutations in katG315 gene sequence but none of the INH susceptible strains (n = 225) had shown mutation in the same gene sequence.
The sensitivities and specificities of MAS‐PCR assay compared to that of PM were observed to be 93 and 100% for RIF and 83 and 100% for INH, respectively. Further, negative predictive values and positive predictive values were found to be 97 and 100% for RIF and 91 and 100% for INH, respectively. The accuracy of MAS‐PCR assay was appreciable with the standard PM for both the drugs, i.e., INH (94%) and RIF (98%) (Supporting information Table 3 file 3).
Results of DNA sequencing showed that all 14 INH, RIF‐susceptible and 16 of 20 INH, RIF‐resistant strains produced same results as compared to PM and MAS‐PCR assays. Another four INH strains that were detected resistant by PM and susceptible by MAS‐PCR showed no mutation in 435 bp katG region targeting codon 315. Further, among four selected RIF strains that were detected resistant by PM and susceptible by MAS‐PCR; two strains showed no mutation in RRDR while in another two, one showed deletion within RRDR and another mutation at codon 522 (TCG to CCG alteration).
DISCUSSION
Understanding the nature and frequency of mutations associated with drug resistance in MTB in different clinical settings is important for the development and large‐scale implementation of rapid, genetics‐based assays for the diagnosis of drug resistance.
While performing the PM for DST of MTB isolates in this study, no significant differences between the resistance rates of STR (34.75%), INH (36.44%), RIF (33.1%), EMB (31.92%), and MDR (29.38%) were found. The percentage of drug resistance cases and MDR‐TB was observed to be quite high because our samples were obtained from a tertiary care center; sampling bias cannot be ruled out.
The present study revealed that 93.16% of RIF‐resistant isolates had rpoB gene mutations in RRDR, 73.5, 16.24, and 3.42% of these mutations were single‐, double‐, and triple‐point mutations, respectively. Prevalence of two‐ to three‐point mutations was high in our region with 19.66% (23 of 117) cases. In a study from India 18, the prevalence of double–triple point mutations was found in 48.57% (17 of 35), which was twofold in comparison to our setting. Further, a study from Thailand 11 showed 5.2% and another study from China 19 observed 12.4% prevalence of two‐ to three‐point mutations, which was low in comparison to our findings.
In the present study, among RIF‐resistant isolates, 59.83% had mutation in codon 531 of rpoB gene, which was similar to other studies; 58% 11, 60% 20, and 59% 21. However, a higher proportion, i.e., 70.5% was reported by Barnard et al. 22. The frequency of mutation at codon 526 was observed among 45.29% of RIF‐resistant isolates, which was found to be much higher than that of the studies from four east Asian countries 23, Latvia 24, and India 20, which ranged between 10% and 27%. The rate of mutation at 516 was higher (22.22%) than the range reported elsewhere 20, 22, 25. However, our observation is in accordance with reports from East Asia 23 and Latvia 24, where higher mutational frequencies in codon 516 (15–32%) along with a predominant Beijing genotype were reported. We have also observed a higher frequency of Beijing genotype in our region (data not shown).
In our study, 107 of 129 INH‐resistant isolates (82.95%) had mutations at katG315. Similar results were reported in Latvia and Iran 24, 26. However, the frequency of katGS315T mutation may vary according to geographical area, even within a single country. A study from India has reported 64% of 70 isolates with the same mutation 27. Similarly, 62% of 79 isolates from Spain and 67% of 37 isolates from the North of Mexico have been reported 28, 29. In partial agreement, in another study, no variability was observed in the mutation frequency of codon 315; a mutation was observed in 100% of 92 isolates from Kazakhstan, Belarus 30. Hence, this mutation needs to be analyzed in a local context, not by international comparison. Furthermore, in our study, the wild‐type codon, AGC (Ser), was altered to ACC (Thr) in 97 (77%) strains and ACA (Thr) in nine (7.14%) strains while a study from Australia 12 reported the alteration of wild‐type codon, AGC (Ser) to ACC (Thr) in 31 (60%) and ACA (Thr) in three (6%) strains.
Present study observed a very good sensitivities and specificities of MAS‐PCR assay compared to that of PM with 93 and 100% for RIF and 83 and 100% for INH, respectively (Supporting information Table 3). Similar results were also observed by Prammananan et al. 11. Further, a study from Turkey 31 showed sensitivity and specificity to be 81.1 and 97.5% for INH, 93.0 and 98.9% for RIF respectively. Moreover, Tho et al. 32 found the sensitivity and specificity of the MAS‐PCR to be 83.7 and 100%, respectively for RIF.
We were able to sequence only a limited number of MTB isolates because of low resource set up but that 34 strains are representative strains and succeeded to give the answer of our almost all queries. Similarly, Leung et al. 33 sequenced only 20 MTB isolates (ten INH susceptible and ten INH resistant) to verify the point mutations detected by PCR‐RFLP in his setting and found 100% sequence accuracy.
Among those RIF/INH‐resistant isolates, in which we were unable to detect the expected mutations employing the above protocol of MAS PCR assay, there might be presence of unusual mutations responsible for the resistance with the same drugs. RIF resistance might be due to mutations in other codons such as 511, 513, 516, 518, 522, and 533 of RRDR of the rpoB gene 4. Similarly, INH resistance could be due to mutations in inhA regulatory region or ahpC‐oxyR intergenic region, or katG mutations outside of codon 315 5, 6. Further, simultaneous presence of susceptible and resistant isolates in culture from a single patient, a condition termed as heteroresistance, could be an obstacle against the sensitivity of molecular drug resistance testing and successive therapy 34. Hence, results indicating susceptibility should be interpreted carefully.
CONCLUSION
It may, therefore, be concluded that in our region the combinations of two‐ to three‐point mutations were prevalent in RIF‐resistant MTB strains. MAS‐PCR assay is rapid, cost effective, and easy to perform DNA‐based protocol for the detection of RIF and INH resistance. Its implementation would be useful for early diagnosis of drug‐resistant TB, which may facilitate provision of individualized treatment regimens in patients harboring them.
Supporting information
Disclaimer: Supplementary materials have been peer‐reviewed but not copyedited.
Figure 2
Table 2. Frequency and type of rpoB gene alterations in RIF resistance isolates of MTB
Table 3. Results of the MAS‐PCR compared with those obtained using the proportion method
ACKNOWLEDGEMENTS
We are thankful to all the patients and volunteers who were enrolled in the present study. Financial assistance from DST – PURSE GRANT, Department of Microbiology; IMS, BHU, Varanasi; and ICMR, New Delhi, India has been thankfully acknowledged.
Grant sponsor: DST – PURSE GRANT, Department of Microbiology; Grant sponsor: IMS, BHU; Grant sponsor: ICMR.
REFERENCES
- 1. RNTCP, Annual Status Report 2009. Available from: http://www.tbcindia.org (http://planningcommission.nic.in/reports/genrep/health/RNTCP_2011.pdf) Accessed on 2012 July 20.
- 2. Centers for Disease Control and Prevention (CDC) . Trends in tuberculosis – United States, 2008. Morb Mortal Wkly Rep 2009;58:249–253. [PubMed] [Google Scholar]
- 3. WHO . Global Tuberculosis Control: Epidemiology, Strategy, Financing WHO Report 2009. Geneva: World Health Organization; 2009. (WHO /HTM/ TB/2009.411). [Google Scholar]
- 4. Ramaswamy S, Musser JM. Molecular genetic basis of antimicrobial agent resistance in Mycobacterium tuberculosis: 1998 update. Tuberc Lung Dis 1998;79:3–29. [DOI] [PubMed] [Google Scholar]
- 5. Musser JM, Kapur V, Williams DL, Kreiswirth BN, van Soolingen D, van Embden JD. Characterization of the catalase‐peroxidase gene (katG) and inhA locus in isoniazid‐resistant and ‐susceptible strains of Mycobacterium tuberculosis by automated DNA sequencing: Restricted array of mutations associated with drug resistance. J Infect Dis 1996:173:196–202. [DOI] [PubMed] [Google Scholar]
- 6. Hillemann D, Weizenegger M, Kubica T, Richter E, Niemann S. Use of the genotype MTBDR assay for rapid detection of rifampin and isoniazid resistance in Mycobacterium tuberculosis complex isolates. J Clin Microbiol 2005;43:3699–3703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Stop TB Partnership WHO . Working with the media: How to make your messages on tuberculosis count. World Health Organization, Geneva, Switzerland; 2010. (http://www.stoptb.org/assets/documents/resources/publications/acsm/Working%20with%20the%20Media%20Final%20Web.pdf) Accessed on 2012 July 20. [Google Scholar]
- 8. Stop TB Department, World Health Organization . 7th meeting of the strategic and technical advisory group for tuberculosis (STAG‐TB). Report on conclusions and recommendations. Geneva: Switzerland, The Organization; 2007. [Google Scholar]
- 9. WHO . Policy statement Molecular line probe assays for rapid screening of patients at risk of multidrug‐resistant tuberculosis (MDR‐TB). Geneva: World Health Organization; 2008. [Google Scholar]
- 10. Mokrousov I, Narvskaya O, Limeschenko E, Otten T, Vyshnevskiy B. Detection of ethambutol‐resistant Mycobacterium tuberculosis strains by multiplex allele‐specific PCR assay targeting embB306 mutations. J Clin Microbiol 2002;40:1617–1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Prammananan T, Cheunoy W, Taechamahapun D, et al. Distribution of rpoB mutations among multidrug‐resistant Mycobacterium tuberculosis (MDRTB) strains from Thailand and development of a rapid method for mutation detection. Clin Microbiol Infect 2008;14:446–453. [DOI] [PubMed] [Google Scholar]
- 12. Lavender C, Globan M, Sievers A, Billman‐Jacobe H, Fyfe J. Molecular characterization of isoniazid‐resistant Mycobacterium tuberculosis isolates collected in Australia. Antimicrob Agents Chemother 2005;10:4068–4074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Canetti G, Froman S, Grosset J, et al. Mycobacteria: Laboratory methods for testing drug sensitivity and resistance. Bull World Health Organ 1963;29:565–578. [PMC free article] [PubMed] [Google Scholar]
- 14. Canetti G, Fox W, Khomenko A, et al. Advances in techniques of testing mycobacterial drug sensitivity, and the use of sensitivity tests in tuberculosis control programmes. Bull World Health Organ 1969;41:21–43. [PMC free article] [PubMed] [Google Scholar]
- 15. Van Embden JDA, Cave MD, Crawford JT, et al. Strain identification of Mycobacterium tuberculosis by DNA fingerprinting: Recommendations for a standardized methodology. J Clin Microbiol 1993;31:406–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mokrousov I, Otten T, Filipenko M, et al. Detection of isoniazid‐resistant Mycobacterium tuberculosis strains by a multiplex allele‐specific PCR assay targeting katG codon 315 variation. J Clin Microbiol 2002;40:2509–2512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mokrousov I, Otten T, Vyshnevskiy B, Narvskaya O. Allele‐specific rpoB PCR assays for detection of rifampin‐resistant Mycobacterium tuberculosis in sputum smears. Antimicrob Agents Chemother 2003;47:2231–2235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Srivastava K, Das R, Jakhmola P, et al. Correlation of mutations detected by INNO‐LiPA with levels of rifampicin resistance in Mycobacterium tuberculosis . Indian J Med Res 2004;120:100–105. [PubMed] [Google Scholar]
- 19. Huang H, Jin Q, Ma Y, Chen X, Zhuang Y. Characterization of rpoB mutations in rifampicin‐resistant Mycobacterium tuberculosis isolated in China. Tuberculosis 2002;82:79–83. [DOI] [PubMed] [Google Scholar]
- 20. Patra SK, Jain A, Sherwal BL, Khanna A. Rapid detection of mutation in RRDR of rpo B gene for rifampicin resistance in MDR‐pulmonary tuberculosis by DNA sequencing. Ind J Clin Biochem 2010;25:315–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Suresh N, Singh UB, Arora J, et al. rpoB gene sequencing and spoligotyping of multidrug‐resistant Mycobacterium tuberculosis isolates from India. Infect Genet Evol 2006;6:474–483. [DOI] [PubMed] [Google Scholar]
- 22. Barnard M, Albert H, Coetzee G, O'Brien R, Bosman ME. Rapid molecular screening for multidrug‐resistant tuberculosis in a high‐volume public health laboratory in South Africa. Am J Respir Crit Care Med 2008;177:787–792. [DOI] [PubMed] [Google Scholar]
- 23. Qian L, Abe C, Lin TP, et al. rpoB genotypes of Mycobacterium tuberculosis Beijing family isolates from East Asian countries. J Clin Microbiol 2002;40:1091–1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Tracevska T, Jansone I, Broka L, Marga O, Baumanis V. Mutations in the rpoB and katG genes leading to drug resistance in Mycobacterium tuberculosis in Latvia. J Clin Microbiol 2002;40:3789–3792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lee ASG, Lim IHK, Tang LLH. High frequency of mutations in the rpoB gene in rifampin‐resistant clinical isolates of Mycobacterium tuberculosis from Singapore. J Clin Microbiol 2005;43:2026–2027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bostanabad S Z, Titov LP, Bahrmand A, Nojoumi SA. Detection of mutation in isoniazid‐resistant Mycobacterium tuberculosis isolates from M. tuberculosis patients in Belarus. Indian J Med Microbiol 2008;26:143–147. [DOI] [PubMed] [Google Scholar]
- 27. Nusrath‐Unissa A, Selvakumar N, Narayanan S, Narayanan PR. Molecular analysis of isoniazid‐resistant clinical isolates of Mycobacterium tuberculosis from India. Int J Antimicrob Agents 2008;31:71–75. [DOI] [PubMed] [Google Scholar]
- 28. Torres M, Criado A, Gónzalez N, Palomares JC, Aznar J. Rifampin and isoniazid resistance associated mutations in Mycobacterium tuberculosis clinical isolates in Seville, Spain. Int J Tuberc Lung Dis 2002;6:160–163. [PubMed] [Google Scholar]
- 29. Ramaswamy SV, Dou SJ, Rendon A, Yang Z, Cave MD, Graviss EA. Genotypic analysis of multidrug‐resistant Mycobacterium tuberculosis isolates from Monterrey, Mexico. J Med Microbiol 2004;53:107–113. [DOI] [PubMed] [Google Scholar]
- 30. Hillemann D, Kubica T, Agzamova R, Venera B, Rusch‐Gerdes S, Niemann S. Rifampicin and isoniazid resistance mutations in Mycobacterium tuberculosis strains isolated from patients in Kazakhstan. Int J Tuberc Lung Dis 2005;9:1161–1167. [PubMed] [Google Scholar]
- 31. Yang Z, Durmaz R, Yang D, et al. Simultaneous detection of isoniazid, rifampin, and ethambutol resistance of Mycobacterium tuberculosis by a single multiplex allele‐specific polymerase chain reaction (PCR) assay. Diagn Microbiol Infect Dis 2005;53:201–208. [DOI] [PubMed] [Google Scholar]
- 32. Tho DQ, Ha DT, Duy PM, et al. Comparison of MAS‐PCR and GenoType MTBDR assay for the detection of rifampicin‐resistant Mycobacterium tuberculosis . Int J Tuberc Lung Dis 2008;12:1306–1312. [PubMed] [Google Scholar]
- 33. Leung ETY, Kam KM, Chiu A, et al. Detection of katG Ser315Thr substitution in respiratory specimens from patients with isoniazid‐resistant Mycobacterium tuberculosis using PCR‐RFLP. J Med Micobiol 2003;52:999–1003. [DOI] [PubMed] [Google Scholar]
- 34. Rinder H, Mieskes KT, Loscher T. Heteroresistance in Mycobacterium tuberculosis . Int J Tuberc Lung Dis 2001;5:339–345. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Disclaimer: Supplementary materials have been peer‐reviewed but not copyedited.
Figure 2
Table 2. Frequency and type of rpoB gene alterations in RIF resistance isolates of MTB
Table 3. Results of the MAS‐PCR compared with those obtained using the proportion method


