Abstract
Malnutrition as a lack of several substances containing antioxidants such as vitamins and micronutrients, while showing a predisposition for lipid peroxidation and DNA damage, is also characterized by a slowing down of the metabolic processes, which may then have protective properties against DNA damage due to a reduction in endogenous free radical production. This study aimed to examine the oxidative status and DNA damage in cases of marasmus. The study comprised 28 infants aged 6–24 months with marasmus only and 28 age‐matched healthy infants. DNA damage was examined by the alkali single cell electrophoresis method (Comet assay) on mononuclear leukocytes. The total oxidant status (TOS) and total antioxidant status (TAS) were measured by colormetric auto‐analyzer and the oxidative stress index (OSI) was calculated. The TOS, TAS, and OSI levels of the patient group were found to be significantly lower compared to the control group (P < 0.01, P < 0.01, P < 0.01, respectively). No statistically significant difference was found between the two groups in terms of mononuclear leukocyte DNA damage (P > 0.05). The findings of this study showed that in marasmus cases, the oxidative and antioxidative processes, which have a counteractive effect, decreased together. The other results of the study indicate that there is no increase in DNA damage in marasmus cases. J. Clin. Lab. Anal. 26:161‐166, 2012. © 2012 Wiley Periodicals, Inc.
Keywords: infant, marasmus, oxidative status, DNA damage
INTRODUCTION
Malnutrition, which is defined as an inbalance between nutritional requirements and intake, continues to be a leading cause of infant mortality in some countries 1. Malnutrition develops in children who are not nourished in sufficient quantity or quality. It is defined as 2 SD below the normal body weight for size due to a lack of protein or energy, or both protein and energy 2, 3, 4.
In the biological system, molecules that take electrons are called oxidant or free radicals. By taking the electrons from the target molecules, the free radicals damage the structure and function of those molecules 5, 6. Hydrogen peroxide, nitric oxide, lipidhydroperoxide, and nitrogen dioxide molecules can be counted as oxidants. Endogenous antioxidants can be examined as two groups: those working as enzymes (such as superoxide dismutase, glutation peroxidase, glutation–S transferase, catalase) and nonenzyme antioxidants (such as bilirubin, albumin, uric acid, α‐tocopherol, ascorbic acid, ceruloplasmin, transferrin, ferritin, and glutation). These form the first line of defense against free oxygen radicals 7, 8. Exogenous originating antioxidants include vitamin C, vitamin E, vitamin A, vitamin B12, vitamin B6, folic acid, copper, zinc, and selenium 9. Oxidative stress is described as an increase in oxidants and/or a decrease in antioxidant capacity against oxidants 5, 6.
To date there have been several epidemiological studies on the relationship between DNA mutations and nutrition 8, 10, 11, 12, 13, 14. The hypothesis of the study is based on a predicted lack of energy in marasmus slowing down basal metabolic processes, the result of which is reduced oxidative stress and DNA damage triggered by oxidative stress.
MATERIAL AND METHODS
The study comprised 28 infants aged 6–24 months diagnosed with marasmus at Sanliurfa Paediatric Hospital and the Paediatric Polyclinic of Harran University Medical Faculty, and 28 healthy infants aged 6–24 months of similar sociocultural background. Informed consent was obtained from all the parents. Approval for the study was obtained from Harran University Ethics Committee. A detailed history was taken for all the infants and detailed physical examinations and laboratory tests were carried out. For height and weight evaluation, the height and weight percentile charts developed for Turkey by Neyzi et al. 15, 16 were used. Using the length and weight percentile tables developed for Turkey, 3% children below their weight and length were admitted to the study as having malnutrition. The Gomez classification was used for malnutrition criteria. Cases that weighed less than 89% of the age‐matched 50th percentile were accepted as cases of malnutrition. The endogenous mobilization of all available energy and nutrients resulting in loss of subcutaneous fat and muscle tissue is defined as marasmus 17.
Following physical examination and laboratory tests, infants were excluded from the study if there were found to be additional pathologies apart from clinical marasmic malnutrition, such as exposure to cigarette smoke, iron or vitamin B12 deficiency, infection, antibiotic use, or kwashiorkor (characterized by edema, acid, changes in hair and skin color, anemia, hepatosplenomegaly, and lethargy). Venous blood samples were collected in heparinized tubes from all the infants in the study, in order to examine DNA damage and oxidant and total antioxidant capacity.
At the start of the study, a full blood count of all the children was examined using an automatic blood count instrument (CELL‐DYN 3500; Abbott Laboratories; Abbott Park, Illinois). To evaluate DNA damage, oxidant and antioxidant capacity, venous blood samples were taken from the child into heparinized tubes. The blood samples were placed in iced water immediately until reaching the laboratory. First, the samples were used for the measurement of DNA damage with the separation of mononuclear leukocytes. For biochemical analysis, the venous blood samples were separated by 10 min centrifuge at 3500 rpm, then the formed elements were discarded together with the tube and the serum samples were stored at –80°C.
The total antioxidant status (TAS) and total oxidant status (TOS) were measured on the study day colormetrically using the Erel method by auto‐analyzer (Abbott Aeroset, Abbott Diagnostics, Abbott Park, IL). For the TAS results, mmol Trolox Eqv/l units were used and for the TOS results, μmol H2O2 Eqv/l units were used. The oxidative stress index (OSI) was defined as percentage rate of TAS values to TOS values. Before the calculation, the TAS test millimol unit value was translated to micromol units as in the TOS test. The results were expressed as arbitrary units (AU), calculated by the following formula: OSI (arbitrary unit) = TOS (μmol H2O2 Eqv/l)/TAS (mmol Trolox Eqv/l) × 10.
Determination of DNA Damage by the Alkaline Comet Assay
Mononuclear leukocytes isolation for the comet assay was performed by density gradient separation (Histopaque 1077, Sigma‐Aldrich, Inc., St. LouisMO). Heparinized blood of 1 ml was carefully layered over 1 ml Histopaque and centrifuged for 35 min at 500 × g and at 25°C. The interface band containing lymphocytes was washed with phosphate‐buffered saline (PBS) and then collected by 15 min centrifugation at 400 × g. The resulting pellets were resuspended in PBS to obtain 20,000 cells in 10 μl. Membrane integrity was assessed by trypan blue exclusion.
Endogenous DNA damage in mononuclear leukocytes was analyzed by the alkaline comet assay as described by Singh et al. 18, with minor modifications. Fresh lymphocyte cell suspension of 10 μl (about 20,000 cells) was mixed with 80 μl of 0.7% low‐melting point agarose (LMPA) (Sigma T‐1378; Sigma‐Aldrich, St. Louis, Missouri.) in PBS at 37°C. Subsequently, 80 μl of this mixture was layered onto slides that had previously been coated with 1.0% hot (60°C) normal‐melting point agarose (NMPA) and the slides were covered with a cover slip at 4°C for at least 5 min to allow the agarose to solidify. After removing the cover slips, the slides were submersed in freshly prepared cold (4°C) lysing solution (2.5 M NaCl, 100 mM EDTA‐2Na; 10 mM Tris‐HCl, pH 10–10.5; 1% Triton X‐100 and 10% DMSO were added just before use) for at least 1 h. The slides were then immersed in freshly prepared alkaline electrophoresis buffer (0.3 mol/l NaOH and 1 mmol/l Na2ETDA, pH >13) at 4°C for unwinding (40 min) and then electrophoresed (25 V/300 mA, 25 min). All these steps were conducted under red light or without direct light in order to prevent additional DNA damage. After electrophoresis, the slides were stained with ethidium bromide (2 μ/ml in distilled H2O; 70 μl/slide), covered with a cover slip, and analyzed using a epifluorescence microscope (Olympus, Japan) equipped with rhodamine filter (excitation wavelength 546 nm, barrier filter 580 nm). The images of 100 randomly chosen nuclei were analyzed visually. Each image was classified according to the intensity of the fluorescence in the comet tail, which was rated from 0 to 4 (undamaged to maximally damaged) (Fig. 1, so that the total score of slides could be between 0 and 400 AU. All procedures were performed by the same biochemistry staff and DNA damage was assessed by a single observer who was not aware of the diagnosis.
Figure 1.
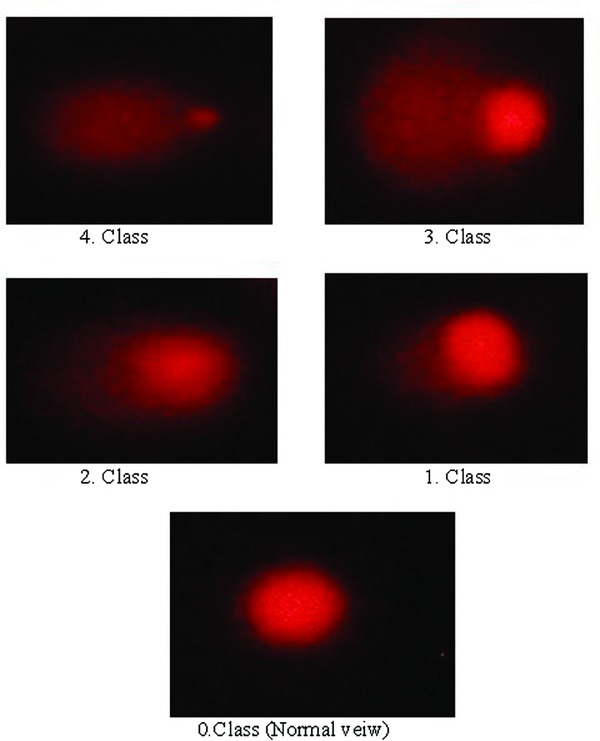
Photomicrographs showing varying intensities of the fluorescence in the comet tail (class O, undamaged; class 4, maximally damaged).
MEASUREMENT OF TAS
TAS of plasma was determined using a novel automated measurement method, developed by Erel 19. In this method, the most potent biological radical, hydroxyl radical, is produced. In the assay, ferrous ion solution, which is present in reagent 1 [o‐dianisidine (10 mM), ferrous ion (45 AM) in the Clark and Lubs solution (75 mM, pH 1.8] is mixed with hydrogen peroxide, which is present in reagent 2 [H2O2 (7.5 mM) in the Clark and Lubs solution]. The sequentially produced radicals such as brown colored dianisidinyl radical cation, produced by the hydroxyl radical, are also potent radicals. Using this method, the antioxidative effect of the sample against the potent free radical reactions that is initiated by the produced hydroxyl radical, is measured. The assay has excellent precision values of lower than 3%. The results were expressed as mmol Trolox Eqv/l.
MEASUREMENT OF TOS
TOS of plasma was determined using a novel automated measurement method, developed by Erel 6. Oxidants present in the sample oxidize the ferrous ion‐o‐dianisidine complex to ferric ion. The oxidation reaction is enhanced by glycerol molecules, which are abundantly present in the reaction medium. The ferric ion makes a colored complex with xylenol orange in an acidic medium. The color intensity, which can be measured spectrophotometrically, is related to the total amount of oxidant molecules present in the sample. The assay is calibrated with hydrogen peroxide and the results were expressed in terms of micromolar hydrogen peroxide equivalent per liter (l mol H2O2 Eqv/l).
OXIDATIVE STRESS INDEX
The ratio percentage of TOS level to TAS level gave the OSI, an indicator of the degree of oxidative stress 6.
STATISTICAL ANALYSIS
Data were analyzed using SPSS (Statistical Package for the Social Sciences, version 11.5 for Windows, SPSS® Inc, Chicago, IL). The results were presented as mean ± standard deviation. Distribution of parametric variables was assessed with one‐sample Kolmogorov–Smirnov test and all parametric variables were found to be normally distributed. The comparison of parameters between the patient group and control group was performed by Student t test for independent samples. A two‐tailed P value of less than 0.05 was considered statistically significant.
RESULTS
The 28 infants in the study group comprised 19 males (67.9%) and nine females (32.1%) with a mean age of 13.3 ± 4.9 months (range 6–24 months). The control group comprised 19 males (67.9%) and nine females (32.1%) with a mean age of 13.6 ± 4.2 months (range 6–24 months). There was no statistically significant difference between the marasmus group and the control group in terms of age (P > 0.05).
There was found to be a statistically significant difference when the values of mean length (67.8 ± 5.5 cm) and weight (7.0 ± 1.5 kg) of the patients, were compared with the values of mean height (77.9 ± 6.1 cm) and weight (10.5±1.4 kg) for the control group. The results are shown in Table 1.
Table 1.
Height and Weight Values of the Patient Group and the Control Group
| Control group (n = 28) Mean ± SD | Patient group (n = 28) Mean ± SD | P | |
|---|---|---|---|
| Height (cm) | 77.9 ± 6.1 | 67.8 ± 5.5 | <0.01 |
| Weight (kg) | 10.5 ± 1.4 | 7.0 ± 1.5 | <0.01 |
According to the Gomez classification (60–74%), our patients were at a mid level of malnutrition.
Student t test for independent samples was used.
When the mean TAS (0.95 ± 0.1), TOS (6.7 ± 1.6), OSI (0.72 ± 0.2), and DNA damage values (8.44 ± 7.79) of the patients were compared with the mean TAS (1.05 ± 0.1), TOS (12.4 ± 3.3), OSI (1.2 ± 0.3), and DNA damage values (9.43 ± 8.70) of the control group, statistical significance was found in respect of TAS, TOS, and OSI (all values P < 0.01) and there was no statistical significance in respect of DNA damage (P > 0.05). The results are shown in Table 2.
Table 2.
DNA Damage, TAS, TOS, and OSI Values of the Patient Group and the Control Group
| Control group Mean ± SD | Patient group Mean ± SD | ||
|---|---|---|---|
| TOS (μmol H2O2 Eqv./L) | 12.4 ± 3.3 | 6.7 ± 1.6 | <0.01 |
| TAS (mmol Troloks Eqv./L) | 1.05 ± 0.1 | 0.95 ± 0.1 | <0.01 |
| OSI (AU) | 1.2 ± 0.3 | 0.72 ± 0.2 | <0.01 |
| DNA damage (AU) | 9.43 ± 8.70 | 8.44 ± 7.79 | >0.05 |
Student t test for independent samples was used.
Significance was defined as P < 0.05.
TOS, total oxidant stress; TAS, total antioxidant status; OSI, oxidative stress index; AU, arbitrary units.
DISCUSSION
Marasmus is a lack of energy occurring in the body due to poor calorific nutrition 2, 20. Experimental studies have shown that limiting calories decreases oxidative stress 21, 22, 23. Another study has reported increased oxidative stress in marasmic children with an acute infection and that there may be a relationship between that increasedinfection and the antibiotics used to treat the infection 12, 24. In the current study, taking the presence of infection as exclusion criteria enabled a clearer evaluation of the effect of marasmus on the oxidant status and antioxidant status systems. The TOS and OSI levels of the marasmus cases were found to be significantly lower compared to the control group (P < 0.01). In these circumstances, it is thought that decreased production of endogenous free radicals was related to the slowing down of the body's metabolic processes and energy consumption in malnutrition.
Several studies have reported a decrease in TOS and antioxidant levels in conditions of malnutrition 25, 26. In this study, the total antioxidant capacity values of the marasmus group were found to be significantly lower compared to the control group (P < 0.01). This situation, which shows the antioxidant effect, is thought to be related to an insufficient intake of micronutrients.
In an experimental study, 24‐month‐old rats had calories reduced by 40%, a significantly decreased (24%) rate of mitochondrial H2O2 production in the brains of the rats given limited calories was seen compared to that of those given unlimited calories, and mitochondrial DNA disorders associated with oxidative damage were seen to be lower (23%) 27. Dietary restriction can lower reactive oxygen species formation, and thereby lower oxidative damage in the brain. The brain consists of a diverse group of neurons with varying functions. However, attenuating role of dietary restriction on oxidative stress in different regions of brain is not well known. Another study demonstrated that by caloric restriction intake for a period of 6 months, mice lowered the endogenous levels of oxidative stress markedly by decreasing lipid peroxidation and protein carbonyl contents in cerebral cortex, hippocampus, and striatum regions of the brain 28. The DNA damage that developed associated with increased oxidative stress in the rats fed on an excessive calorie diet, was seen to decrease with a restricted calorie diet 27. In the current study, no significant difference was found between the marasmus group and the control group in terms of DNA damage (P > 0.05). Because malnourished children with additional pathologies that cause DNA damage (infection, antibiotic use, acute and chronic diseases, passive smoking) were excluded from the study, the reduced free radical production may be related to the association of insufficient energy resources and a slowing down of oxygen respiration.
A limitation of this study was that the insufficient number in the malnutrition subgroups (mild, medium, severe) did not allow for the evaluation of the severity of malnutrition on DNA damage.
CONCLUSION
In marasmus cases with no additional clinical pathologies apart from malnutrition, the level of oxidative stress was found to be significantly lower compared to the control group. Findings were obtained, suggesting a possible reduction in the oxidative threat to DNA caused by a possible reduction in the production of endogenous free radicals and slowing down of the metabolic processes. From the results of this study there is thought to be a need for more inclusive studies evaluating DNA damage together with oxidative and antioxidative processes in marasmus cases.
REFERENCES
- 1. Food and Agriculture Organization of the United Nations . Undernourishment around the world. In: The State of Food Insecurity in the World 2004. Rome: Food and Agricultural Organization of the United Nations; 2004. [Google Scholar]
- 2. Müller O, Krawinkel M. Malnutrition and health in developing countries. CMAJ 2005;173(3):279–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Udall JN Jr, Bhutta ZA, Firmansyah A, Goyens P, Lentze MJ, Lifschitz C. Malnutrition and diarrhea: Working Group Report of the First World Congress of Pediatric Gastroenterology, Hepatology, and Nutrition. J Pediatr Gastroenterol Nutr 2002;35(2):S173–S179. [DOI] [PubMed] [Google Scholar]
- 4. Walker‐Smith J, Barnard J, Bhutta Z, Heubi J, Reeves Z, Schmitz J. Chronic diarrhea and malabsorption (including short gut syndrome): Working Group Report of the First World Congress of Pediatric Gastroenterology, Hepatology, and Nutrition. J Pediatr Gastroenterol Nutr 2002;35(2):S98–S105. [DOI] [PubMed] [Google Scholar]
- 5. Cochrane CG. Cellular injury by oxidants. Am J Med 1991;91(3C):23S–30S. [DOI] [PubMed] [Google Scholar]
- 6. Erel O. A new automated colorimetric method for measuring total oxidantstatus. Clin Biochem 2005;38(12):1103–1111. [DOI] [PubMed] [Google Scholar]
- 7. Buonocore G, Perrone S, Bracci R. Free radicals and brain damage in the newborn. Biol Neonate 2001;79(3–4):180–186. [DOI] [PubMed] [Google Scholar]
- 8. Buhimschi IA, Buhimschi CS, Pupkin M, Weiner CP. Beneficial impact of term labor: Nonenzymatic antioxidant reserve in the human fetus. Am J Obstet Gynecol 2003;189(1):181–188. [DOI] [PubMed] [Google Scholar]
- 9. Ames BN. Micronutrients prevent cancer and delay aging. Toxicol Lett 1998;28(102–103):5–18. [DOI] [PubMed] [Google Scholar]
- 10. Klein EA, Thompson IM Jr, Tangen CM, et al. Vitamin E and the risk of prostate cancer: The selenium and vitamin E cancer prevention trial (SELECT). JAMA 2011;306(14):1549–1556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Betancourt M, Ortiz R, González C, et al. Assessment of DNA damage in leukocytes from infected and malnourished children by single cell gel electrophoresis/comet assay. Mutat Res 1995;331(1):65–77. [DOI] [PubMed] [Google Scholar]
- 12. González C, Nájera O, Cortés E, et al. Hydrogen peroxide‐induced DNA damage and DNA repair in lymphocytes from malnourished children. Environ Mol Mutagen 2002;39(1):33–42. [DOI] [PubMed] [Google Scholar]
- 13. Crott JW, Mashiyama ST, Ames BN, Fenech M. The effect of folic acid deficiency and MTHFR C677T polymorphism on chromosome damage in human lymphocytes in vitro. Cancer Epidemiol Biomarkers Prev 2001;10(10):1089–1096. [PubMed] [Google Scholar]
- 14. Fenech M. Micronutrients and genomic stability: A new paradigm for recommended dietary allowances (RDAs). Food Chem Toxicol 2002;40(8):1113–1117. [DOI] [PubMed] [Google Scholar]
- 15. Neyzi O, Yalcindag A, Alp H. Heights and weights of Turkish children. J Trop Pediatr Environ Child Health 1973;19(1):5–13. [DOI] [PubMed] [Google Scholar]
- 16. Neyzi O, Türkan E. Pediatri cilt, İstanbul: Nobel tıp. 2002; 1:91–99, 183–185. [Google Scholar]
- 17. Bhan MK, Bhandari N, Bahl R. Management of the severely malnourished child: Perspective from developing countries. BMJ 2003;326(7381):146–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Singh NP, McCoy MT, Tice RR, Schneider EL. A simple technique for quantitation of low levels of DNA damage in individual cells. Exp Cell Res 1988;175(1):184–191. [DOI] [PubMed] [Google Scholar]
- 19. Erel O. A novel automated method to measure total antioxidant response against potent free radical reactions. Clin Biochem 2004;37(2):112–119. [DOI] [PubMed] [Google Scholar]
- 20. Schaible UE, Kaufmann SH. Malnutrition and infection: Complex mechanisms and global impacts. PLoS Med 2007;4(5):115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gredilla R, Sanz A, Lopez‐Torres M, Barja G. Caloric restriction decreases mitochondrial free radical generation at complex I and lowers oxidative damage to mitochondrial DNA in the rat heart. FASEB J 2001;15(9):1589–1591. [DOI] [PubMed] [Google Scholar]
- 22. Gredilla R, Barja G, López‐Torres M. Effect of short‐term caloric restriction on H2O2 production and oxidative DNA damage in rat liver mitochondria and location of the free radical source. J Bioenerg Biomembr 2001;33(4):279–287. [DOI] [PubMed] [Google Scholar]
- 23. Bevilacqua L, Ramsey JJ, Hagopian K, Weindruch R, Harper ME. Effects of short‐ and medium‐term calorie restriction on muscle mitochondrial proton leak and reactive oxygen species production. Am J Physiol Endocrinol Metab 2004;286(5):E852–E861. [DOI] [PubMed] [Google Scholar]
- 24. González C, Nájera O, Cortés E, et al. Susceptibility to DNA damage induced by antibiotics in lymphocytes from malnourished children. Teratog Carcinog Mutagen 2002;22(2):147–158. [PubMed] [Google Scholar]
- 25. Tatli MM, Vural H, Koc A, Kosecik M, Atas A. Altered anti‐oxidant status and increased lipid peroxidation in marasmic children. Pediatr Int 2000;42(3):289–292. [DOI] [PubMed] [Google Scholar]
- 26. Catal F, Avci A, Karadag A, Alioglu B, Avci Z. Oxidant and antioxidant status of Turkish marasmic children: A single center study. J Trace Elem Med Biol 2007;21(2):108–112. [DOI] [PubMed] [Google Scholar]
- 27. Sanz A, Caro P, Ibañez J, Gómez J, Gredilla R, Barja G. Dietary restriction at old age lowers mitochondrial oxygen radical production and leak at complex I and oxidative DNA damage in rat brain. J Bioenerg Biomembr 2005;37(2):83–90. [DOI] [PubMed] [Google Scholar]
- 28. Rathod P, Hemnani TH, Parihar SK. Dietary restriction lowers endogenous levels of oxidative stress in different brain regions of adult mice. Cell Mol Biol 2011;27(57):OL1575–OL1580. [PubMed] [Google Scholar]


