Abstract
Background
Identification of dermatophytes at the species level, relying on macro‐ and microscopic properties of the colonies is time‐consuming, questioned in many circumstances, and requires considerable expertise. In this study, we examined the potency of a new genetic marker, β‐tubulin (BT2) gene, for differentiation of dermatophytes in an in silico and experimental restriction fragment length polymorphism (RFLP) profile.
Methods
The BT2 sequences of dermatophyte species were retrieved from GenBank and analyzed using bioinformatics softwares to choose suitable restriction enzyme(s). Forty reference culture collections and 100 clinical isolates were PCR‐amplified using the primers T1 and Bt2b and consequently subjected to virtual RFLP analysis. The dermatophytes were identified according to specific lengths of bands in agarose gel electrophoresis.
Results
After digestion of partially amplified β‐tubulin gene with the restriction enzyme FatI, three dermatophyte species, that is, Microsporum gypseum, M. canis, and Trichophyton verrucosum yielded unique restriction maps while the remaining species including T. interdigitale, T. rubrum, T. tonsurans, T. schoenleinii, and T. violaceum, were identified by further restriction digestion by Alw21I, MwoI, and HpyCH4V endonucleases. The length of RFLP products was same as of those expected by computer analysis.
Conclusion
The two‐step BT2 restriction mapping used in this study is an effective tool for reliable differentiation of the clinically relevant species of dermatophytes.
Keywords: dermatophytes, β‐tubulin gene, identification, RFLP
INTRODUCTION
Dermatophytes comprise a group of closely related fungi, correlating to three anamorphic genera of Trichophyton, Microsporum, and Epidermophyton, each includes several distinct species. They can colonize or infect the keratinized tissues (skin, hair, and nails) of humans or animals, causing clinically localized to extensively generalized lesions termed as dermatophytosis 1, 2. From etiological, therapeutic, and epidemiological standpoints, identification of the clinically isolated dermatophytes at the species level is important to verify the diagnosis, organize the appropriate therapy, establish the preventive strategies for infection, as well as to extend our knowledge in the realm of dermatophytes ecology and epidemiology 2, 3. The current scenario for species identification of these fungi relies on macromorphology and microscopic properties of the colonies on specific media like Sabouraud's glucose agar with cycloheximide and chloramphenicol, along with analysis of some nutritional requirements or biochemical characters 1, 3. However, due to the high similarity and variability in morphology, the pleomorphism phenomenon, and a long time required for emergence of phenotypic characteristics, identification of these molds based on phenotypic criteria is time‐consuming, requires a significant degree of experience, and remains imprecise in many circumstances 4, 5, 6. During the last decades, the development of molecular biology techniques has provided more rapid and precise alternatives for species delineation of dermatophytes and in this context, molecular targets like internal transcribed spacer (ITS) regions of rDNA 3, 7, 8, DNA topoisomerase II 9, chitin synthase 1 (CHS1; 10, 11), and actin genes 12 have met with some success. In the present study, we conducted a PCR‐RFLP (PCR‐restriction fragment length polymorphism) assay on β‐tubulin (BT2) gene aiming to evaluate the efficacy of this new marker for accurate differentiation of pathogenic dermatophytes, along with special impression on the species of clinical relevance in Iran.
MATERIALS AND METHODS
Computer‐Simulated RFLP Analysis
The BT2 sequences for dermatophyte species were retrieved from NCBI (http://www.ncbi.nlm.nih.gov/pubmed/), exported to the MEGA 4 software (http://www.megasoftware.net), and aligned with T1/Bt2b primers. Each related trimmed sequence was exported to DNASIS program (Hitachi DNASIS® MAX version 3.0) for in silico digestion with all restriction endonucleases included in the software. The enzymes presenting the best‐resolved species‐specific RFLP patterns were chosen as candidates for next evaluations.
Fungal Strains and Growth Conditions
The PCR‐RFLP was optimized using 40 culture collections and 100 clinical isolates. The reference strains were kindly prepared by Teikyo University Institute of Medical Mycology (TIMM) and the Centraalbureau voor Schimmelcultures (CBS, Utrecht, The Netherlands). The reference strains were T. interdigitale (CBS 130788, CBS 130789, CBS 130790, CBS 130791, CBS 130792, CBS 130794, CBS 130796, CBS 130799, CBS 130801, CBS 130803, CBS 130804, NBRC 5809, NBRC 5466, NBRC 5812, NBRC 5974), T. schoenleinii (NBRC 8192, NBRC 8191), T. tonsurans (CBS 130800, CBS 130814, NBRC 5928, NBRC 5945), T. rubrum (CBS 130927, NBRC 5808, NBRC 5467), T. violaceum (CBS 130937, NBRC 31064, CBS 376.92), T. verrucosum (CBS 562.50, CBS134.66), E. floccosum (CBS 130793, CBS 130802, NBRC 9095, NBRC 9045), M. gypseum (CBS 130939, IFO 5948, IFO 8228), and M. canis (CBS 130795, CBS 130797, CBS 130798, NBRC 9182). All strains were cultured on Mycobiotic Agar (Difco, Detroit, MI, USA) and incubated at room temperature for 3 weeks. The identity of all used clinical isolates, including T. interdigitale (n = 37), T. rubrum (n = 25), T. schoenleinii (n = 2), T. violaceum (n = 1), E. floccosum (n = 10), T. tonsurans (n = 10), M. canis (n = 13), and M. gypseum (n = 2) was delineated by ITS‐RFLP and sequencing 6.
DNA Isolation
Total DNA of each strain was extracted from the colony using the previously described method 13. Briefly, a small plug of young colony was put into an 1.5 ml tube containing 300 μl of lysis buffer (200 mM Tris‐HCl (pH 7.5), 25 mM EDTA (Ethylenediaminetetraacetic Acid), 0.5% w/v SDS (Sodium Dodecyl Sulfate), 250 mM NaCl), and crushed with a grinder and mixed with phenol‐chloroform (1:1), vortexed in few seconds, and centrifuged at 10,000 rpm for 10 min. Then the supernatant was mixed with chloroform and centrifuged. The DNA was precipitated with 1/10 volume of 3.0 M sodium acetate and equal volume of iso‐propanol at −20°C for 10 min, washed with 70% ethanol, dried, and suspended in 50 μl of ultrapure water.
PCR Reaction
The universal primers T1 (5′‐AACATGCGTGAGATTGTAAGT‐3′) 14 and Bt2b (5′‐ACCCTCAGTGTAGTGACCCTTGGC‐3′) 15 were used for partial amplification of BT2 gene. PCR reactions consisted of 2 μl template DNA, 2.5 μl 10× reaction buffer, 1.5 mM MgCl2, 400 μM deoxynucleotide triphosphates, 1.25 U Taq DNA polymerase, 25 pmol of each forward and reverse primers, and enough water up to a final volume of 25 μl. Thermal cycling program was an initial denaturation at 94°C, followed by 30 s at 94°C, 30 s at 58°C, and 1 min at 72°C for 35 cycles and a terminal extension of 72°C for 10 min.
Experimental Validation of Restriction Analysis
To verify the computationally calculated restriction patterns, the BT2 PCR amplicons were preliminarily subjected to cleavage with FatI (New England Biolabs Ltd., NHitchin, UK). For differentiation of T. interdigitale/T. tonsurans and T. schoenleinii/E. floccosum strains, which had the same RFLP profile with FatI, two new digestions were performed by the enzymes Alw21I (Fermentas, Vilnius, Lithuania) and HpyCH4V (New England Biolabs Ltd.), respectively. Digestion reaction was carried out in a mixture containing 1.5 μl of 10× buffer, 5 U of each enzyme, 5 μl of PCR product, and ultrapure water to a final volume of 15 μl. Incubation temperature was based on the enzyme manufacturer's instructions.
Assessment of Length Polymorphisms by Electrophoresis
Ten microliters of amplified products or digested fractions were separated by electrophoresis through an 1.5 and 2% agarose gel (w⁄v), respectively, for 60 min at 100 V in TBE buffer (Tris 90 mM, boric acid 90 mM, EDTA 2 mM) containing ethidium bromide stain (0.5 μg/ml) and visualized under a UV light. A 100 base pair (bp) DNA ladder was used as molecular size marker in each run. Identification of the strains was performed by comparing the restriction maps observed in experimental RFLP with those deduced from in silico analysis.
RESULTS
Sequence analysis by MEGA 4 and DNASIS programs indicated that polymorphism in the BT2 sequences was enough to develop a PCR‐RFLP profile for identification of common pathogenic dermatophytes. Additionally, except T. interdigitale, no intraspecies sequence variation was found among strains of each species (data not shown). The results of BT2 sequence analysis were summarized in detail in Table 1. Partial amplification of BT2 gene by the primer T1 and Bt2b yields product range from 770 bp for M. canis to 798 bp for M. gypseum, T. schoenleinii, and T. verrucosum. Among more than 600 restriction enzymes included in DNASIS software, four restriction enzymes, namely, FatI, Alw21I, MwoI, and HpyCH4V were selected as the best enzymes for discrimination of pathogenic dermatophytes. These enzymes were used in a two‐step RFLP set. In the first round, both computer‐assisted and experimental digestion by FatI, M. gypseum, T. verrucosum, and M. canis, produced specific unique restriction maps while the other species shared fragments with similar length (Table 1, Fig. 1). The remaining species were undertaken with further restriction analyses, therefore the enzymes Alw21I, MwoI, and HpyCH4V were selected as the best candidates for discrimination between T. interdigitale/T. tonsurans, T. rubrum/T. violaceum, and T. schoenleinii/E. floccosum pair species, respectively. As illustrated in Figures 2 and 3, electrophoresis of fragmented PCR products on agarose gel confirmed that BT2 digestion with Alw21I is clearly discriminative for T. interdigitale and T. tonsurans, while enzymatic cutting of the products with HpyCH4V helps the differentiation of T. schoenleinii and E. floccosum. Hence, in the BT2‐RFLP profiles by selected enzymes, 40 culture collections and 100 clinical isolates produced electrophoretic patterns congruent with expected species‐specific schema achieved from sequence analysis.
Table 1.
Results of Computer and Experimental BT2‐RFLP Analysis With FatI, Alw21I, HpyCH4V, and MwoI for Differentiation of Dermatophyte Strains Tested in This Study
| Species | BT2 GenBank accession number used for in silico analysis | Total number of tested strains in this study | Size of the BT2 amplicon (bp) | BT2 profile after digestion with FatI (bp) | BT2 profile after digestion with Alw21I (bp) | BT2 profile after digestion with HpyCH4V (bp) | Profile after digestion with MwoI | Species identification by BT2‐RFLP/ sequencing |
|---|---|---|---|---|---|---|---|---|
| T. interdigitale | JF731050–JF731054 | 55 | 790 | 2, 202, 246, 340 | 152, 217, 421 | NA | NA | T. interdigitale |
| T. tonsurans | JF731070–JF731074 | 9 | 790 | 2, 202, 246, 340 | 217, 573 | NA | NA | T. tonsurans |
| T. rubrum | JF731063–JF731067 | 28 | 796 | 2, 63, 125, 246, 360 | NA | NA | 9, 97, 180, 510 | T. rubrum |
| T. violaceum | JF731088–JF731089 | 5 | 796 | 2, 63, 125, 246, 360 | NA | NA | 9, 98, 689 | T. violaceum |
| M. gypseum | JF731096–JF731098 | 5 | 798 | 2, 124, 672 | NA | NA | NA | M. gypseum |
| M. canis | JF731101–JF731103 | 10 | 770 | 2, 68, 111, 117, 226, 246 | NA | NA | NA | M. canis |
| T. schoenleinii | JF731081–JF731082 | 2 | 798 | 2, 246, 550 | NA | 83, 118, 125, 219, 253 | NA | T. schoenleinii |
| E. floccosum | JF731122–JF731123 | 24 | 789 | 2, 246, 541 | NA | 81, 219, 489 | NA | E. floccosum |
| T. verrucosum | Undeposited data | 2 | 798 | 124, 250, 424 | NA | NA | NA | T. verrucosum |
NA, not applicable.
Figure 1.
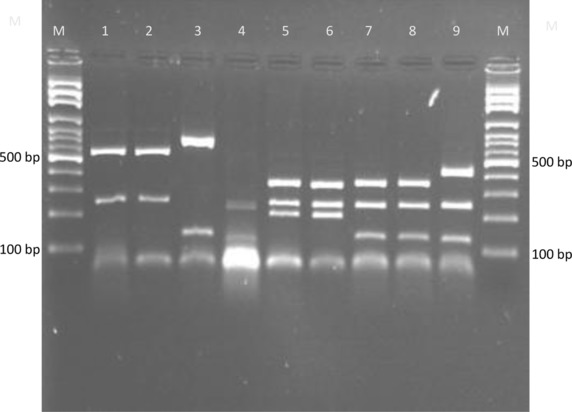
Restriction profiles of BT2 gene by FatI for the common pathogenic dermatophytes. Lane 1, T. schoenleinii (NBRC 8192); lane 2, E. floccosum (NBRC 9045); lane 3, M. gypseum (NBRC 8228); lane 4, M. canis (NBRC 9182); lane 5, T. tonsurans (NBRC 5928); lane 6, T. interdigitale (NBRC 5809); lane 7, T. rubrum (NBRC 5808); lane 8, T. violaceum (CBS 376.92); lane 9, T. verrucosum (CBS 562.50). M, 100‐bp molecular size marker.
Figure 2.
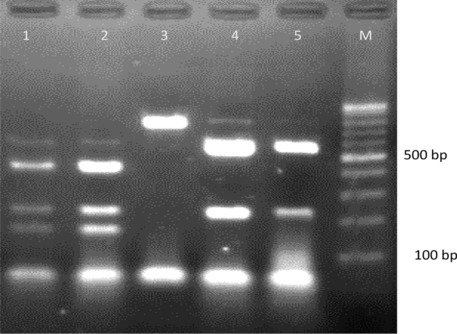
Restriction maps of BT2 gene by Alw21I. Lane 1, T. interdigitale (NBRC 5809); lane 2, T. interdigitale (NBRC 5466); lane 3, M. canis (NBRC 9182); lane 4, T. tonsurans (NBRC 5928); lane 5, T. tonsurans (NBRC 5945). Contrary to T. interdigitale and T. tonsurans, the BT2 sequence of M. canis has no cutting site for Alw21I. M, 100‐bp molecular size marker.
Figure 3.
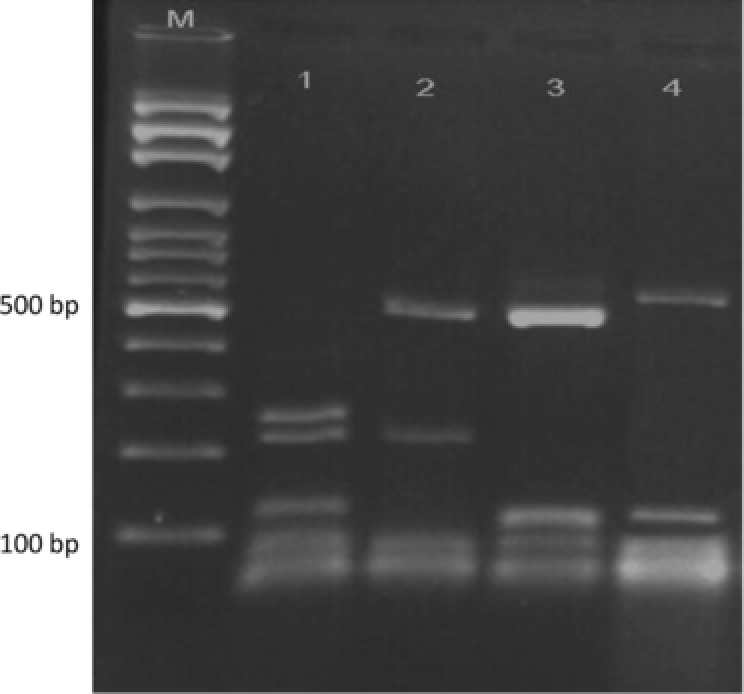
Restriction patterns of BT2 gene by HpyCH4V for discrimination between T. schoenleinii and E. floccosum. Lane 1, T. schoenleinii (NBRC 8191); lane 2, E. floccosum (NBRC 9045); lane 3, M. gypseum (CBS 8228), lane 4, M. canis (NBRC 9182). M, 100‐bp molecular size marker.
DISCUSSION
Phenotypic criteria conventionally used for species identification of dermatophytes are known to be slow and insufficiently discriminative because dermatophytes typically undergo cultural variability 5, 6, 16, 17. Recently, to address these inadequacies, some DNA‐based procedures targeting different genetic markers have been used as more rapid and accurate alternatives for dermatophyte identification 6, 8, 9, 10, 11, 12. In this study, we developed a novel and simple RFLP scheme to evaluate the potential and applicable utility of BT2 gene for quick species distinction of the most frequently encountered dermatophytes in Iran 6, 18, 19, 20, 21. BT2 protein‐encoding gene has been broadly used in fungal phylogenetic analyses because it contains both variable and highly conserved regions 22. It was shown that BT2 digestion provides an easy route for species discrimination among the agriculturally important genera of Cupressus, Ceratocystis, and Phaeoacremonium 23, 24, 25, however, there was no similar experience in the realm of medically important fungi. Currently, ITS‐rDNA sequencing is the golden standard for species delineation of dermatophytes; however, the method is expensive and may be impracticable for large‐scale analysis 16, 17. To overcome these limitations, several comprehensive and sequencing‐independent platforms have been developed, in which most of them have used the ITS‐rDNA as genetic screening/identification marker 3, 6, 7, 8, 26, 27.
Kamiya et al., using combination of a nested‐PCR and PCR‐RFLP of DNA topoisomerase II gene, developed a protocol for species identification of dermatophytes; however, the introduced procedure exclusively could delineate six species of T. rubrum, T. mentagrophytes, T. tonsurans, M. canis, M. gypseum, and E. floccosum 28. As exemplified in Figures 1, 2, 3 and in accordance with our theoretical analysis, restriction fragment length determination of BT2 revealed that a combination of restriction digestion by FatI, Alw21I, and HpyCH4V is useful and applicable for identifying the nine species, M. gypseum, M. canis, T. verrucosum, T. rubrum T. violaceum, T. interdigitale, T. tonsurans, T. schoenleinii, and E. floccosum. The mentioned species are the predominant agents of dermatophytosis in Iran and worldwide 6, 18, 19, 20, 21.
M. canis, M. ferrugineum, and M. audouinii are three closely‐related members of the Arthroderma otae complex 16, 18 and it was recently found that interspecies variation of BT2 sequence is higher than ITS for differentiation among the members of A. otae complex, however, the two latter species are uncommon species in Iran 18 and we eliminated them in our study. T. interdigitale alongside T. tonsurans and T. equinum comprise three species correlated to A. vanbreuseghemii teleomorph 16. The two former species are, respectively, the prevalent agents of tinea pedis and endothrix type of tinea capitis in Iran 6 and were easily distinguished by Alw21I in our study but given that human dermatophytosis by T. equinum is rare throughout the world, we excluded the species from our restriction analysis. The strains of T. interdigitale had intraspecies variations in BT2 sequence, however, surprisingly these variations were not reflected in RFLP and in contrast to Rezaei‐Matehkolaei et al. who obtained two ITS‐RFLP profiles for T. interdigitale isolates 3, 6 we found a unique BT2‐RFLP barcode for the species.
The human‐adapted dermatophytes, T. rubrum and T. violaceum, are two closely related species in the T. rubrum complex 29. While the former species is the most ubiquitous cause of dermatophytosis worldwide 1, 2, 29 and amongst the common species in Iran, prevalence of the latter fungus has substantially decreased in the country 6, 21. Discrimination between the two species was taken into consideration in some studies. Rezaei‐Matehkolaei et al. 3 and Jackson et al. 7 noted that ITS‐RFLP with MvaI can differentiate these closely related Trichophyton species, however, due to the poor resolution of low molecular weight fragments, the observed patterns in gel electrophoresis were not fruitful for this purpose. Conversely, in our investigation the BT2 restriction fragmentation by MwoI was more informative for differentiation of T. rubrum and T. violaceum especially that a good separation was found by high molecular weight fragments (9, 97, 180, 508 bp for T. rubrum vs. 9, 98, 687 bp for T. violaceum; Table 1). Owing to some limitations, we did not experimentally perform the discrimination between T. rubrum and T. violaceum species. But regarding to the fully congruence of the restriction profiles by FatI, Alw21I, and HpyCH4V (Figs. 1, 2, 3) with the fragments sizes deduced from computational analysis, it is certainly predictable that the estimated fragments sizes of BT2 after digestion with MwoI can be applicable for differentiation of the mentioned species. It was shown that enzymatic digestion of ITS‐rDNA regions with MvaI generates band patterns characteristic of the anthropophilic species, E. floccosum and T. schoenleinii 3, 7. Likewise, in the current study, two species were nicely distinguished through BT2 fractionization by HpyCH4V (Table 1, Fig. 3). The geophilic dermatophyte, M. gypseum, which is less frequently cited as a source of human glabrous skin and scalp infection 2 and the common zoophilic species, T. verrucosum were also distinguishable by BT2 digestion.
Conclusively, given that the species assignment of all studied strains by BT2‐RFLP was concordant with ITS‐RFLP/ITS‐sequencing, the proposed restriction assay would be an affordable, reliable, and relatively rapid screening tool for differentiating the clinically relevant dermatophytes in Iran. The findings of the study emphasize that the BT2, as a new genetic marker other than ITS, has the potency of species differentiation of medically important dermatophytes.
ACKNOWLEDGMENTS
This project was supported by Tehran University of Medical Sciences, Tehran, Iran.
REFERENCES
- 1. Weitzman I, Summerbell RC. The dermatophytes. Clin Microbiol Rev 1995;8:240–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Padhye AA, Summerbell RC. The dermatophytes In: Merz WG, Hay RJ, editors. Topley and Wilson's Microbiology and Microbial Infections: Medical Mycology, tenth edition, London: Hodder Arnold; 2005. p. 220–243. [Google Scholar]
- 3. Rezaei‐Matehkolaei A, Makimura K, Shidfar MR, et al. Use of single‐enzyme PCR‐restriction digestion barcode targeting the internal transcribed spacers (ITS rDNA) to identify dermatophyte species. Iranian J Publ Health 2012;41(3):82–94. [PMC free article] [PubMed] [Google Scholar]
- 4. Kanbe T. Molecular approaches in the diagnosis of dermatophytosis. Mycopathologia 2008;166:307–317. [DOI] [PubMed] [Google Scholar]
- 5. Graser Y, De Hoog S, Summerbell RC. Dermatophytes: Recognizing species of clonal fungi. Med Mycol 2006;44:199–209. [DOI] [PubMed] [Google Scholar]
- 6. Rezaei‐Matehkolahei A, Makimura K, De Hoog S, et al. Molecular epidemiology of dermatophytosis in Tehran, Iran, a clinical and microbial survey. Med Mycol 2013;51:203–207. [DOI] [PubMed] [Google Scholar]
- 7. Jackson CJ, Barton RC, Evans EG. Species identification and strain differntiation of dermatophyte fungi by analysis of ribosomal DNA intergenic spacer regions. J Clin Microbiol 1999;37:936–931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Shin JH, Sung JH, Park SJ, et al. Species identification and strain differentiation of dermatophyte fungi using polymerase chain reaction ampliction and restriction enzyme analysis. J Am Acad Dermatol 2003;48:857–865. [DOI] [PubMed] [Google Scholar]
- 9. Kanbe T, Suzuki Y, Kamiya A, Mochizuki T, Fujihiro M, Kikuchi A. PCR‐based identification of common dermatophyte species using primer sets specific for the DNA topoisomerase II genes. J Dermatol Sci 2003;32:151–161. [DOI] [PubMed] [Google Scholar]
- 10. Hirai A, Kano R, Nakamura Y, Watanabe S, Hasegawa A. Molecular taxonomy of dermatophytes and related fungi by chitin synthase 1 (CHS1) gene sequences. Antonie van Leeuwenhoek 2003;83:11–20. [DOI] [PubMed] [Google Scholar]
- 11. Kano R, Nakamura Y, Watari T, et al. Phylogenetic analysis of 8 dermatophyte species using chitin synthase 1 gene sequences. Mycoses 1997;40:411–414. [DOI] [PubMed] [Google Scholar]
- 12. Okeke CN, Tsuboi R, Kawai M, Hiruma M, Ogawa H. Isolation of an intron‐containing partial sequence of the gene encoding dermatophyte actin (ACT) and detection of a fragment of the transcript by reverse transcription‐nested PCR as a means of assessing the viability of dermatophytes in skin scales. J Clin Microbiol 2001;39:101–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Makimura K, Tamura Y, Mochizuki T, et al. Phylogenetic classification and species identification of dermatophyte strains based on DNA sequences of nuclear ribosomal internal transcribed spacer 1 regions. J Clin Microbiol 1999;37:920–924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. O'Donnell K, Cigelnik E. Two different intragenomic rDNA ITS2 types within a monophyletic lineage of the fungus Fusarium are nonorthologous. Mol Phylogenet Evol 1997;7:103–116. [DOI] [PubMed] [Google Scholar]
- 15. Glass NL, Donaldson GC. Development of primer sets designed for use with the PCR to amplify conserved genes from filamentous ascomycetes. Appl Environ Microbiol 1995;61:1323–1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Graser Y, Scott J, Summerbell R. The new species concept in dermatophytes–A polyphasic approach. Mycopathologia 2008;166:239–256. [DOI] [PubMed] [Google Scholar]
- 17. Kong F, Tong Z, Chen X, et al. Rapid identification and differentiation of Trichophyton species, based on sequence polymorphisms of the ribosomal internal transcribed spacer regions, by rolling‐circle amplification. J Clin Microbiol 2008;46:1192–1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Rezaei‐Matehkolaei A, Makimura K, de Hoog GS, et al. Multilocus differentiation of the related dermatophytes Microsporum canis, Microsporum ferrugineum and Microsporum audouinii . J Med Microbiol 2012;61:57–63. [DOI] [PubMed] [Google Scholar]
- 19. Rezaei‐Matehkolaei A, Makimura K, de Hoog GS, et al. Discrimination of Trichophyton tonsurans and Trichophyton equinum by PCR‐RFLP and by β‐tubulin and translation elongation factor 1‐α sequencing. Med Mycol 2012;50:760–764. [DOI] [PubMed] [Google Scholar]
- 20. Mahmoudabadi AZ. Study of dermatophytosis in South West of Iran (Ahwaz). Mycopathologia 2005;160:21–24. [DOI] [PubMed] [Google Scholar]
- 21. Aghamirian MR, Ghiasian SA. Dermatophytoses in outpatients attending the dermatology center of avicenna hospital in Qazvin, Iran. Mycoses 2008;51:155–160. [DOI] [PubMed] [Google Scholar]
- 22. Myburg H, Gryzenhout M, Wingfield BD, Wingfield MJ. Beta‐tubulin and histone H3 gene sequences distinguish Cryphonectria cubensis from South Africa, Asia, and South America. Can J Bot 2002;80:590–596. [Google Scholar]
- 23. Krokene P, Barnes I, Wingfield BD, Wingfield MJ. A PCR‐RFLP based diagnostic technique to rapidly identify Seiridium species causing cypress canker. Mycologia 2004;96:1352–1354. [PubMed] [Google Scholar]
- 24. Loppnau PA, Breuil C. Species level identification of conifer associated Ceratocystis sapstain fungi by PCR‐RFLP on a beta‐tubulin gene fragment. FEMS Microbiol Lett 2003;222:143–147. [DOI] [PubMed] [Google Scholar]
- 25. Dupont J, Magini S, Cesari C, Gatica M. ITS and β‐tubulin markers help delineate Phaeoacremonium species, and the occurrence of P. parasiticum in grapevine disease in Argentina. Mycol Res 2002;106:1143–1150. [Google Scholar]
- 26. De Baere T, Summerbell R, Theelen B, Boekhout T, Vaneechoutte M. Evaluation of internal transcribed spacer 2‐RFLP analysis for the identification of dermatophytes. J Med Microbiol 2010;59:48–54. [DOI] [PubMed] [Google Scholar]
- 27. Arabatzis M, Bruijnesteijn van Coppenraet LE, Kuijper EJ, et al. Diagnosis of common dermatophyte infections by a novel multiplex real‐time polymerase chain reaction detection/identification scheme. Br J Dermatol 2007;157:681–689. [DOI] [PubMed] [Google Scholar]
- 28. Kamiya A, Kikuchi A, Tomita Y, Kanbe T. PCR and PCR‐RFLP techniques targeting the DNA topoisomerase II gene for rapid clinical diagnosis of the etiologic agent of dermatophytosis. J Dermatol Sci 2004;34:35–48. [DOI] [PubMed] [Google Scholar]
- 29. Graser Y, Kuijpers AF, Presber W, de Hoog GS. Molecular taxonomy of the Trichophyton rubrum complex. J Clin Microbiol 2000;38:3329–3336. [DOI] [PMC free article] [PubMed] [Google Scholar]


