Abstract
Background
Vascular endothelial growth factor (VEGF), transforming growth factor β1 (TGF‐β1), and nitric oxide (NO) have been reported to be contributory factors to the pathogenesis of psoriasis vulgaris. In the current study, we aimed to investigate the association between the levels of VEGF, TGF‐β1, and NO and psoriasis severity (as expressed by psoriasis area severity index, PASI).
Methods
Fifty‐eight patients with psoriasis vulgaris and twenty‐two controls were included in the study. The serum levels of VEGF and TGF‐β1 were estimated by ELISA technique. The serum levels of NO were determined by colorimetric method.
Results
The serum levels of VEGF, TGF‐β1, and NO were significantly higher in patients than controls. Moreover, the serum levels of the studied biochemical variables in patients with severe disease activity were significantly higher than mild cases. The duration of disease showed significant positive correlations with each VEGF (r = 0.35, P < 0.01) and TGF‐β1 (r = 0.41, P < 0.05). In addition, the PASI score was significantly positively correlated with VEGF (r = 0.65, P < 0.001), TGF‐β1 (r = 0.31, P < 0.05), and NO (r = 0.51, P < 0.001).
Conclusion
These findings suggest an association between psoriasis disease severity and serum levels of VEGF, TGF‐β1, and NO, which can be recognized as markers of the psoriasis severity. The modulation of their production may represent a therapeutic potential strategy for psoriasis.
Keywords: patients, psoriasis vulgaris, VEGF, TGF‐β1, NO
INTRODUCTION
Psoriasis is an immune‐mediated, multifactorial skin disease with hyperproliferation and altered differentiations of keratinocytes, linking the pathways of angiogenesis and inflammation 1. Vessels' expansion plays an important role in the evolution of psoriatic plaques. The dysregulated angiogenesis has been observed in inflammatory diseases and might underlie chronic cutaneous inflammation in psoriasis 2.
Several growth factors are recognized as pivotal factors responsible for angiogenesis in different tissues. Vascular endothelial growth factor (VEGF) and transforming growth factors (TGFs) α and β demonstrate angiogenic activity 3. VEGF and its high‐affinity tyrosine kinase receptors VEGFR‐1 (Flt‐1) and VEGFR‐2 (KDR in humans⁄Flk‐1 in mice) are essentially involved in vascular embryogenesis and adult neovascularization 4. VEGF, first described as vascular permeability factor, represents in its active form a homodimeric glycoprotein of 40–45 kDa 5. VEGFR‐1 and VEGFR‐2 are primarily expressed by vascular endothelial cells. VEGF binding to either of these receptors leads to receptor activation and intracellular signal transduction 6. It is known that VEGF contributes to angiogenesis by both direct and indirect mechanisms. On the one hand, VEGF stimulates the endothelial cells to proliferate, to migrate, and to alter their pattern of gene expression. On the other hand, it renders these endothelial cells hyperpermeable so that they spill plasma proteins into the extravascular space, leading to profound alterations in the extracellular matrix that favor angiogenesis 7.
Overexpression of VEGF can promote new blood vessel formation and account for the chronicity in psoriatic lesion 8. In vitro culture studies revealed that VEGF in the skin is secreted predominantly by keratinocytes and its concentration is enhanced in skin of patients with psoriasis 9. Moreover, constant delivery of VEGF to the skin in the transgenic VEGF mouse results in development of psoriasis‐like inflammation 10.
TGF‐β1 is a pleiotropic cytokine that is produced by almost all cell types, including activated inflammatory cells and keratinocytes 11. It possesses strong immunoregulatory properties, acts in autocrine and paracrine modes, and controls the differentiation, proliferation, and activation state of immune cells 12. TGF‐β1 controls immune responses in a complex and often context‐dependent manner 13. Its effects on immune cells depend on the type of cell, state of differentiation of the cell, and environment of the cytokines present 14. TGF‐β1 can modulate expression of adhesion molecules, and provide a chemotactic gradient for leucocytes and other cells participating in an inflammatory response 12. TGF‐β1 is linked to keratinocyte proliferation, which stimulates fibroblast production in the extracellular matrix and activates angiogenesis 14. Consequently, TGF‐β1 may play an important role in the pathogenesis of psoriasis 11.
Nitric oxide (NO) is an active molecule generated in many cells including fibroblasts and endothelial cells, participating in psoriatic inflammatory processes. In context of dermal vascular dilatation and increased blood flow, characteristic features of psoriasis, the contribution of NO deserves special attention; however literature data on NO production in psoriasis are inconsistent 15.
In the current study, we aimed to investigate the association between the serum levels of VEGF, TGF‐β1, and NO in patients with psoriasis vulgaris and disease severity.
PATIENTS AND METHODS
Fifty‐eight patients with psoriasis vulgaris (36 males and 22 females) were enrolled in this study. The study was conducted at the Dermatology Clinics of a hospital affiliated to Qassim University, Buraydah, Saudi Arabia, between January 2011 and January 2012. The patients' age was (mean ± SE) 30.17 ± 1.406 years. The control group consisted of 22 age‐ and sex‐matched healthy subjects. Their age was (mean ± SE) 29.36 ± 1.882 years. They included 11 males and 11 females. The patients did not receive topical treatment for 1 week or systemic treatment for 1 month such as steroids, methotraxate, Psoralen‐Ultraviolet A (PUVA), retenoids, or cyclosporin. All patients were free of infections. Exclusion criteria were hypertension; obesity; diabetes mellitus; connective tissue diseases; and disorders of thyroid, kidney, and liver functions. In order to assess the severity of psoriasis, estimation of psoriasis area severity index (PASI) was done according to Fredrikson and Petterson 16. According to PASI score, the patients were classified into mild (PASI 0–3; n = 23), moderate (PASI >3–15; n = 24), and severe (PASI >15; n = 11) cases.
All patients were subjected to the following: thorough history‐taking and clinical examination, full blood picture, hemoglobin concentration, erythrocytic sedimentation rate (ESR), leukocytic count, liver and kidney function tests, ECG, and abdominal sonography. After approval by the ethics committee of the College of Medicine, Qassim University, an informed consent was obtained from each subject enrolled in the study.
Ten milliliters of venous blood was collected from all patients and controls and allowed to clot at room temperature. After centrifugation, serum was collected and stored at −80°C until biochemical analyses. The serum levels of VEGF were determined using enzyme‐linked immunosorbant assay (ELISA) kit (DRG International Inc., East Mountainside, NJ, USA). The serum levels of TGF‐β1 were also detected using the ELISA kit (DRG International Inc., East Mountainside, NJ, USA). As NO is an unstable molecule, it is rapidly converted to nitrates and nitrites in the body; hence their concentration is parallel to NO levels. Total nitrite was quantified by the Griess reaction after reduction of nitrate to nitrite using Escherichia coli nitrate reductase 17, 18. The results were given as micromole per liter.
Statistical Analysis
The statistical analysis was performed using Prism Statistical Package, version 5.0 (Graphpad, San Diego, CA). Data comparisons were performed by Student's t‐test and ANOVA with Bonferroni's post multiple comparisons test, and the correlations among the clinical and biochemical parameters were performed using Spearman's rank correlation coefficient. The levels of significance were accepted with P < 0.05 and the results were presented in tables as mean ± SEM.
RESULTS
The clinical characteristics of patients with psoriasis vulgaris and controls are shown in Table 1. The routine laboratory investigations for patients (mean ± SEM) were the following: hemoglobin (13.44 ± 0.25 mg/dl), leukocyte count (7.88 ± 1.58 mm3), and ESR (19.71 ± 3.13 mm/hr for first hour and 37.25 ± 5.06 mm/hr for second hour). The levels of aspartate transaminase (AST), alanine transaminase (ALT), and alkaline phosphatase were in the normal range.
Table 1.
Clinical Characteristics of Controls and Patients With Psoriasis Vulgaris
| Variables | Controls (n = 22) | Patients (n = 58) |
|---|---|---|
| Age (years) | 29.36 ± 1.882 | 30.17 ± 1.406 |
| Male/Female | 11/11 | 36/22 |
| BMI (Kg/m2) | 29.58 ± 1.235 | 28.89 ± 0.7813 |
| Illness duration (years) | 10.34 ± 0.879 |
The serum levels of VEGF, TGF‐β1, and NO were significantly higher in patients than the corresponding levels in controls (Table 2). The serum levels of the studied biochemical variables, VEGF, TGF‐β1, and NO, in patients with severe disease activity were significantly increased in comparison with mild cases (P < 0.001, P < 0.05, and P < 0.001, respectively; Figs. 1, 2, 3). In addition, the levels of VEGF and TGF‐β1 did not show any significant differences in the moderate cases in comparison with mild cases.
Table 2.
Serum Levels of VEGF, TGF‐β1, and NO in Patients With Psoriasis Vulgaris Comparing With Controls
| Variables | Controls (n = 22) | Patients (n = 58) |
|---|---|---|
| VEGF (pg/ml) | 229.3 ± 16.82 | 357.5 ± 15.04** |
| TGF‐β1 (ng/ml) | 25.44 ± 2.289 | 34.81 ± 2.719* |
| NO (μmol/l) | 46.74 ± 3.539 | 132.1 ± 10.68** |
Values are means ± SE.
*P < 0.05 and **P < 0.001, respectively, for comparison between patients and controls.
Figure 1.
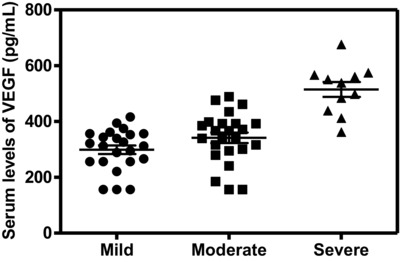
Serum levels of VEGF were significantly higher in patients with severe psoriasis activity than in mild ones (P < 0.001).
Figure 2.
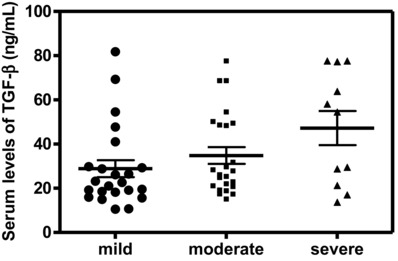
Serum levels of TGF‐β1 were significantly higher in patients with severe psoriasis activity than in mild ones (P < 0.05).
Figure 3.
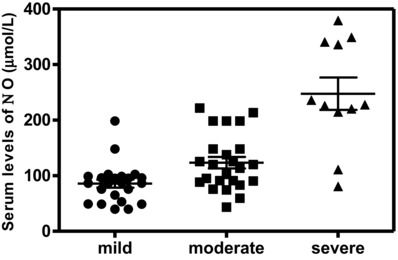
Serum levels of NO were significantly higher in patients with moderate and severe psoriasis activities than in mild ones (P < 0.01 and P < 0.001, respectively).
The VEGF and TGF‐β1 showed significant positive correlation with the duration of disease. The significant positive correlations between PASI score and each VEGF (P ≤ 0.001, r = 0.65), TGF‐β1 (P ≤ 0.05, r = 0.31), and NO (P ≤ 0.001, r = 0.51) are shown in Table 3. Moreover, a significant positive correlation between VEGF and NO was shown (P ≤ 0.05, r = 0.28). The other clinical variables such as ESR, leukocyte count, and age of the patient were not significantly correlated to the serum levels of VEGF, TGF‐β1, and NO.
Table 3.
Correlation Coefficients Between the Measured Biochemical Variables and Clinical Parameters in Patients With Psoriasis Vulgaris
| Variables | VEGF | TGF‐β1 | NO |
|---|---|---|---|
| PASI | r = 0.65 | r = 0.31 | r = 0.51 |
| P < 0.001 | P < 0.05 | P < 0.001 | |
| Illness duration | r = 0.35 | r = 0.42 | r = −0.08 |
| P < 0.01 | P < 0.05 | P > 0.773 |
Pearson's rank correlation analysis was conducted to investigate the relationship between variables. Data are presented as correlation coefficient (r) and the level of statistically significance (P).
DISCUSSION
Psoriasis is a common inflammatory skin disease seen in dermatological clinics around the world. It is very common in Saudi Arabia, and Saudi psoriatic patients are expected to have an etiological base on both environmental and genetic factors 19. The most frequently seen form of psoriasis is psoriasis vulgaris, occurring in 90% of cases. It is rarely life‐threatening; however, it has a severe negative impact on the patient's quality of life and can be an economic burden. Psoriasis vulgaris is characterized by scaly papulosquemous plaque lesions 20. It has a complex pathogenesis involving inflammation, hyperproliferation of keratinocytes, and enhanced angiogenesis. Angiogenic activity is driven by several growth factors including VEGF and TGF‐β1. The most active is VEGF, which induces vascular hyperpermeability resulting in enhanced migration of inflammatory cells from blood vessels into psoriatic lesions 21.
Our results revealed that the levels of VEGF in patients were significantly higher than the corresponding levels in controls. In addition, a significant positive correlation between PASI score and VEGF was shown. There is increasing evidence that VEGF is the primary angiogenic factor involved in the pathogenesis of psoriasis 22. Similarly, many investigators 23 found that baseline mean serum levels of VEGF were significantly higher in patients than in healthy controls. Also, the authors demonstrated a significant correlation between VEGF and PASI score. Previous studies 24, 25 confirmed the significant elevations of VEGF in psoriatic patients and its significant correlation with PASI score. The elevated levels of VEGF in the sera of psoriatic patients may reflect overproduction, either in the skin with overflow into the circulation 9 or as a result of distinct genetic polymorphism 26. Conversely, some investigators 27 have found nonsignificant difference in the mean serum levels of VEGF between patients and controls, and insignificant correlation between serum levels of VEGF and PASI scores. They believed that VEGF plasma levels could not be a useful monitor of psoriasis severity. Another study did not confirm association between VEGF plasma concentration and severity of the disease 28.
The precise role of VEGF in the evolution of psoriatic lesions is not fully explained. It promotes vascular permeability that enhances leucocyte traffic into the dermis of psoriatic lesions 29, induces capillary dilatation that helps to nourish the hyperplasic epidermis 30, alters the dermal capillaries to express leucocyte chemoattractant molecules such as intercellular adhesion molecule 1 31, and mediates high endothelial venules formation that may be important for T‐lymphocyte extravasation and trafficking 32. The previous findings suggest that VEGF is likely a key factor in the link between inflammation and angiogenesis in psoriasis 1.
TGF‐β1 is a member of a family of dimeric polypeptide growth factors and directly stimulates angiogenesis 33. Previous study 34 showed a relationship regarding TGF‐β1 plasma levels, which was linked to keratinocytes proliferation and differentiation, as well as to skin inflammation associated with psoriasis activity.
Our results revealed that the levels of TGF‐β1 were significantly higher in patients than the corresponding levels in controls. In addition, a significant positive correlation between PASI score and TGF‐β1 was shown. Several investigators found higher levels of TGF‐β1 in patients with psoriasis than controls 35, 36. Unlikely, other investigators 37 found that TGF‐β1 levels are higher in serum of psoriatic patients in comparison to controls but insignificant. Flisiak et al. 38 found that in patients with chronic psoriasis vulgaris, baseline TGF‐β1 plasma concentrations were significantly higher in only severe cases than control values. In the previous study 39, patients with severe active psoriasis vulgaris showed increased serum concentrations of TGF‐β1 and a positive correlation between serum TGF‐β1 levels and the intensity of psoriatic lesions.
Parallel to the findings of many studies 35, 40, we observed that serum TGF‐β1 was correlated significantly and positively with PASI score. The possible reason for the increased circulating TGF‐β1 with respect to the disease severity can be inflammation 40 or vascular expansion associated with activation of endothelial cells and fibroblasts that are important sources of TGF‐β1 41. In this respect, serum levels of TGF‐β1 are also increased in other inflammatory diseases, such as rheumatoid arthritis and systemic lupus erythematosus, and thus are not specific for psoriasis 42. It is also not clear why serum TGF‐β1 levels are correlated with psoriasis severity as measured by PASI, as shown in this study and other studies 35, 38.
Dysregulation of TGF‐β1 signaling has been reported in human psoriasis 43. The mechanism for increased serum levels of TGF‐β1 in patients with psoriasis remains unclear. Increased TGF‐β1 in the epidermis and the serum has been found in psoriatic patients 40 and the TGF‐β1 serum level was closely correlated with disease severity 34, 35. In contrast, TGF‐β1 is barely detectable in normal skin epidermis because of its short half‐life time 43, 44. The increased TGF‐β1 could come from activated endothelial cells, fibroblasts, or inflammatory cells in psoriasis patients; all of which can produce more TGF‐β1 34. However, based on clinical data, it is difficult to determine if increased TGF‐β1 plays a causal role in psoriasis, or it is simply a consequence of psoriasis pathogenesis.
NO has been shown to play an important role in the pathogenesis of psoriasis. Previous studies have demonstrated raised levels of NO in psoriatic plaques, which may be attributed to its effect on keratinocytes, local cyclic guanosine monophosphate (cGMP) levels, or its ability to induce angiogenesis 45. Our results revealed that the levels of NO were significantly higher in patients than the corresponding levels in controls. In addition, a significant positive correlation between PASI score and NO was shown. Similarly, several investigators found the same findings 46. This is clearly indicating that the patients with psoriasis were under severe oxidative stress. This may also be indicating that oxidative stress plays an important role in the pathogenesis of psoriasis 46. Moreover, several investigators 45 found that NO levels were significantly increased in patients with psoriasis and these levels showed a positive correlation with severity and duration in the chronic plaque‐type group.
Cals‐Gierson and Ormerod 47 have stated that NO can stimulate epithelial cells to produce and release chemokines and other growth mediators, such as VEGF, that appear to be important for keratinocyte proliferation and angiogenesis. NO is also found to increase the level of cGMP, which may act as a secondary mediator and bring about proliferation of keratinocytes. Ormerod 48 also demonstrated decreased NO production in psoriatic plaque after application of iNOS inhibitor, NG monomethyl L‐arginine. In the current study, the VEGF and NO were shown significantly positively correlated. In this respect, many investigators 49 provide more evidence that NO plays a critical role in angiogenesis. Cooke and Lsordo 50 reported that angiogenesis is attenuated when NO bioactivity is reduced. The mechanisms by which NO promotes angiogenesis are not fully elucidated. NO is an endothelial survival factor, inhibiting apoptosis and enhancing endothelial cell proliferation, perhaps in part by increasing the expression of VEGF or fibroblast growth factor 50. NO may suppress the production of angiostatin, an endogenous antagonist of angiogenesis 49.
CONCLUSIONS
The elevated serum levels of VEGF, TGF‐β1, and NO in our psoriatic patients and their significant correlation with psoriasis severity (PASI score) strongly support the proposed role of these bio‐indices in the pathogenesis of psoriasis. Accordingly, the serum levels of VEGF, TGF‐β1, and NO might be recognized as indicators for clinical evaluation of disease severity. It has been assumed that direct targeting of angiogenesis, particularly the main player VEGF, may help to develop new strategies to treat psoriasis by influencing the angiogenesis required for the inflammatory disease. Further studies must take place to clarify the exact role of these studied growth factors and NO in psoriasis and their possible usefulness from a therapeutic standpoint.
CONFLICT OF INTEREST
None declared.
ACKNOWLEDGMENTS
This study was supported by a grant (SR‐D‐011–877) from Deanship of Scientific Research, Qassim University, Saudi Arabia. We thank our lab specialists for helping in the experiment performed in the lab.
Grant sponsor: Qassim University Deanship of Scientific Research; Grant number: SR‐D‐011–877.
Permanent address: Abdel‐Raheim M. A. Meki, Department of Medical Biochemistry, Faculty of Medicine, Assiut University, Assiut, Egypt. E‐mail: Meki202000@Yahoo.com.
REFERENCES
- 1. Simonetti O, Lucarini G, Goteri G, et al. VEGF is likely a key factor in the link between inflammation and angiogenesis in psoriasis: Results of an immunohistochemical study. Int J Immunopathol Pharmacol 2006;19:751–760. [DOI] [PubMed] [Google Scholar]
- 2. Henno A, Blacher S, Lambert CA, et al. Histological and transcriptional study of angiogenesis and lymphangiogenesis in uninvolved skin, acute pinpoint lesions and established psoriasis plaques: An approach of vascular development chronology in psoriasis. J Dermatol Sci 2010;57:162–169. [DOI] [PubMed] [Google Scholar]
- 3. Micali G, Lacarrubba F, Musumeci ML, Massimino D, Nasca MR. Cutaneous vascular patterns in psoriasis. Int J Dermatol 2010;49(3):249–256. [DOI] [PubMed] [Google Scholar]
- 4. Zhu JW, Wu XJ, Lu ZF, Luo D, Cai SQ, Zheng M. Role of VEGF receptors in normal and psoriatic human keratinocytes: Evidence from irradiation with different UV sources. PLoS One 2013;8(1):e55463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Keck PJ, Hauser SD, Krivi G, et al. Vascular permeability factor, an endothelial cell mitogen related to PDGF. Science 1989;246:1309–1312. [DOI] [PubMed] [Google Scholar]
- 6. Shibuya M, Claesson‐Welsh L. Signal transduction by VEGF receptors in regulation of angiogenesis and lymphan‐giogenesis. Exp Cell Res 2006;312:549–560. [DOI] [PubMed] [Google Scholar]
- 7. Brown LF, Detmar M, Claffey K, et al. Vascular permeability factor/vascular endothelial growth factor: A multifunctional angiogenic cytokine. EXS 1997;79:233–269. [DOI] [PubMed] [Google Scholar]
- 8. Chua RA, Arbiser JL. The role of angiogenesis in the pathogenesis of psoriasis. Autoimmunity 2009;42:574–579. [DOI] [PubMed] [Google Scholar]
- 9. Bhushan M, McLaughlin B, Weiss JB, Griffiths CE. Levels of endothelial cell stimulating angiogenesis factor and vascular endothelial growth factor are elevated in psoriasis. Br J Dermatol 1999;141:1054–1060. [DOI] [PubMed] [Google Scholar]
- 10. Teige I, Hvid H, Svensson L, Kvist PH, Kemp K. Regulatory T cells control VEGF‐dependent skin inflammation. J Invest Dermatol 2009;129:1437–1445. [DOI] [PubMed] [Google Scholar]
- 11. Lawrence DA. Transforming growth factor‐beta: general review. Eur Cytokine Netw 1996;7:363–374. [PubMed] [Google Scholar]
- 12. Letterio JJ, Roberts AB. Regulation of immune responses by TGF‐b. Annu Rev Immunol 1998;16:137–161. [DOI] [PubMed] [Google Scholar]
- 13. Sporn MB, Roberts AB. Autocrine secretion—10 years later. Ann Intern Med 1992;117:408–414. [DOI] [PubMed] [Google Scholar]
- 14. Wahl SM, McCarteney‐Francis N, Mergenhagen SE. Inflammatory and immunomodulatory roles of TGF‐β. Immunol Today 1989;10:258–261. [DOI] [PubMed] [Google Scholar]
- 15. Bruch‐Gerharz D, Schnorr O, Suschek C, et al. Arginase 1 overexpression in psoriasis. Limitation of inducible nitric oxide oxide synthase activity as a molecular mechanism of keratinocyte hyperproliferation. Am J Pathol 2003;162:203–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Fredrikson T, Petterson U. Severe psoriasis: Oral therapy with a new retinoid. Dermatologica 1978;157:238–244. [DOI] [PubMed] [Google Scholar]
- 17. Granger DL, Taintor RR, Boockvar KS, Hibbs JB, Jr. Measurement of nitrate and nitrite in biological samples using nitrate reductase and Griess reaction. Methods Enzymol 1996;268:142–151. [DOI] [PubMed] [Google Scholar]
- 18. Balat A, Cekmen M, Yürekli M, et al. Adrenomedullin and nitrite levels in children with Bartter syndrome. Pediatr Nephrol 2000;15(3–4):266–270. [DOI] [PubMed] [Google Scholar]
- 19. Al Robaee AA. Molecular genetics of psoriasis (principles, technology, gene location, genetic polymorphism and gene expression). Int J Health Sci 2010;4(2):103–127. [PMC free article] [PubMed] [Google Scholar]
- 20. Griffiths CE, Barker JN. Pathogenesis and clinical features of psoriasis. Lancet 2007;370:263–271. [DOI] [PubMed] [Google Scholar]
- 21. Creamer D, Allen M, Jaggar R, Stevens R, Bicknell R, Barker J. Mediation of systemic vascular hyperpermeability in severe psoriasis by circulating vascular endothelial growth factor. Arch Dermatol 2002;138:791–796. [DOI] [PubMed] [Google Scholar]
- 22. Detmar M. Evidence for vascular endothelial growth factor (VEGF) as a modifier gene in psoriasis. J Invest Dermatol 2004;122:xiv–xv. [DOI] [PubMed] [Google Scholar]
- 23. Flisiak I, Zaniewski P, Rogalska‐Taranta M, Chodynicka B. Effect of psoriasis therapy on VEGF and its soluble receptors serum concentrations. J Eur Acad Dermatol Venereol 2012;26(3):302–307. [DOI] [PubMed] [Google Scholar]
- 24. Nofal A, Al‐Makhzangy I, Attwa E, Nassar A, Abdalmoati A. Vascular endothelial growth factor in psoriasis: An indicator of disease severity and control. J Eur Acad Dermatol Venereol 2009;23:803–806. [DOI] [PubMed] [Google Scholar]
- 25. Flisiak I, Zaniewski P, Rogalska M, Myśliwiec H, Jaroszewicz J, Chodynicka B. Effect of psoriasis activity on VEGF and its soluble receptors concentrations in serum and plaque scales.Cytokine 2010;52(3):225–229. [DOI] [PubMed] [Google Scholar]
- 26. Young HS, Summers AM, Bhushan M, Brenchley PE, Griffiths CE. Single‐nucleotide polymorphisms of vascular endothelial growth factor in psoriasis of early onset. J Invest Dermatol 2004;22:209–215. [DOI] [PubMed] [Google Scholar]
- 27. Akman A, Dicle O, Yilmaz F, Coskun M, Yilmaz E. Discrepant levels of vascular endothelial growth factor in psoriasis patients treated with PUVA, Re‐PUVA and narrow‐band UVB. Photodermatol Photoimmunol Photomed 2008;24:123–127. [DOI] [PubMed] [Google Scholar]
- 28. Barile S, Medda E, Nisticò L, et al. Vascular endothelial growth factor gene polymorphisms increase the risk to develop psoriasis. Exp Dermatol 2006;15:368–376. [DOI] [PubMed] [Google Scholar]
- 29. Barker JN. The pathophysioology of psoriasis. Lancet 1993;338:227–230. [DOI] [PubMed] [Google Scholar]
- 30. Krueger G, Ellis CN. Psoriasis – Recent advances in understanding its pathogenesis and treatment. J Am Acad Dermatol 2005;53:S94–S100. [DOI] [PubMed] [Google Scholar]
- 31. Guenther LC, Ortonne JP. Pathophysiology of psoriasis: Science behind therapy. J Cutan Med Surg 2002;6:2–7. [DOI] [PubMed] [Google Scholar]
- 32. Saurat JH, Geiger JM, Amblard P, et al. Randomized double‐blind multicenter study comparing acitretin‐PUVA, etretinate‐PUVA and placebo‐PUVA in the treatment of severe psoriasis. Dermatologica 1988;177:218–224. [DOI] [PubMed] [Google Scholar]
- 33. Blobe GC, Schiemann WP, Lodish HF. Role of transforming growth factor beta in human disease. N Engl J Med 2000;342:1350–1358. [DOI] [PubMed] [Google Scholar]
- 34. Flisiak I, Zaniewski P, Chodynicka B. Plasma TGF‐ β1, TIMP‐1, MMP‐1 and IL‐18 as combined biomarker of psoriasis activity. Biomarkers 2008;13:549–556. [DOI] [PubMed] [Google Scholar]
- 35. Nockowski P, Szepietowski JC, Ziarkiewicz M, Baran E. Serum concentrations of transforming growth factor beta 1 in patients with psoriasis vulgaris. Acta Dermatovenerol Croat 2004;12:2–6. [PubMed] [Google Scholar]
- 36. Kallimanis PG, Xenos K, Markantonis SL, et al. Serum levels of transforming growth factor‐beta1 in patients with mild psoriasis vulgaris and effect of treatment with biological drugs. Clin Exp Dermatol 2009;34(5):582–586. [DOI] [PubMed] [Google Scholar]
- 37. Zaher H, Shaker OG, EL‐Komy MH, El‐Tawdi A, Fawzi M, Kadry D. Serum and tissue expression of transforming growth factor beta 1 in psoriasis. J Eur Acad Dermatol Venereol 2009;23(4):406–409. [DOI] [PubMed] [Google Scholar]
- 38. Flisiak I, Porebski P, Flisiak R, Chodynicka B. Plasma transforming growth factor beta1 as a biomarker of psoriasis activity and treatment efficacy. Biomarkers 2003;8:437–443. [DOI] [PubMed] [Google Scholar]
- 39. Bonifati C, Carduci M, Mussi A, et al. The levels of transforming growth factor‐ b1 are increased in the serum of patients and correlate with disease severity. Eur J Dermatol 1996;6:486–490. [Google Scholar]
- 40. Flisiak I, Chodynicka B, Porebski P, Flisiak R. Association between psoriasis severity and transforming growth factor beta (1) and beta (2) in plasma and scales from psoriatic lesions. Cytokine 2002;19:121–125. [DOI] [PubMed] [Google Scholar]
- 41. Oyama N, Iwatsuki K, Satoh M, Akiba H, Kaneko F. Dermal fibroblasts are one of the therapeutic targets for topical application of 1alpha, 25‐dihydroxyvitamin D3: the possible involvement of transforming growth factor‐beta induction. Br J Dermatol 2000;143:1140–1148. [DOI] [PubMed] [Google Scholar]
- 42. Border WA, Ruoslahti E. Transforming growth factor‐beta in disease: The dark side of tissue repair. J Clin Invest 1992;90:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Han G, Lu SL, Li AG, et al. Distinct mechanisms of TGF‐beta1‐mediated epithelial‐to‐mesenchymal transition and metastasis during skin carcinogenesis. J Clin Invest 2005;115:1714–1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Quan T, He T, Kang S, Voorhees JJ, Fisher GJ. Ultraviolet irradiation alters transforming growth factor beta/smad pathway in human skin in vivo. J Invest Dermatol 2002;119:499–506. [DOI] [PubMed] [Google Scholar]
- 45. Gokhale NR, Belgaumkar VA, Pandit DP, Deshpande S, Damle DK. A study of serum nitric oxide levels in psoriasis. Indian J Dermatol Venereol Leprol 2005;71(3):175–178. [DOI] [PubMed] [Google Scholar]
- 46.Sikar Aktürk A, Özdoğan HK, Bayramgürler D, Çekmen MB, Bilen N, Kıran R. Nitric oxide and malondialdehyde levels in plasma and tissue of psoriasis patients. J Eur Acad Dermatol Venereol 2012;26(7):833–837. [DOI] [PubMed] [Google Scholar]
- 47. Cals‐Gierson MM, Ormerod AD. Nitric oxide function in the skin. Nitric Oxide 2004;10:179–193. [DOI] [PubMed] [Google Scholar]
- 48. Ormerod AD, Copeland P, Shah SA. Treatment of psoriasis with topical NG‐monomethyl‐L‐arginine, an inhibitor of nitric oxide synthesis. Br J Dermatol 2000;145:985–990. [DOI] [PubMed] [Google Scholar]
- 49. Matsunaga T, Weihrauch DW, Moniz MC, Tessmer J, Warltier DC, Chilian WM. Angiostatin inhibits coronary angiogenesis during impaired production of nitric oxide. Circulation 2002;105:2185–2191. [DOI] [PubMed] [Google Scholar]
- 50. Cooke JP, Losordo DW. Nitric oxide and angiogenesis. Circulation 2002;105(18):2133–2135. [DOI] [PubMed] [Google Scholar]


