Abstract
Introduction
To date, no study reports the implication of YKL‐40 in pelvic inflammatory disease (PID). Therefore, we investigate the levels of plasma YKL‐40 in patients with PID and further associate its expression with the severity of disease.
Methods
We designed a hospital‐based case‐control study with approximate 1:1 ratio and consecutively recruited 64 patients with PID and 70 control women. We collected blood samples from 64 women with PID before and after they received treatment and 70 control women to detect levels of plasma YKL‐40 and C‐reactive protein (CRP) as well as white blood cell and neutrophil counts.
Results
The results revealed that levels of plasma YKL‐40 were significantly elevated in patients with PID as compared to those in controls (38.36 vs. 21.69 ng/ml, P = 0.001) but the significant difference was restricted to women aged 30 years or old after age stratification (56.75 vs. 23.61 ng/ml, P ≤ 0.001). It declined significantly after they received treatment (median: 38.36 vs. 27.54 ng/ml; P ≤ 0.001). Although both plasma YKL‐40 and CRP were elevated in patients with tubo‐ovarian abscess, PID patients with surgery exhibited higher YKL‐40 concentration than those without surgery (median: 82.05 vs. 30.19 ng/ml, P = 0.005) and only plasma YKL‐40 was significantly associated with the length of the hospital stay (P ≤ 0.001, R = 0.604).
Conclusion
We conclude that once individuals are diagnosed to have PID, YKL‐40 may act as a biomarker to predict the severity and clinical outcome of the disease. J. Clin. Lab. Anal. 26:136‐142, 2012. © 2012 Wiley Periodicals, Inc.
Keywords: YKL‐40, pelvic inflammatory disease, CRP
INTRODUCTION
Pelvic inflammatory disease (PID) refers to the infection of uterus, fallopian tubes, and ovaries. It often involves the neighboring pelvic organs. Lower abdominal pain is the main presenting symptom in women with PID although the character of the pain may be quite subtle. If PID is not treated early and accurately, its sequelae are severe. Tubo‐ovarian abscess (TOA) may form or more seriously, sepsis takes place. When the infection itself is eradicated too late, the damaged genital organs or neighboring pelvic organs are probably accompanied by scarring and adhesion formation in pelvis, which causes female infertility of tubal factor. Women with tubal factor infertility apparently induced by past episodes of PID often give no history of PID 1, 2. Patient may stay in hospital for a long time or receive surgery. In order to prevent the delay in diagnosis of PID that leads to severe sequelae, it is necessary to diagnose and treat the women with PID early. Therefore, it is reasonable to introduce biologic factors to offer early diagnosis and correlate their expression with the severity of PID.
YKL‐40 means the first three N‐terminal amino acids and 40 denotes its molecular mass in kilodaltons (KDa). It is a heparin‐, collagen‐, and chitin‐binding plasma glycoprotein and belongs to the chitinase protein family 3, 4, 5. YKL‐40 is secreted by activated macrophages and macrophages during late stage of differentiation 6, 7, neutrophils 8, vascular smooth muscle cells 9, arthritic chondrocytes 10, and cancer cells 11. The concentration of YKL‐40 increases in patients by more than 25% following an inflammatory stimulus and regarded as an acute phase protein 12, 13. It is emerging as a new biomarker of severe disease activity and poor prognosis in patients with diseases related to the degree of inflammation, pathological tissue remodeling, and ongoing fibrosis such as rheumatoid arthritis, inflammatory bowel disease, ischemic cardiovascular disease, chronic obstructive pulmonary disease, diabetes, and cancer 14, 15, 16, 17, 18, 19, 20.
We hypothesized that the level of plasma YKL‐40 is elevated in women with PID and correlated with disease process. To date, no reports delineate the level of plasma YKL‐40 in PID and its implication in the severity of the disease. Therefore, the objectives of our investigation were to compare the concentration of plasma YKL‐40 between women with PID and normal controls and further determine its cutoff level to differentiate women with PID from normal women. Moreover, we correlate the level of plasma YKL‐40 with the severity and clinical outcome of PID.
MATERIALS AND METHODS
Individuals and Samples Collection
This was a hospital‐based case‐control study with approximate 1:1 ratio. We consecutively recruited 64 patients with PID, who received routine treatment protocols at Department of Obstetrics and Gynecology, as well as 70 healthy women, who visited Department of Family and Community Medicine for health examination in Chung Shan Medical University Hospital, between April 2006 and September 2007. No significant difference for age was found between patients with PID and normal controls (mean ± SD: 37.4 ± 11.0 vs. 40.6 ± 10.3; P = 0.081). The controls were included as age‐group matched and further matched in clinical data such as socioeconomic, area, occupation, cigarette smoking, and alcohol drinking.
PID were diagnosed according to the characteristic criteria of Centers for Disease Control and Prevention (CDC) for PID if the patients had lower abdominal pain or pelvic pain of no other origin, with one of the following criteria: uterine tenderness, adnexal tenderness, or cervical motion tenderness 21. Blood samples were collected from 70 healthy women as well as 64 patients with PID before and 3 days after they received the treatment for 3 days based on the routine protocols in our hospital based on the CDC. The healthy women visited our hospital for health examination and agreed to accept our study protocols. Therefore, plasma YKL‐40 and C‐reactive protein (CRP) may be measured from the blood samples that were obtained for CBC (complete blood count) and biochemistry examination. Plasma samples were collected from healthy women and patients in vacuum tubes with EDTA and stored within 30 min. After centrifugation at 3,000 ×g for 10 min, plasma was isolated, aliquoted, and stored in a −80°C freezer. All samples were analyzed for YKL‐40 concentration, white blood cell (WBC) and neutrophil counts, as well asCRP concentration. Both the technicians, who measured the YKL‐40 levels, as well as the clinical laboratory staff, who measured WBCs and neutrophil counts and CRP levels, were blinded to this study. The study was approval by the Chung Shan University Hospital Institutional Review Board, and informed consent was obtained from each subject.
Measurements of Plasma YKL‐40 Levels by Enzyme‐Linked Immunosorbent Assay (ELISA)
Detection of the plasma YKL‐40 levels was analyzed by human YKL‐40 ELISA kits (R&D Systems, Abingdon, UK) in 64 patients with PID and 70 healthy individuals. One hundred microlitres were directly transferred to the microtest strip wells of the ELISA plate from each plasma sample, and then incubated for 2 hr at room temperature. The detection antibody was incubated for 2 hr at room temperature after three washing steps. Antibody binding was detected with streptavidin‐conjugated horseradish peroxidase and developed with a substrate solution. The reaction was stopped and optical density was determined with a microplate reader set at 450 nm. Wavelength correction was set to 570 nm. Sample results were calculated from a standard curve generated by dilutions of a known amount of recombinant YKL‐40 protein. Each standard or sample was assayed in duplicate.
Statistical Analysis
Mann–Whitney U‐test was used to test the statistical significance regarding difference in the levels of plasma YKL‐40 and CRP as well as WBC and neutrophil counts between 64 patients with PID before they received treatment and 70 normal controls. Since YKL‐40 levels rise in a linear fashion with increasing age 22, the comparison of plasma YKL‐40 levels is stratified by the age of 30 in these subjects. A Wilcoxon signed‐rank test was used to detect the difference of these parameters between pretreatment and posttreatment plasma in the same patients with PID. Spearman's rank correlation analysis was used to evaluate the correlations of these parameters. Odds ratios (ORs) and their 95% confident intervals (CIs) of levels of plasma YKL‐40 and CRP as well as WBC and neutrophil counts for PID risk were determined by the cutoff levels estimation based on receiver‐operating characteristic curves (ROCs). They were calculated via Chi‐square test using WinPepi software, Version 10.0. The adjusted odds ratios (AORs) with their 95% CIs of these parameters were analyzed using logistic regression model.
We tried to plot the ROCs to determine the cutoff levels of plasma YKL‐40 to differentiate patients with PID from normal individuals. The cutoff levels of plasma YKL‐40 were determined based on its pretreatment plasma levels in 64 patients with PID and its levels in 70 control women. Sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), accuracy, and likelihood ratios of positive result were then calculated. Furthermore, we evaluate the correlation of the levels of plasma YKL‐40 with disease severity and clinical course of PID. Severe signs of infection include TOA, sepsis, and organ failures. However, there were no cases of sepsis and organ failure in our studied samples. Mann–Whitney U‐test was used to test the statistical significance among plasma YKL‐40 levels, incidence of TOA, and surgery. Spearman's rank correlation analysis was used to correlate the levels of plasma YKL‐40 with the length of the hospital stay. We further used multiple linear regression model for adjusting our parameter for the length of the hospital stay. The SPSS statistical package version 12.0 (SPSS Inc., Chicago, IL) was used for statistical analysis. A P‐value of less than 0.05 was considered.
RESULTS
The level of plasma YKL‐40 was significantly elevated in 64 patients with PID before they received treatment as compared to that in 70 control subjects (median: 38.36 vs. 21.69 ng/ml, P = 0.001; Table 1). When the comparison of plasma YKL‐40 was stratified by the age of 30, the YKL‐40 level was significantly elevated in 41 patients with PID, as compared to 44 control women in these individuals aged 30 years age or older (56.75 vs. 23.61 ng/ml, P ≤ 0.001). However, there was no significant difference between 23 patients with PID and 26 control women for those younger than 30 years old (P = 0.795). In addition, the level of plasma YKL‐40 in patients with PID before they received treatment was significantly elevated as compared to that in same patients after they received treatment (median: 38.36 vs. 27.54 ng/ml; P < 0.001). WBC and neutrophil counts were significantly decreased after they received treatment among the 64 patients with PID (WBC count was from 11,085 to 6,120, P < 0.001; neutrophil count was from 8,927 to 3,595, P < 0.001, respectively; Table 1). After treatment, the level of plasma CRP was also significantly decreased (from 4.87 to 0.94 mg/dl, P < 0.001).
Table 1.
The Laboratory Data of Both Controls and Patients With Pelvic Inflammatory Disease (PID) Before and After They Received Treatmenta
| Clinical variables | Controls (n = 70) Median (range) | Pretreated (n = 64) Median (range) | Posttreated (n = 64) Median (range) | P‐value UT/Cb | P‐value UT/Tc |
|---|---|---|---|---|---|
| YKL‐40 (ng/ml) | 21.69 | 38.36 | 27.54 | P = 0.001 | P < 0.001 |
| (2.85–79.66) | (5.25–187.61) | (5.20–180.47) | |||
| CRP (mg/dl) | 0.30 (0.02–1.65) | 4.87 (0.30–24.60) | 0.94 (0.30–11.30) | P < 0.001 | P < 0.001 |
| WBC (/mm3) | 6,775 | 11,085 | 6,120 | P < 0.001 | P < 0.001 |
| (3,390–16,400) | (3,880–25,950) | (2,350–13,240) | |||
| Neutrophils (/mm3) | 4,250 | 8,927 | 3,595 | P < 0.001 | P < 0.001 |
| (1,733–12,464) | (2,091–21,625) | (1,130–11,122) |
CRP, C‐reactive protein; WBC, white blood cell; C, controls; UT, patients with PID before they received treatment; T, patients with PID after they received treatment.
P‐value < 0.05 was considered significant.
The statistical difference was analyzed by Mann–Whitney U‐test.
The statistical difference was analyzed by Wilcoxon signed‐ranks test.
The correlations between the pretreatment level of plasma YKL‐40 and WBC counts as well as between the level of YKL‐40 and neutrophil counts were significant (Spearman's rank correlation coefficient r = 0.457, P < 0.001; r = 0.493, P < 0.001, respectively; Fig. 1A and B). Furthermore, the correlation between the levels of plasma
Figure 1.
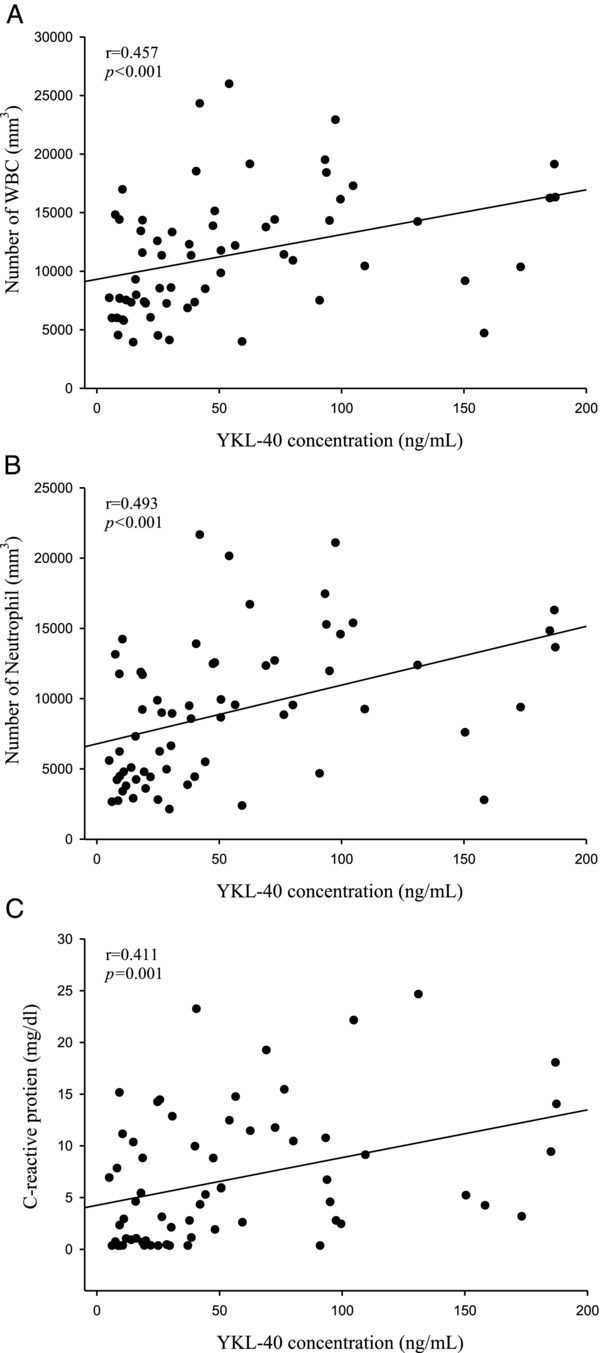
The correlations among levels of plasma YKL‐40 and C reactive protein (CRP) as well as white blood cell (WBC) and neutrophil counts in patients with pelvic inflammatory disease (PID) before they received treatment. (A) There was a significant correlation between plasma YKL‐40 and WBC counts (r = 0.457, P < 0.001) in 64 patients with PID. (B) There was a significant correlation between plasma YKL‐40 and neutrophil counts (r = 0.493, P < 0.001) in 64 patients with PID. (C) There was a significant correlation between levels of plasma YKL‐40 and CRP (Spearman correlation coefficients r = 0.411, P = 0.001) in 64 patients with PID.
YKL‐40 and CRP was also significant (r = 0.411, P = 0.001; Fig. 1C).
When the cutoff levels of the plasma YKL‐40 and CRP as well as WBC and neutrophil counts were set to be 24.82 ng/ml and 0.67 mg/dl as well as 7,800 and 5,500/mm3 by plotting the ROCs, they were all significant factors to predict PID by univariate analyses (Table 2). The areas under curves of plasma YKL‐40 and CRP were 0.670 (P = 0.001; 95% CI: 0.573–0.766), 0.902 (P < 0.001; 95% CI: 0.846–0.959), respectively.
Table 2.
The Adjusted Odds Ratios (AORs) with 95% Confidence Intervals (CIs) of Plasma Parameters for the Risk of Pelvic Inflammatory Disease (PID)a
| Parameters | PID (n = 64) | Controls (n = 70) | P‐value | ORs (95% CIs) | AORs (95% CIs) |
|---|---|---|---|---|---|
| YKL‐40b (ng/ml) | <0.001 | 3.66 | 2.55 | ||
| >24.82 | 42 | 24 | (1.69–7.96) | (0.91–7.15) | |
| ≤24.82 | 22 | 46 | |||
| CRPb (mg/dl) | <0.001 | 39.00 | 29.68 | ||
| >0.67 | 52 | 7 | (13.13– | (9.83–89.58) | |
| ≤0.67 | 12 | 63 | 122.49) | ||
| WBCb (/mm3) | <0.001 | 4.77 | 0.28 | ||
| >7,800 | 42 | 20 | (2.16–10.60) | (0.04–1.87) | |
| ≤7,800 | 22 | 50 | |||
| Neutrophilb (/mm3) | <0.001 | 7.00 | 5.45 | ||
| >5,500 | 42 | 15 | (3.05–16.32) | (0.86–34.64) | |
| ≤5,500 | 22 | 55 |
CRP, C‐reactive protein; WBC, white blood cell.
Statistical analysis: Chi‐square test was used to analyze P‐value and ORs. WinPepi Software, Version 10.0 was applied. The AORs with their 95% CIs were calculated after controlling for plasma YKL‐40 and CRP concentrations as well as WBC and neutrophil counts using logistic regression model. SPSS statistical package version 12.0 was applied.
Cutoff levels were estimated according to receiver‐operating characteristic curves of these parameters.
After these parameters were adjusted using logistic regression model, only the level of plasma CRP had significant association with PID although the level of plasma YKL‐40 tended to be elevated in patients with PID (Table 2). When the cutoff levels of the plasma YKL‐40 and CRP were determined, their sensitivities were 65.63 and 81.25% (Table 3). Their NPVs were 67.65 and 84.00%. Their likelihood ratios of positive result were 1.914 and 8.125, respectively. If the cutoff level of plasma YKL‐40 was set to be 35.63 ng/ml in subjects, who aged 30 years or older, the areas under curve was 0.827 (P ≤ 0.001; 95% CI: 0.733–0.922). The sensitivity was 78.05 and likelihood ratio of positive result was 3.817 (Table 3).
Table 3.
The Sensitivity, Specificity, Positive and Negative Predictive Values (PPV and NPV), Accuracy, and Likelihood Ratios of Positive Result (LR+) of the Plasma YKL‐4 and CRP for Pelvic Inflammatory Disease (PID)
| Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | Accuracy (%) | LR+ | |
|---|---|---|---|---|---|---|
| YKL‐40 | 65.63 | 65.71 | 63.64 | 67.65 | 65.67 | 1.914 |
| YKL‐40 (aged 30 or older)a | 78.05 | 79.55 | 78.05 | 79.55 | 78.82 | 3.817 |
| CRP | 81.25 | 90.00 | 88.14 | 84.00 | 85.82 | 8.125 |
The cutoff levels of plasma YKL‐40 and CRP for PID are 24.82 ng/ml and 0.67 mg/dl, respectively, based on 64 patients with PID and 70 healthy women using the receiver‐operating characteristic curves. When the levels of plasma YKL‐40 or CRP are higher than the cutoff levels, they are regarded as positive results.
The data are analyzed in women aged 30 years or older and the cutoff level is set to be 35.63 ng/ml.
Areas under curves for YKL‐40 and CRP were 0.670 and 0.902, respectively (P = 0.001; 95% CI: 0.573–0.766; P < 0.001; 95% CI: 0.846–0.959). Sensitivity = the number of patients with PID, who have positive results/the number of patients with PID; Specificity = the number of healthy women, who have negative results/the number of healthy women; PPV = the number of women with positive results, who have PID/the number of PID patients and healthy women, who have positive results; NPV = the number of women with negative results, who are healthy/the number of PID patients and healthy women, who have negative results; Accuracy = the number of patients with PID, who have positive results plus the number of healthy women, who have negative results/number of all PID patients plus all healthy women; LR+ = the probability of positive results for patients with PID /the probability of positive results for healthy women.
Among the 64 PID patients, 25 patients had TOA and 12 received surgical intervention. We found that PID patients with TOA had higher plasma YKL‐40 and CRP concentrations than those without TOA (median: 69.33 vs. 38.36 ng/ml, P = 0.005 for YKL‐40; 9.38 vs. 4.87 mg/dl, P = 0.001 for CRP). PID patients with surgery exhibited higher YKL‐40 concentration than those without surgery (median: 82.05 vs. 30.19 ng/ml, P = 0.005). But, PID patients with surgery did not have higher CRP concentration than those without surgery (median: 9.07 vs. 4.40 ng/ml, P = 0.16). Plasma YKL‐40 and CRP also exhibited a significant correlation with the length of the hospital stay (Spearman correlation coefficient r = 0.540 and 0.529; P < 0.001 and P < 0.001, respectively; Fig. 2). Using multiple linear regression model for adjusting the concentrations of plasma YKL‐40 and CRP as well as WBC and neutrophil counts, only plasma YKL‐40 was significantly associated with the length of the hospital stay (P < 0.001, B estimate = 0.046, standard error = 0.012, R = 0.604).
Figure 2.
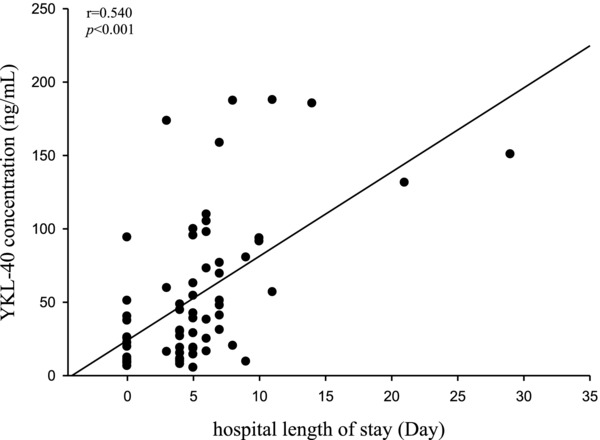
The correlation between level of plasma YKL‐40 and length of the hospital stay. Plasma YKL‐40 exhibited a significant correlation with the length of the hospital stay (Spearman correlation coefficient r = 0.540; P < 0.001).
DISCUSSION
In this study, we found that the level of plasma YKL‐40 in patients with PID was elevated, as compared to that in healthy women. After treatment, the level of YKL‐40 was significantly reduced. YKL‐40 is an alternatively spliced 40‐kDa variant of chitinase 1 and reported to be expressed and stored in intracellular lysosomes and lysosome‐related organelles 23. YKL‐40 may be released by exocytosis from specific granules at the site of inflammation, mainly expressed by activated macrophages and neutrophils 24. Therefore, it can be used as a diagnostic and prognostic serologic marker of specific bacterial infections. Elevated level of YKL‐40 was demonstrated to be in lysosomal storage diseases, infections (fungal and bacterial infection), and chronic inflammation (atherosclerosis) 25, 26. YKL‐40 still can bind to chitin, type I collagen, heparin, as well as hyaluronan and is thought to play a role in the process of inflammation although it lacks chitinolytic activity 27. In articular chondrocytes, Recklies et al. found that TNF‐α and IL‐1β, the inflammatory cytokines, stimulate the expression of YKL‐40 28. In contrast, it was suggested that the induction of YKL‐40 feeds back to control local tissue responses through the inhibition of cellular responses induced by IL‐1 and TNF‐α 29. These mechanisms may contribute to the dysregulation of YKL‐40 seen in acute or chronic inflammation. It is reasonable that YKL‐40 may be released by neutrophils and serves as a marker of the process of inflammation in PID.
The univariate analyses showed that women with elevated parameters (≥cutoff level for each parameter) have more risk of developing PID (OR: 3.66, 39.0, 4.77, and 7.00, for YKL‐40, CRP, WBC, and neutrophil counts, respectively). Although plasma YKL‐40 tended to be higher in patients with PID, multivariate analysis revealed that only plasma CRP can significantly predict PID and exhibit better sensitivity and specificity than plasma YKL‐40. Plasma CRP may serve as a better diagnostic biomarker for PID than plasma YKL‐40 based on the better likelihood ratio of positive result (8.125 vs. 1.914). Since YKL‐40 levels rise in a linear fashion with increasing age 22, it is investigated by the stratification of age 30. Our results further revealed that plasma YKL‐40 is only predictable for PID in women aged 30 years or older but not in those younger than 30 years old. However, it is still inferior to CRP in distinguishing PID patients from healthy women.
YKL‐40 levels have been found elevated and correlated with disease activity in inflammatory diseases such as rheumatoid arthritis and inflammatory bowel disease 30, 31. Conrozier et al. revealed significant relationships among serum YKL‐40 and CRP levels and disease activity in patients with rheumatoid arthritis 32. Furthermore, Hattori et al. indicated the possibility of involvement of YKL‐40 in the sepsis and suggested that YKL‐40 is a biomarker of sepsis 33. In our study, there were no cases of sepsis and organ failure. However, in TOA cases, more severe form of PID, we found that PID patients with TOA had higher plasma YKL‐40 and CRP concentrations than those without TOA. The level of plasma YKL‐40 also has a significant correlation with that of CRP.
Živanović et al. indicated that increased levels of YKL‐40 can reflect the degree of the destructive changes in knee osteoarthritis. This indicates that the biomarker can be used to assess joint destruction 34. High serum YKL‐40 is also reported to be associated with poor prognosis in patients with Streptococcus pneumonia bacteraemia 35. To date, our investigation is the first one to put emphasis on the association of plasma YKL‐40 with severity and prolonged inflammation of PID. We found that plasma YKL‐40 is elevated in TOA, more severe form of PID. Furthermore, plasma YKL‐40 exhibited a significant correlation with hospital length of stay. It means that PID patients with higher concentration of plasma YKL‐40 need more admission time to treat. Moreover, plasma YKL‐40 was associated with the incidence of surgery in patients with PID. This implies that only medication cannot relieve the manifestation of PID because the disease progresses. These findings indicate that PID patients with higher levels of plasma YKL‐40 have poor clinical courses. Although plasma YKL‐40 level is limited in the diagnosis of PID, it may be used as a predictor for the severity and clinical outcome of this disease.
In conclusion, levels of plasma YKL‐40 are significantly elevated only in patients with PID aged 30 years or older as compared to healthy counterparts. Once individuals are diagnosed to have PID based on clinical manifestation, physical examination and history as well as plasma CRP, plasma YKL‐40 may act as a biomarker to predict the severity and clinical outcome of the disease.
Conflict of Interest
We wish to confirm that there are no known conflicts of interest associated with this publication and there has been no significant financial support for this work that could have influenced its outcome.
Grant sponsor: Chung Shan Medical University Hospital; Grant number: CSH‐97‐A‐33
REFERENCES
- 1. Moore DE, Spadoni LR, Foy HM, et al. Increased frequency of serum antibodies to Chlamydia trachomatis in infertility due to distal tubal disease. Lancet 1982;2:574. [DOI] [PubMed] [Google Scholar]
- 2. Wolner‐Hanssen P. Silent pelvic inflammatory disease: Is it overstated. Obstet Gynecol 1995;86:321. [DOI] [PubMed] [Google Scholar]
- 3. Renkema GH, Boot RG, Au FL, et al. Chitotriosidase, a chitinase, and the 39‐kDa human cartilage glycoprotein, a chitin‐binding lectin, are homologues of family 18 glycosyl hydrolases secreted by human macrophages. Eur J Biochem 1998;251:504–509. [DOI] [PubMed] [Google Scholar]
- 4. Shackelton LM, Mann DM, Millis AJ. Identification of a 38‐kDa heparin binding glycoprotein (gp38k) in differentiating vascular smooth muscle cells as a member of a group of proteins associated with tissue remodeling. J Biol Chem 1995;270:13076–13083. [DOI] [PubMed] [Google Scholar]
- 5. Badariotti F, Kypriotou M, Lelong C, et al. The phylogenetically conserved molluscan chitinase‐like protein 1 (Cg‐Clp1), homologue of human HC‐gp39, stimulates proliferation and regulates synthesis of extracellular matrix components of mammalian chondrocytes. J Biol Chem 2006;281: 29583–29596. [DOI] [PubMed] [Google Scholar]
- 6. Rehli M, Krause SW, Andreesen R. Molecular characterization of the gene for human cartilage gp‐39 (CHI3L1), a member of the chitinase protein family and marker for late stages of macrophage differentiation. Genomics 1997;43:221–225. [DOI] [PubMed] [Google Scholar]
- 7. Johansen JS, Stoltenberg M, Hansen M, et al. Serum YKL‐40 concentrations in patients with rheumatoid arthritis: Relation to disease activity. Rheumatology (Oxford) 1999;38:618–626. [DOI] [PubMed] [Google Scholar]
- 8. Volck B, Price PA, Johansen JS, et al. YKL‐40, a mammalian member of the chitinase family, is a matrix protein of specific granules in human neutrophils. Proc Assoc Am Physicians 1998;110:351–360. [PubMed] [Google Scholar]
- 9. Malinda KM, Ponce L, Kleinman HK, Shackelton LM, Millis AJ. Gp38k, a protein synthesized by vascular smooth muscle cells, stimulates directional migration of human umbilical vein endothelial cells. Exp Cell Res 1999;250:168–173. [DOI] [PubMed] [Google Scholar]
- 10. Johansen JS, Olee T, Price PA, Hashimoto S, Ochs RL, Lotz M. Regulation of YKL‐40 production by human articular chondrocytes. Arthritis Rheum 2001;44:826–837. [DOI] [PubMed] [Google Scholar]
- 11. Johansen JS, Jensen BV, Roslind A, Nielsen D, Price PA. Serum YKL‐40, a new prognostic biomarker in cancer patients? Cancer Epidemiol Biomarkers Prev 2006;15:194–202. [DOI] [PubMed] [Google Scholar]
- 12. Roslind A, Johansen JS. YKL‐40: a novel marker shared by chronic inflammation and oncogenic transformation. Methods Mol Biol 2009;511:159–184. [DOI] [PubMed] [Google Scholar]
- 13. Johansen JS. Studies on serum YKL‐40 as a biomarker in diseases with inflammation, tissue remodelling, fibroses and cancer. Dan Med Bull 2006;53:172–209. [PubMed] [Google Scholar]
- 14. Güngen G, Ardic F, Fιndıkoğlu G, Rota S. The effect of mud pack therapy on serum YKL‐40 and hsCRP levels in patients with knee osteoarthritis. Rheumatol Int 2011. in press. [DOI] [PubMed]
- 15. Syversen SW, Goll GL, van der Heijde D, et al. Cartilage and bone biomarkers in rheumatoid arthritis: Prediction of 10‐year radiographic progression. J Rheumatol 2009;36:266–272. [DOI] [PubMed] [Google Scholar]
- 16. Mizoguchi E, Mizoguchi A. Is the sugar always sweet in intestinal inflammation? Immunol Res 2007;37:47–60. [DOI] [PubMed] [Google Scholar]
- 17. Kastrup J, Johansen JS, Winkel P, et al. High serum YKL‐40 concentration in patients with stable coronary artery disease is associated with increased risk of myocardial infarction, cardiovascular death, and all‐cause mortality. Eur Heart J 2009;30:1066–1072. [DOI] [PubMed] [Google Scholar]
- 18. Letuve S, Kozhich A, Arouche N, et al. YKL‐40 is elevated in patients with chronic obstructive pulmonary disease and activates alveolar macrophages. J Immunol 2008;181:5167–5173. [DOI] [PubMed] [Google Scholar]
- 19. Nielsen AR, Erikstrup C, Johansen JS, et al. Plasma YKL‐40: A BMI‐independent marker of type 2 diabetes. Diabetes 2008;57:3078–3082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Johansen JS, Schultz NA, Jensen BV. Plasma YKL‐40: A potential new cancer biomarker? Rev Future Oncol 2009;5:1065–1082. [DOI] [PubMed] [Google Scholar]
- 21. Workowski KA, Berman SM. Sexually transmitted diseases treatment guidelines, 2006. MMWR Recomm Rep 2006;55(RR‐11):1–94. [PubMed] [Google Scholar]
- 22. Bojesen SE, Johansen JS, Børge G. Nordestgaard BG. Plasma YKL‐40 levels in healthy subjects from the general population. Clinica Chimica Acta 2011;412:709–712. [DOI] [PubMed] [Google Scholar]
- 23. Renkema GH, Boot RG, Strijland A, et al. Synthesis, sorting, and processing into distinct isoforms of human macrophage chitotriosidase. Eur J Biochem 1997;244:279–285. [DOI] [PubMed] [Google Scholar]
- 24. Coffman FD. Chitinase 3‐Like‐1 (CHI3L1): A putative disease marker at the interface of proteomics and glycomics. Crit Rev Clin Lab Sci 2008;45:531–562. [DOI] [PubMed] [Google Scholar]
- 25. Kzhyshkowska J, Gratchev A, Goerdt S. Human chitinases and chitinase‐like proteins as indicators for inflammation and cancer. Biomark Insights 2007;2:128–146. [PMC free article] [PubMed] [Google Scholar]
- 26. Malaguarnera L, Di Rosa M, Zambito AM, dell'Ombra N, Nicoletti F, Malaguarnera M. Chitotriosidase gene expression in Kupffer cells from patients with nonalcoholic fatty liver disease. Gut 2006;55:1313–1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kawada M, Hachiya Y, Arihiro A, Mizoguchi E. Role of mammalian chitinases in inlammatory conditions. Keio J Med 2007;56:21–27. [DOI] [PubMed] [Google Scholar]
- 28. Recklies AD, Ling H, White C, Bernier SM. Inflammatory cytokines induce production of CHI3L1 by articular chondrocytes. J Biol Chem 2005;280:41213–41221. [DOI] [PubMed] [Google Scholar]
- 29. Ling H, Recklies AD. The chitinase 3‐like protein human cartilage glycoprotein 39 inhibits cellular responses to the inflammatory cytokines interleukin‐1 and tumor necrosis factor‐α. Biochem J 2004;380:651–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Zivanovic S, Rackov LP, Vojvodic D, Vucetic C. Human cartilage glycoprotein 39—Biomarker of joint damage in knee osteoarthritis. Int Orthop 2009;33:1165–1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Koutroubakis IE, Petinaki E, Dimoulios P. Increased serum levels of YKL‐40 in patients with inflammatory bowel disease. Int J Colorectal Dis 2003;18:254–259. [DOI] [PubMed] [Google Scholar]
- 32. Conrozier T, Carlier MC, Mathieu P, et al. Serum levels of YKL‐40 and C reactive protein in patients with hip osteoarthritis and healthy subjects: A cross sectional study. Ann Rheum Dis 2000;59:828–831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hattori N, Oda S, Sadahiro T et al. YKL‐40 identifie by proteomic analysis as a biomarker of sepsis. Shock 2009;32:393–400. [DOI] [PubMed] [Google Scholar]
- 34. Živanović S, Rackov LP, Vojvodić D, Vučetić D. Human cartilage glycoprotein 39—Biomarker of joint damage in knee osteoarthritis. Int Orthop 2009;33:1165–1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kronborg G, Østergaard C, Weis N, et al. Serum level of YKL‐40 is elevated in patients with Streptococcus pneumonia bacteremia and is associated with the outcome of the disease. Scand J Infect Dis 2002;34,323–326. [DOI] [PubMed] [Google Scholar]


