Abstract
Background
The clinical usefulness of [−2]pro‐PSA (where PSA is prostate‐specific antigen) in prostate cancer diagnosis has been emphasized in recent studies. To determine proper blood sample handling conditions for [−2]pro‐PSA evaluation, we analyzed the preanalytical stability of [−2]pro‐PSA.
Methods
Blood samples from 22 Japanese males were stored under various conditions before total PSA (tPSA), free PSA, and [−2]pro‐PSA concentrations were measured, and the preanalytical stability of [−2]pro‐PSA and the changes in the Prostate Health Index (phi) were assessed.
Results
[−2]Pro‐PSA was stable in serum for at least 24 hr at both room temperature (RT) and at 4°C. However, [−2]pro‐PSA levels in whole blood increased rapidly over time, particularly at RT. Mean recovery (%) of [−2]pro‐PSA in whole blood at RT was >110% at 1 hr after drawing of blood. The phi tended to increase over time in a pattern similar to the change in[−2]pro‐PSA.
Conclusions
Preanalytical stability was lower for [−2]pro‐PSA than for free PSA or tPSA. Whole‐blood [−2]pro‐PSA increased in a time‐dependent manner, particularly at RT. Thus, whole blood samples collected at RT should be centrifuged within 1 hr after drawing. The [−2]pro‐PSA in serum is stable for at least 24 hr at both RT and at 4°C.
Keywords: [−2]pro‐PSA, prostate cancer, free PSA, Prostate Health Index, preanalytical stability
INTRODUCTION
Prostate‐specific antigen (PSA) is widely used as a serum marker for prostate cancer (PCa; 1, 2). However, its use is limited and it lacks cancer specificity, particularly when PSA < 10 ng/ml. Therefore, new markers that can accurately detect PCa are required. Recent studies have focused on the “fraction of free PSA” (fPSA), also known as “pro‐PSA,” for this purpose 3, 4, 5. Native pro‐PSA (also known as [−7]pro‐PSA) exists in a truncated form and contains a seven‐amino acid N‐terminal proleader peptide 6. [−7]Pro‐PSA is partially cleaved by human kallikrein family or other proteases, resulting in the generation of three truncated forms of pro‐PSA: [−5]pro‐PSA, [−4]pro‐PSA, and [−2]pro‐PSA. [−5]Pro‐PSA and [−4]pro‐PSA are further cleaved to the active form of PSA 7. However, [−2]pro‐PSA cannot be cleaved further. In addition, pro‐PSA is more often associated with peripheral zone cancer than transition zone hyperplasia 8. Thus, [−2]pro‐PSA may be a more cancer‐specific isoform of pro‐PSA. Several studies on the clinical utility of [−2]pro‐PSA for early PCa detection have been reported 3, 5, 9. For example, Catalona et al. analyzed serum specimens obtained from 1,091 men who underwent prostate biopsy. They concluded that percent pro‐PSA was superior to total PSA (tPSA) or percent free PSA within a PSA range of 2–10 ng/ml for predicting PCa 5. Moreover, percent [−2]pro‐PSA (defined as [−2]pro‐PSA/free PSA) showed the highest cancer specificity among all other variables in a PSA range of 2–4 ng/ml. Ito et al. reported that laboratory‐based indices containing [−2]pro‐PSA, particularly the Prostate Health Index (phi), in serum samples from Japanese males have greater specificity for PCa in comparison to percent free PSA 9.
As described above, [−2]pro‐PSA has high clinical potential to improve the quality of PCa screening, but it is important to determine the measurement accuracy and reproducibility of this strategy. Thus, it is important to evaluate preanalytical stability under various conditions. For example, free PSA is less stable than tPSA under storage conditions in both a time‐ and temperature‐dependent manner 10. Only one study from Europe investigated [−2]pro‐PSA stability; the results indicated that mean [−2]pro‐PSA increased with time in whole blood at room temperature (RT; 11). As the epidemiology of PCa and basic PSA behaviors show ethnic variations, evaluation of [−2]pro‐PSA stability in the Asian population is an interesting area of investigation. Therefore, the present study was performed to evaluate the short‐term [−2]pro‐PSA preanalytical stability in serum and whole blood from Japanese males to determine the parameters for optimal specimen handling.
MATERIALS AND METHODS
Specimen Collection and PSA Measurement
Twenty‐two Japanese patients who were referred to Nagasaki University Hospital from May to September 2011 due to elevation of tPSA were enrolled in this study. Blood samples were collected from each patient under an Internal Review Board‐approved protocol after receiving informed consent and in accordance with the practices and ethical standards of the Declaration of Helsinki and Nagasaki University. The basic characteristics of the 22 patients are shown in Table 1. Blood was drawn in the morning, and samples were divided into two groups. In group 1, after allowing for clotting for about 10 min, blood sample was then centrifuged, and part of the collected serum was frozen at −70°C within 15 min after centrifugation; this was used as a reference sample, because two freeze‐thaw cycles do not affect [−2]pro‐PSA stability 11. The remaining serum was aliquoted and stored at 21°C (RT) or 4°C for 1, 3, 8, or 24 hr before being frozen at −70°C. In group 2, blood samples were stored as whole blood at RT or at 4°C for 1, 3, 8, or 24 hr. The serum was then isolated and frozen at ‑70°C until PSA measurement. The tPSA, fPSA, and [−2]pro‐PSA measurements were performed simultaneously, including reference samples for baseline values, using an Access Immunoassay Analyzer (Beckman Coulter, Brea, CA). Each measurement result represents the mean of duplicate determinations. To evaluate preanalytical stability, data from each of the 22 patient samples at the different time points were transformed to percentage of recovery from the respective baseline value. The phi was defined as ([−2]pro‐PSA/fPSA)×(square root of tPSA; 7, 9).
Table 1.
Patient Characteristics
| Age (years) | |
| Median | 63.5 |
| Range | 52–75 |
| tPSA (ng/ml) | |
| Median | 5.40 |
| Range | 3.62–43.30 |
| fPSA (ng/ml) at baseline | |
| Median | 0.92 |
| Range | 0.23–5.89 |
| [−2]Pro‐PSA (pg/ml) at baseline | |
| Median | 12.06 |
| Range | 3.55–71.04 |
| phi at baseline | |
| Median | 26.13 |
| Range | 16.53–89.47 |
| Prostate volume (cc) | |
| Median | 34.3 |
| Range | 16.9–67.8 |
| Prostate biopsy | |
| Yes (cancer positive/negative) | 21 (7/14) |
| No | 1 |
phi, Prostate Health Index; PSA, prostate‐specific antigen.
Statistical Analysis
Data for the percentage recovery of [−2]pro‐PSA, fPSA, and phi are expressed as means ± SD. Comparisons between baseline and the values at each time point were conducted using ANOVA for repeated measurements. A value of P < 0.05 was considered to indicate statistical significance.
RESULTS
The time course of [−2]pro‐PSA percentage recovery is summarized in Table 2. The [−2]pro‐PSA preanalytical stability in serum was favorable at time points within 24 hr regardless of temperature (RT or 4°C). The actual recovery percentage increased slightly over time (Fig. 1A). In contrast, the percentage recovery of [−2]pro‐PSA in whole blood increased significantly, particularly at RT. As shown in Figure 1B, the mean recovery percentage at 1 hr was already >110% and increased continuously with time thereafter. The changes in percentage recovery of [−2]pro‐PSA in whole blood at 4°C showed a trend similar to that at RT; however, the increases in percentage recovery were much smaller than those at RT. We also evaluated the stabilities of tPSA and fPSA under the same specimen handling conditions. Changes in mean recovery percent of fPSA are shown in Table 2. Both tPSA (data not shown) and fPSA were almost stable for at least 24 hr, and the percentage recovery of fPSA decreased gradually with time. This tendency of fPSA to decrease was consistent with patterns described in previous reports 12, 13.
Table 2.
Percentage Recovery of [−2]Pro‐PSA, Free PSA, and the Prostate Health Index (phi) at Various Time Points and Temperatures
| Hours | In serum at RT | In serum at 4°C | In whole blood at RT | In whole blood at 4°C |
|---|---|---|---|---|
| Mean ± SD [−2]pro‐PSA recovery % | ||||
| Baseline (0 hr) | 100 | 100 | 100 | 100 |
| 1 hr | 101.7 ± 6.2 | 104.3 ± 5.6 | 111.0 ± 9.3a | 103.6 ± 9.1 |
| 3 hr | 104.7 ± 8.9 | 104.9 ± 9.1 | 123.1 ± 14.1a | 106.5 ± 7.4 |
| 8 hr | 104.8 ± 7.0 | 103.9 ± 8.7 | 142.3 ± 24.9a | 114.1 ± 11.0a |
| 24 hr | 104.5 ± 8.9 | 103.9 ± 6.7 | 244.7 ± 135.3a | 116.3 ± 20.9a |
| Mean ± SD free PSA recovery % | ||||
| Baseline (0 hr) | 100 | 100 | 100 | 100 |
| 1 hr | 96.9 ± 5.0 | 97.3 ± 5.9 | 97.8 ± 5.3 | 98.1 ± 5.5 |
| 3 hr | 94.9 ± 4.5 | 97.1 ± 4.6 | 96.5 ± 3.8 | 99.1 ± 5.0 |
| 8 hr | 93.2 ± 4.3a | 95.7 ± 3.9 | 93.5 ± 4.5a | 98.4 ± 4.3 |
| 24 hr | 91.9 ± 6.5a | 92.5 ± 5.3a | 91.9 ± 4.8a | 93.9 ± 5.5a |
| Mean ± SD phi recovery % | ||||
| Baseline (0 hr) | 100 | 100 | 100 | 100 |
| 1 hr | 105.1 ± 6.9 | 107.0 ± 6.7 | 113.3 ± 8.3a | 104.0 ± 9.0 |
| 3 hr | 110.2 ± 8.5a | 108.4 ± 7.4 | 127.2 ± 16.8a | 107.9 ± 8.7 |
| 8 hr | 111.7 ± 8.7a | 107.3 ± 8.1 | 151.9 ± 27.9a | 114.0 ± 10.8a |
| 24 hr | 113.3 ± 8.6a | 112.2 ± 8.0a | 265.3 ± 144.5a | 120.2 ± 19.0a |
PSA, prostate‐specific antigen; RT, room temperature.
P < 0.05 compared with baseline.
Figure 1.
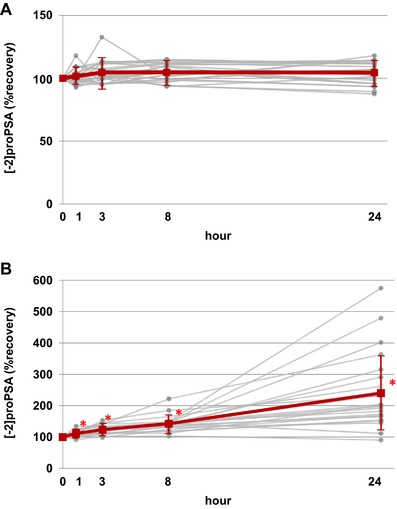
Time course of percentage recovery of [−2]pro‐PSA (where PSA is prostate‐specific antigen) in serum (A) and whole blood (B) at room temperature. Gray lines indicate individual values, and the red line indicates mean ± SD. *P < 0.05 compared with baseline value.
The phi value, which is used in an early PCa detection mathematical algorithm in Europe, was also evaluated. As shown in Table 2 and Figure 2, the changes in mean phi were similar to those in [−2]pro‐PSA under each handling condition. The percentage change in phi in whole blood at RT increased rapidly over time. In contrast, the percentage change of phi in serum was relatively stable for 24 hr after drawing blood. The increase in the phi value in serum reached almost +10% by 3 hr after drawing of blood but was stable until 24 hr.
Figure 2.
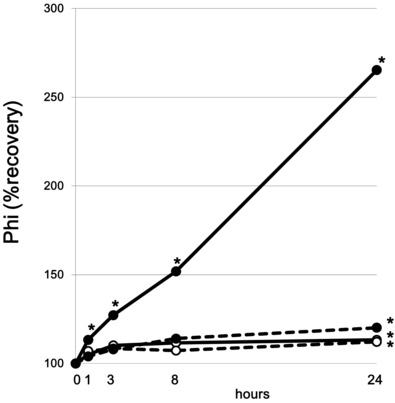
Changes in Prostate Health Index (phi) over time in serum at 4°C (‐‐‐○‐‐‐) and at room temperature (—○—), and in whole blood at 4°C (‐‐‐●‐‐‐) and at room temperature (—●—). *P < 0.05 compared with baseline value.
DISCUSSION
Preanalytical and analytical stability of a tumor marker is essential for accurate cancer diagnosis 14. PSA is a reliable and useful serum marker for PCa, but its clinical utility is limited by its moderate cancer specificity. Free PSA is usually used as a supplemental serum marker, which may improve the specificity of tPSA during the PCa detection process 15, 16, 17. Among the different forms of free PSA, [−2]pro‐PSA is a cancer‐specific pro‐PSA that can serve as a new screening marker, particularly in the PSA range of 2–10 ng/ml. In this study, the [−2]pro‐PSA preanalytical stability in serum was acceptable for at least 24 hr after drawing of blood in Japanese males, regardless of sample temperature (4°C or RT). Moreover, unlike fPSA, changes in percentage recovery of [−2]pro‐PSA in whole blood increased significantly with time and temperature. This same trend in [−2]pro‐PSA preanalytical stability was reported by Semjonow et al. in Caucasian males 11. Thus, this indicates that ethnic differences do not affect the preanalytical stability of [−2]pro‐PSA. To our knowledge, this is the first report regarding [−2]pro‐PSA preanalytical stability in Asian males.
Regarding [−2]pro‐PSA increase in whole blood at RT, Semjonow suggested that the proteolytic activity of the blood, possibly mediated by human kallikrein‐related or other endopeptidases, resulted in an increase in the [−2]pro‐PSA value. Human kallikrein‐related peptidases (hKs) consist of a single family of 15 highly conserved trypsin‐ or chymotrypsin‐like serine proteases 18, 19. hK3 or PSA is the most widely known hK due to its clinical application. PSA and other hKs are synthesized as inactive pre–pro forms that are proteolytically processed to secreted inactive pro‐forms. Then, pro‐hKs are subsequently activated to mature forms by autocatalytic activity or by another hK or other endopeptidase. It has been demonstrated that hK2, hK4, hK5, and hK15 can cleave pro‐PSA to the active form of PSA 19, 20, 21. Following activation, mature hK enzymes are inactivated by endogenous inhibitors, such as α2‐macrogloblin, antichymotrypsin, or kallistatin. Interestingly, after internal cleavage and activation of pro‐PSA by hK5, hK5 also deactivates this cleavage process upon prolonged incubation 22. These findings highlight the complexity of hK regulatory mechanisms, particularly with regard to pro‐PSA in the human prostate or human serum. Specific enzymes serially cleave [−7]pro‐PSA to the active form of PSA or to other truncated forms of pro‐PSA, such as [−5]pro‐PSA, [−4]pro‐PSA, or [−2]pro‐PSA. As hK2 and trypsin are unable to activate [−2]pro‐PSA further, [−2]pro‐PSA is the most stable form of pro‐PSA in prostate tissue and serum 19. Thus, the [−2]pro‐PSA molecule accumulates, and levels of [−2]pro‐PSA eventually increase in whole blood. However, the difference in proteolytic activity (i.e., the hKs/endopeptidase content) between serum and whole blood is unknown. To ensure optimal clinical application of [−2]pro‐PSA, the precise mechanism of [−2]pro‐PSA alteration in blood specimens must be elucidated. The present study did not assess preanalytical stability at time points beyond 24 hr because clinical blood samples are nearly always processed within 24 hr after being drawn.
We also characterized changes in phi. It has been suggested that higher phi values are associated with an increased probability of PCa detection 7, 9. In fact, phi and percent [−2]pro‐PSA were the strongest predictors of a positive prostate biopsy when compared with tPSA, fPSA, and percent free PSA. In the present study, changes in phi were similar to those of [−2]pro‐PSA. Based on the results of [−2]pro‐PSA or fPSA preanalytical stability, and on the phi calculation formula, the calculated phi results were most affected by the [−2]pro‐PSA or fPSA values. As [−2]pro‐PSA increased and fPSA decreased over time in the sample after the drawing of blood, the tendency of phi to increase increases with time. Thus, the phi was affected by blood sample handling conditions over the short term after drawing of blood. As the increase of phi in the serum specimen was almost constant, around +10%, until 24 hr after drawing blood, this change in phi in the serum specimen may be acceptable for clinical use. However, it should be noted that the results of phi in whole blood, particularly at RT, increased markedly with time. This may considerably affect clinical decision‐making for PCa diagnosis. Blood sample handling conditions should be taken into consideration when assessing [−2]pro‐PSA or phi. This may be the first report of changes of phi in clinical blood specimens under various handling conditions.
CONCLUSIONS
In summary, [−2]pro‐PSA is relatively unstable in comparison to tPSA or fPSA. Whole‐blood samples collected at RT should be centrifuged and separated into serum at least within 1 hr after drawing of blood to obtain accurate [−2]pro‐PSA and phi results. Whole‐blood samples can be kept at 4°C for up to 3 hr until serum separation. Once the serum is separated, the [−2]pro‐PSA value is stable for at least 24 hr. Moreover, as phi values tend to increase over time after drawing of blood, sample handling conditions should be taken into consideration when evaluating phi for PCa diagnosis.
ACKNOWLEDGMENTS
The authors thank Atsuko Yoneda for her excellent technical assistance and all members of the p2PSA focus group, particularly Yoshiyuki Kakehi, Mikio Sugimoto (Kagawa University), Koichiro Akakura (Tokyo Kosei‐Nenkin Hospital), and Akira Yokomizo (Kyushu University) for their helpful comments and Kiyohide Ishikura (Beckman Coulter) for his technical support.
The authors declare no relevant financial interests.
REFERENCES
- 1. Catalona WJ, Smith DS, Ratliff TL, et al. Measurement of prostate‐specific antigen in serum as a screening test for prostate cancer. N Engl J Med 1991;324:1156–1161. [DOI] [PubMed] [Google Scholar]
- 2. Catalona WJ, Richie JP, Ahmann FR, et al. Comparison of digital rectal examination and serum prostate specific antigen in the early detection of prostate cancer: Results of a multicenter clinical trial of 6,630 men. J Urol 1994;151:1283–1290. [DOI] [PubMed] [Google Scholar]
- 3. Sokoll LJ, Chan DW, Mikolajczyk SD, et al. Proenzyme PSA for the early detection of prostate cancer in the 2.5–4.0 ng/ml total PSA range: Preliminary analysis. Urology 2003;61:274–276. [DOI] [PubMed] [Google Scholar]
- 4. Khan MA, Partin AW, Rittenhouse HG, et al. Evaluation of proprostate specific antigen for early detection of prostate cancer in men with a total prostate specific antigen range of 4.0 to 10.0 ng/ml. J Urol 2003;170:723–726. [DOI] [PubMed] [Google Scholar]
- 5. Catalona WJ, Bartsch G, Rittenhouse HG, et al. Serum pro prostate specific antigen improves cancer detection compared to free and complexed prostate specific antigen in men with prostate specific antigen 2 to 4 ng/ml. J Urol 2003;170:2181–2185. [DOI] [PubMed] [Google Scholar]
- 6. Kumar A, Mikolajczyk SD, Goel AS, et al. Expression of pro form of prostate‐specific antigen by mammalian cells and its conversion to mature, active form by human kallikrein 2. Cancer Res 1997;57:3111–3114. [PubMed] [Google Scholar]
- 7. Hori S, Blanchet JS, McLoughlin J. From prostate‐specific antigen (PSA) to precursor PSA (proPSA) isoforms: A review of the emerging role of proPSAs in the detection and management of early prostate cancer. BJU Int 2012. DOI: 10.1111/j.1464-410X.2012.11329.x [DOI] [PubMed] [Google Scholar]
- 8. Chan TY, Mikolajczyk SD, Lecksell K, et al. Immunohistochemical staining of prostate cancer with monoclonal antibodies to the precursor of prostate‐specific antigen. Urology 2003;62:177–181. [DOI] [PubMed] [Google Scholar]
- 9. Ito K, Miyakubo M, Sekine Y, et al. Diagnostic significance of [−2]pro‐PSA and prostate dimension‐adjusted PSA‐related indices in men with total PSA in the 2.0–10.0 ng/mL range. World J Urol 2013;31:305–311. [DOI] [PubMed] [Google Scholar]
- 10. Woodrum D, French C, Shamel LB. Stability of free prostate‐specific antigen in serum samples under a variety of sample collection and sample storage conditions. Urology 1996;48:33–39. [DOI] [PubMed] [Google Scholar]
- 11. Semjonow A, Köpke T, Eltze E, et al. Pre‐analytical in‐vitro stability of [−2]proPSA in blood and serum. Clin Biochem 2010;43:926–928. [DOI] [PubMed] [Google Scholar]
- 12. Sokoll LJ, Bruzek DJ, Dua R, et al. Short‐term stability of the molecular forms of prostate‐specific antigen and effect on percent complexed prostate‐specific antigen and percent free prostate‐specific antigen. Urology 2002;60:24–30. [DOI] [PubMed] [Google Scholar]
- 13. Ulmert D, Becker C, Nilsson JA, et al. Reproducibility and accuracy of measurements of free and total prostate‐specific antigen in serum vs plasma after long‐term storage at −20 degrees C. Clin Chem 2006;52:235–239. [DOI] [PubMed] [Google Scholar]
- 14. Sweep FC, Fritsche HA, Gion M, et al. Considerations on development, validation, application, and quality control of immuno(metric) biomarker assays in clinical cancer research: An EORTC‐NCI working group report. Int J Oncol 2003;23:1715–1726. [PubMed] [Google Scholar]
- 15. Catalona WJ, Smith DS, Wolfert RL, et al. Evaluation of percentage of free serum prostate‐specific antigen to improve specificity of prostate cancer screening. J Am Med Assoc 1995;274:1214–1220. [PubMed] [Google Scholar]
- 16. Prestigiacomo AF, Lilja H, Pettersson K, et al. A comparison of the free fraction of serum prostate specific antigen in men with benign and cancerous prostates: The best case scenario. J Urol 1996;156:350–354. [DOI] [PubMed] [Google Scholar]
- 17. Partin AW, Catalona WJ, Southwick PC, et al. Analysis of percent free prostate‐specific antigen (PSA) for prostate cancer detection: Influence of total PSA, prostate volume, and age. Urology 1996;48:55–61. [DOI] [PubMed] [Google Scholar]
- 18. Lundwall A, Band V, Blaber M, et al. A comprehensive nomenclature for serine proteases with homology to tissue kallikreins. Biol Chem 2006;387:637–641. [DOI] [PubMed] [Google Scholar]
- 19. Sotiropoulou G, Pampalakis G, Diamandis EP. Functional roles of human kallikrein‐related peptidases. J Biol Chem 2009;284:32989–32994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Borgo CA, Diamandis EP. The emerging roles of human tissue kallikreins in cancer. Nat Rev Cancer 2004;4:876–890. [DOI] [PubMed] [Google Scholar]
- 21. Yoon H, Blaber SI, Debela M, et al. A completed KLK activome profile: Investigation of activation profiles of KLK9, 10, and 15. Biol Chem 2009;390:373–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Michael IP, Pampalakis G, Mikolajczyk SD, et al. Human tissue kallikrein 5 is a member of a proteolytic cascade pathway involved in seminal clot liquefaction and potentially in prostate cancer progression. J Biol Chem 2006;281:12743–12750. [DOI] [PubMed] [Google Scholar]


