Abstract
Background
Myoglobin, creatine kinase‐MB isoenzyme (CK‐MB), and cardiac troponin I (cTnI) are cardiac biomarkers that are widely used to assist in the early and late diagnoses of acute myocardial infarction (AMI). Here, we present a clinically applicable fluorescence (FL) immunoassay for cardiac biomarkers.
Methods
Whole blood was mixed with FL‐labeled detector Ab (antibody) and then loaded onto a capture Ab‐immobilized strip in a test cartridge. The FL intensities at test and control line on the strip were obtained and converted in a laser FL scanner to determine the concentration of biomarker. The analytical performance of immunoassay system was evaluated by linearity and imprecision tests. The comparability of the FL immunoassay method was examined with a reference method.
Results
FL intensities and the levels of myoglobin, CK‐MB, and cTnI displayed good linearity and high correlations (r = 0.999, 0.998, and 0.989, respectively). The coefficient of variations (CVs) of imprecision for all cardiac biomarkers were less than 8% in both intra‐ and interassays. When the results from the developed method and bioMerieux VIDAS assay were analyzed by Bland–Altman plot and Passing–Bablok plot, the two assay methods were in good agreement.
Conclusion
The FL immunoassay system can provide a platform for point‐of‐care testing (POCT), and it is an easy, fast, and reliable method for the quantification of cardiac biomarkers.
Keywords: AMI, CK‐MB, cTnI, fluorescence immunochromatographic assay, myoglobin, POCT
Abbreviations
- AMI
acute myocardial infarction
- CK‐MB
creatine kinase‐MB isoenzyme
- cTnI
cardiac troponin I
- CVs
coefficient of variations
- CVD
cardiovascular diseases
- FL‐ICA
fluorescence immunochromatographic assay
- LoD
limit of detection
- mAb
monoclonal antibody
- POCT
point‐of‐care testing
INTRODUCTION
As reported in a consensus document of the European Society of Cardiology (ESC) and the American College of Cardiology (ACC), the criteria for the diagnosis of acute myocardial infarction (AMI) have been recently redefined 1, 2. At least two of the three following characteristics must be met to properly diagnose an AMI: (a) typical symptoms; (b) characteristic rise‐and‐fall pattern of a cardiac panel marker (myoglobin, creatine kinase‐MB isoenzyme [CK‐MB], cardiac troponin I [cTnI]); (c) a typical electrocardiogram pattern involving the development of Q waves. If the typical symptoms are observed, electrocardiographic testing is highly specific for AMI. However, even in these cases, initial examinations show only about 50% sensitivity for AMI 3. These limitations usually result in the majority of patients admitted to the hospital undergoing biochemical testing for cardiac markers to confirm or rule out AMI. Therefore, cardiac marker testing has become very important in the evaluation of patients for the diagnosis and screening of AMI and heart failure, as well as the prognosis of cardiovascular diseases (CVD) 4, 5. Cardiac markers are frequently used not only in central laboratories, but also in emergency departments and outpatient clinics to provide rapid diagnostic reports.
Myoglobin is an early marker in the diagnosis of AMI that can be detected 1–2 hr after symptom onset and remains elevated for up to 24 hr. However, myoglobin has significant limitations, including low specificity for AMI 6, and thus it is usually used in combination with CK‐MB or cTnI. CK‐MB is an 86 kDa cytosolic enzyme that is predominantly located in myocardium and is released into the circulation in the setting of AMI. Typically, CK‐MB becomes elevated in the circulation 3–6 hr after symptom onset in AMI and remains elevated for 24–36 hr. The specificity of CK‐MB for diagnosing AMI is limited by the fact that it may be elevated as a result of acute or chronic muscle damage or during the clearance of abnormalities in renal failure and hypothyroidism 7. Nonetheless, CK‐MB is the one of the cardiac biomarkers frequently used in the diagnosis of AMI. The N‐terminal amino acid sequence of cTnI has specific residues that are not present in the two isotypes of TnI in skeletal muscle; thus, antibodies against these specific residues are used for immunoassays in the evaluation of AMI patients 8. The concentration of cTnI in blood becomes elevated between 4 and 8 hr following an AMI, peaks between 12 and 16 hr, and remains elevated for 5 and 9 days following damage to the myocardium 9. The analytical specificity and the increased duration of the elevation make cTnI an important marker in the diagnosis and evaluation of patients suspected of having an AMI 10.
Combined measurements of myoglobin, CK‐MB, and cTnI have been proposed to greatly help physicians in the diagnosis and management of AMI patients 11, 12. Hospitals and outpatient clinics are now implementing point‐of‐care testing (POCT) devices that allow for the use of diagnostic assays at the site of patient care delivery, thus, facilitating faster decision‐making and rapid treatment. In this study, we introduce an assay platform of POCT for the quantification of myoglobin, CK‐MB, and cTnI in samples, which has reliable analytical performance and allows for a substantial reduction in turnaround time. The developed immunoassay system uses simple lateral‐flow immunochromatography as a separation system and adopts fluorophore dye as a tracer to detect reaction signals for the levels of cardiac markers in the samples 13. The analytic performance of the fluorescence (FL) immunochromatographic assay (FL‐ICA) system was evaluated by various parameters, including imprecision testing, and its comparability was analyzed with a reference immunoassay.
MATERIALS AND METHODS
Materials
Streptavidin and sodium bicarbonate were purchased from Sigma (St. Louis, MO), and human cTnI, CK‐MB, and myoglobin were purchased from Fitzgerald Industries International, Inc. (North Acton, MA). Liquicheck™ cardiac markers were obtained from Bio‐Rad Laboratories, Inc. (Irvine, CA). Sephadex G25 and activated Alexa Fluor 647 were obtained from Amersham Pharmacia Biotech (Piscataway, NJ) and Molecular Probes (Eugene, OR), respectively. Nitrocellulose membrane was obtained from Millipore (Watertown, MA) and the sample pads and absorption pads were obtained from Schleicher and Schuell (Keene, NH).
Labeling of Detector Antibodies
For conjugation of the anti‐cTnI (myoglobin or CK‐MB) monoclonal antibody (mAb) with a FL dye, 100 μl of detector anti‐cTnI mAb (1 mg/l) was mixed with 10 μl of sodium bicarbonate buffer (1 mol/l, pH 8.3) in phosphate‐buffered saline (PBS) and followed by the addition of 1 μl of activated Alexa Fluor 647 (10 g/l). After overnight incubation at 4°C, the mixture was applied onto a Sephadex G25 column to remove the free dye, and FL‐labeled mAb conjugates were collected as elutes after centrifuging the column at 2,500 rpm for 2 min. The biotin‐bovine serum albumin (BSA) complex was similarly conjugated with Alexa Fluor 647, and FL‐labeled biotin–BSA conjugates were purified with the same processes as noted above and were used as an internal control for the assay system. The FL‐labeled mAb and the FL‐labeled biotin–BSA were mixed together with the assay buffer to form the detection buffer and were stored at 4°C until use.
Immunoassay Strip and Cartridge
The FL‐ICA test strip was fabricated in‐house to fit into a disposable cartridge and a laser FL scanner. The sample pad and the absorption pad were cut to a size of 4 × 20 mm and assembled with a mAb‐ and streptavidin‐coated nitrocellulose membrane onto a polystyrene backing card. The capture mAb and streptavidin were dispensed as 1‐mm‐wide lines at the test line and the control line, respectively, using a BioJet dispenser (BioDot, Irvine, CA). The assembled strip was kept in a dry vacuum chamber overnight before being placed into a cartridge (15 × 90 mm), which was designed to fit the holder of the laser FL scanner. The cartridge was then sealed in a foil pouch containing a desiccant and stored at room temperature. Because the appearance of the test cartridge was unique, a laser FL scanner called i‐CHROMA™ (Boditech Med, South Korea) was used to measure the distribution of FL intensity along the strip of cartridge.
Laser FL Scanner
i‐CHROMA™ was constructed to quantify the distribution of FL intensity along the strip. A semiconductor laser WLM‐6305NH from Lanics (Seoul, South Korea) was the illumination source. A focusing lens D640/20× (Chroma Tech, Bellows Falls, VT) was used to move the laser through an excitation filter in order to achieve the best focus on the sample strip. Since the strip was opaque, most of the FL light as well as the diffusely scattered laser light were directed toward the ellipsoidal mirror. The mirror was positioned in such a way that the focus of the laser on the strip was poised on one of two foci of the mirror so that any beam emanating from that focal point would eventually hit the other focal point. FL energy that passed through the emission filter was detected by a photomultiplier tube, H5784–01 (Hamamatsu Photonics, Hamamatsu, Japan), and was converted to a digital‐readout signal. The on‐board computer controlled each operation step and measured the signals via preprogrammed instructions to generate statistically analyzed results. The system components of the laser FL scanner have been previously described in detail 14.
Assay Procedure
The detection buffer was a mixture of FL‐labeled mAb (detector Abs) and FL‐labeled biotin–BSA (internal control) in PBS. For measurement of myoglobin concentration, 10 μl of whole blood (plasma and serum) was added to 150 μl of detection buffer, and 75 μl of the mixture was then loaded onto the sample well of the cartridge. For measurement of CK‐MB and cTnI, 75 μl of whole blood was mixed with 150 μl and 75 μl of detection buffer, respectively, and then 75 μl of the mixture was loaded onto the sample well of the cartridge. After 12 min of incubation for immune reactions, the cartridge was inserted into the laser FL scanner for the detection of FL intensity. The scanner converted FL intensity to numeric data, calculated the relative amount, and displayed the level of cardiac biomarker in the sample as microgram per liter on the screen.
Blood Samples
Blood samples were obtained from patients and healthy individuals who visited at the Hallym University Medical Center in Chuncheon, Korea. Informed consent was obtained from volunteers before their participation in the study. All the female and male participants were between the ages 35 and 75. Venous blood was collected in 5 ml vacuum tubes (Becton‐Dickinson, Franklin Lakes, NJ) containing sodium/lithium heparin. The blood samples were generally tested within 30 min of collection. Otherwise, serum samples were aliquoted in small volumes and frozen at −70°C.
Method of Comparison and Statistics
The concentrations of myoglobin/CK‐MB/cTnI in the blood samples from the same individuals were measured in a side‐by‐side assay using i‐CHROMA™ and bioMerieux VIDAS analyzer. The test results were analyzed and compared with Medcalc version 7.6 (Mariaekerke, Belgium) and Microsoft Excel 2010 software (Redmond, WA) for the Pearson correlation coefficients (r), Bland–Altman difference plot, and Passing–Bablok regression analysis. P values < 0.05 were considered significant.
RESULTS
Characterization of the FL‐ICA System for Cardiac Markers
The developed FL‐ICA system was tested over a wide range of myoglobin (0–500 μg/l), CK‐MB (0–100 μg/l), and cTnI (0–50 μg/l) concentrations. When the relative FL units (RFUs) of the test and the control line were measured from the scanned test cartridge, the RFUs of the test lines gradually increased as the concentrations of cTnI increased, as shown in Figure 1. In contrast, the RFUs of the control lines remained constant at different concentrations of cTnI, indicating that the interaction between FL‐conjugated biotin–BSA and streptavidin was independent of the cTnI concentration in the samples and thus functioned as a good internal standard. Similar results were obtained when the tests were carried out with the FL‐ICA system for myoglobin and CK‐MB (data not shown).
Figure 1.
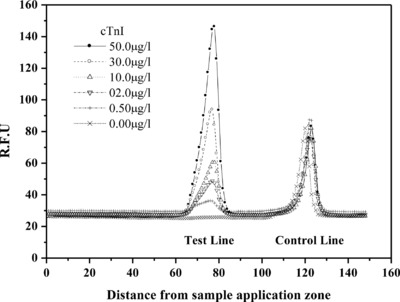
The scanning profiles of the relative FL units (RFUs) in the FL‐ICA system for cTnI. The RFUs at the test and the control lines are plotted on the Y‐axis. An arbitrary distance from the sample well of the cartridge is plotted on the X‐axis to generate two peaks to measure test and control concentrations. The RFUs of the test lines increase as concentrations of cTnI in the samples increase. In contrast, the RFUs of the control lines remain the same at different concentrations of cTnI, indicating that FL‐labeled biotin–BSA and streptavidin in the assay system work properly as an internal control.
For the calibration curve, the RFUs displayed at the test and the control lines were converted into area values (test: A T, control: A C), and then the area ratios (A T /A C) were plotted against the concentrations of cardiac marker. As shown in Figure 2, the correlation coefficient (r) between the area ratio value and the myoglobin, CK‐MB, and cTnI concentrations were 0.999, 0.998, and 0.989, respectively, and good linearity was displayed throughout the entire range of each tested cardiac marker. The coefficient of variation (CV) was less than 7% for ten independent experiments at each concentration of myoglobin and CK‐MB and less than 8% in the concentrations of cTnI. The limit of detection (LoD) of the assay system, calculated as the mean value plus three SD of a zero calibrator, was 1.0 μg/l for myoglobin, 1.0 μg/l for CK‐MB, and 0.01 μg/l for cTnI.
Figure 2.

The calibration curve obtained from the area ratio (A T/A C) against the concentration of cardiac biomarkers, demonstrating the linear coefficient correlation (r) between the area ratio (A T/A C) and cardiac biomarker concentration and the CVs of the area ratio at various cardiac biomarker concentrations. The spiked points for the calibration curves (▪) and for the CVs (○) were obtained from the mean values of ten independent experiments at each cardiac biomarker concentration. The CV was less than 7% at each concentration of myoglobin and CK‐MB and less than 8% for cTnI.
Imprecision Tests of the FL‐ICA System for Cardiac Markers
We evaluated the imprecision of the intra‐assay (within a day) and interassay (between days) variations to determine the reproducibility of the FL‐ICA system for cardiac panel biomarkers (Table 1). To measure the variation of the intra‐assays, 20 replicate tests were performed with known concentrations of myoglobin, CK‐MB, and cTnI. For the variation of the inter‐assays, the same samples were measured on ten sequential days, with two runs per day and 20 replicates at each concentration. Five control samples for each cardiac marker, covering almost the entire dynamic working range, were prepared for the intra‐ and interassay imprecision tests. The CVs of imprecision for myoglobin and CK‐MB were less than 7% and 8%, respectively, in both the intra‐ and interassays at each tested concentration. The intra‐ and interassay CVs for cTnI in the FL‐ICA system were, respectively, 6.36% and 7.64% at 0.53 μg/l; 6.33% and 7.48% at 1.50 μg/l; 6.16% and 6.70% at 3.57 μg/l; 4.26% and 4.62% at 11.50 μg/l; and 4.00% and 4.09% at 45.00 μg/l.
Table 1.
Imprecision Tests of FL‐ICA System for Cardiac Biomarkers
| Intra‐assay | Interassay | |||||
|---|---|---|---|---|---|---|
| Sample (μg/l) | Meana | SD | CV% | Meana | SD | CV% |
| A. Myoglobin | ||||||
| 10.00 | 9.74 | 0.66 | 6.80 | 9.75 | 0.67 | 6.93 |
| 50.00 | 49.64 | 2.60 | 5.24 | 49.84 | 2.82 | 5.65 |
| 100.00 | 102.04 | 5.44 | 5.33 | 101.76 | 5.81 | 5.71 |
| 190.00 | 192.40 | 8.08 | 4.19 | 193.41 | 10.40 | 5.37 |
| 325.00 | 323.52 | 12.25 | 3.79 | 325.98 | 14.34 | 4.39 |
| B. CK‐MB | ||||||
| 3.00 | 3.37 | 0.25 | 7.32 | 3.61 | 0.28 | 7.72 |
| 10.00 | 10.15 | 0.58 | 5.71 | 10.28 | 0.62 | 6.03 |
| 25.00 | 25.19 | 1.35 | 5.37 | 25.37 | 1.50 | 6.01 |
| 50.00 | 50.57 | 2.80 | 5.54 | 50.87 | 2.91 | 5.72 |
| 90.00 | 90.15 | 4.25 | 4.71 | 90.05 | 4.99 | 5.54 |
| C. cTnI | ||||||
| 0.53 | 0.54 | 0.03 | 6.36 | 0.51 | 0.04 | 7.64 |
| 1.50 | 1.51 | 0.10 | 6.33 | 1.54 | 0.11 | 7.48 |
| 3.57 | 3.57 | 0.22 | 6.16 | 3.58 | 0.24 | 6.70 |
| 11.50 | 11.51 | 0.49 | 4.26 | 11.48 | 0.53 | 4.62 |
| 45.00 | 45.09 | 1.78 | 4.00 | 45.39 | 1.85 | 4.09 |
Mean value of 20 replicates.
Comparability of the FL‐ICA System With a Reference Method
The cardiac marker concentrations of 100 samples were measured side‐by‐side with the i‐CHROMA™ FL‐ICA system and the bioMerieux VIDAS analyzer as a reference method. Analysis of the test results showed that the concentrations of the biomarkers were in excellent agreement with significant correlations between the two assay systems. Pearson correlation of coefficients (r) was 0.989 for myoglobin, 0.984 for CK‐MB, and 0.990 for cTnI (data not shown). Bland–Altman difference plots also revealed a good agreement between the two different assay methods, as shown in Figure 3. The maximum difference results between the i‐CHROMA™ FL‐ICA system and the bioMerieux VIDAS assay varied from 34.3 to −41.4 μg/l with a mean difference of −3.5 μg/l for myoglobin, 14.8 to −5.5 μg/l with a mean difference of 4.7 μg/l for CK‐MB, and 4.0 to −3.1 μg/l with a mean difference of 0.5 μg/l for cTnI. The Passing–Bablok regression analysis for myoglobin, CK‐MB, and cTnI yielded, respectively, a slope of 0.91 (95% confidence interval, 0.894–0.936) and a y‐intercept of 7.12 μg/l (95% confidence interval, 6.164–7.945), a slope of 0.97 (95% confidence interval, 0.934–1.008) and a y‐intercept of 5.97 μg/l (95% confidence interval, 5.053–8.041), and a slope of 1.04 (95% confidence interval, 1.005–1.051) and a y‐intercept of 0.14 μg/l (95% confidence interval, −0.040 to 0.227; Table 2).
Figure 3.
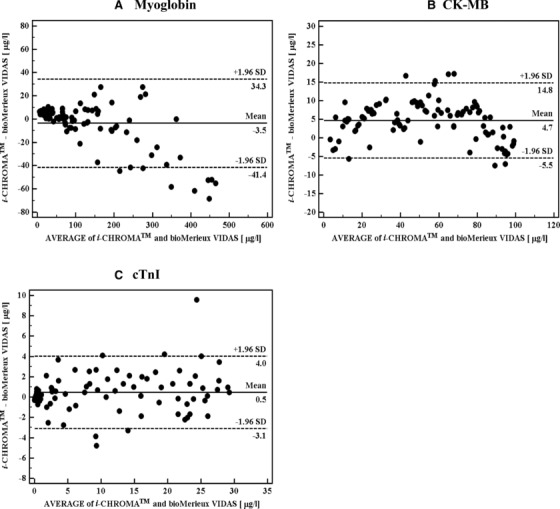
Bland–Altman difference plot comparing cardiac biomarker concentrations obtained from the FL‐ICA system versus the VIDAS assay. The solid line represents the mean difference in measured cardiac biomarker concentration between the methods, and the dashed lines are ±1.96 SD.
Table 2.
Passing–Bablok Regression Analysis
| Slope | Intercept (μg/l) | |||
|---|---|---|---|---|
| (95% CIa) | (95% CIa) | P | ||
| i‐CHROMA™ (Y) versus bioMerieux VIDAS (X) | Myoglobin | 0.9143 | 7.1229 | 0.070 |
| (+0.8974 ∼ 0.9363) | (+6.1639 ∼ 7.9452) | |||
| CK‐MB | 0.9707 | 5.9647 | 0.001 | |
| (+0.9336 ∼ 1.0078) | (+5.0527 ∼ 8.0406) | |||
| cTnI | 1.04 | 0.1442 | 0.014 | |
| (+1.0054 ∼ 1.0505) | (−0.0404 ∼ +0.2273) |
95% confidence interval.
Comparison of Cardiac Marker Concentration With Whole Blood and Serum in the FL‐ICA System
To determine the correlation of the cardiac marker concentration between whole blood and serum from the same individual in the FL‐ICA system, whole blood and serum samples were collected from 50 people, including patients who had been hospitalized due to CVD. As shown in Figure 4, the correlation of coefficient (r) obtained between these two samples was 0.999 for myoglobin, 0.994 for CK‐MB, and 0.989 for cTnI. The slope of the calculated equations was 0.954 for myoglobin, 0.999 for CK‐MB, and 1.009 for cTnI.
Figure 4.
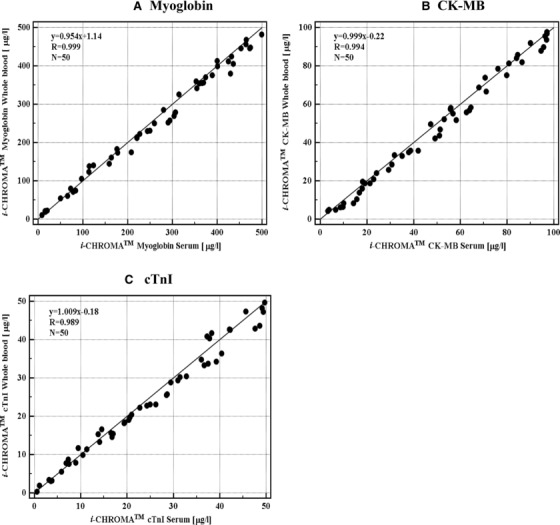
Comparison of cardiac biomarker levels in whole blood and serum with the new FL‐ICA system. Fifty samples were divided into two tubes, with one used for measuring the levels of cardiac biomarkers in whole blood, and the other used for measuring the levels in serum.
DISCUSSION
The testing of cardiac biomarkers is important for the determination of AMI since ECG testing alone is not always available and has a low accuracy of determining AMI 15. Therefore, the testing of these cardiac markers has become standard procedure for determining AMI and has been recognized and recommended by the ESC and ACC for some time. However, due to great variations in sensitivity and specificity, the use of a single biomarker may be inadequate for making clinical decisions. In fact, several reports have focused on the rapid diagnosis of AMI using multiple biomarkers 16, 17. In this study, we developed an FL‐ICA system for the measurement of cardiac panel biomarkers concentrations in blood for clinical applications. As trends in clinical medicine have favored using whole blood and miniaturized devices, the FL‐ICA system was designed as a portable platform that can be applicable with whole blood and serum for POCT and central laboratory use.
The i‐CHROMA™ FL‐ICA system takes advantage of simple ICA principles for the detection of cardiac markers in samples. To capture cardiac markers in the samples using a sandwich‐type method, the capture Ab is immobilized on nitrocellulose strip, and the FL‐conjugated detector Ab is integrated into the detection buffer to mingle with the blood sample. The intensity of the FL emanated by the detector Ab parallels the concentration of the cardiac marker in the sample as measured by an i‐CHROMA™ scanner after the immune reaction (Fig. 1). To ensure that the FL‐ICA system worked flawlessly, the interaction of FL‐labeled biotin–BSA at the control line with immobilized streptavidin was measured in every assay, serving as an internal system control.
Five interfering substances were tested at concentrations beyond physiological levels: bilirubin (150 mg/l), hemoglobin (10 g/l), glucose (1,200 g/l), lipid (1,000 mg/l), and albumin (100 g/l). The recovery of biomarker concentrations did not experience interference with these substances in the FL‐ICA system for cardiac markers (CV < 5%, data not shown) compared to that without these substances. When we determined the LoD, dynamic working range, and linearity of the FL‐ICA system for each cardiac biomarker, as shown in Figure 2, we found the parameters to be comparable with those of other automated assay methods that have been reported 18, 19. In particular, imprecision tests of the FL‐ICA system showed that the CVs of the intra‐ and interassays for all cardiac biomarkers were less than 8% at each tested concentration in the dynamic working range (Table 1). The study group on biomarkers in cardiology that was part of the ESC working group on acute cardiac care endorsed guidelines recommending that the imprecision of cardiac biomarkers should be 10% or less at the 99th percentile 20, 21, 22. In addition, the study group suggested that these cardiac biomarkers could be acceptable tools for risk stratification 23. These test results together demonstrated that the i‐CHROMA™ FL‐ICA system provided a confident performance for the quantification of cardiac panel concentrations in a clinically relevant range and could be used as a tool for diagnostic assessment and risk stratification in patients with signs and symptoms of cardiac emergencies.
Accuracy is a key issue in determining if a cardiac biomarker assay for the evaluation of AMI is extensively reliable and acceptable in all patients. In order to evaluate the accuracy and reliability of the FL‐ICA system, we compared the test results of 100 serum samples obtained with the i‐CHROMA™ FL‐ICA system with those obtained by the well‐known reference assay using bioMerieux VIDAS. As shown by the Bland–Altman difference plots in Figure 3B and C, most values of the mean differences for CK‐MB and cTnI fell within ±1.96 SD, except for one outlier in cTnI. Another analysis by a Passing–Bablok regression plot showed that the P values between the two methods for CK‐MB and cTnI were 0.001 and 0.014, respectively (Table 2), indicating that the concentrations of CK‐MB and cTnI obtained from the two assay systems were in good agreement. In the case of myoglobin, some values of the mean differences at a concentration range of 0–300 μg/l fell within ±1.96 SD, but some values were outside ±1.96 SD at high concentrations of 400–500 μg/l (Fig. 3A). This may influence the slope of 0.914 (95% confidence interval, 0.894–0.936) observed in the Passing–Bablok regression plot and result in some discrepancies (P = 0.070). However, value disagreement does not mean that the two assay methods are not comparable. In fact, there are some reports suggesting that the assays currently in use for the detection of cardiac biomarkers give different results 24. Variations in experimental conditions, including the specificity of the antibody and reagents, indicate that the results obtained from different assay methods are difficult to interchange.
Among the commercially available assays for the diagnosis of cardiac markers, the Triage Cardio Panel by Alere, Inc. (San Diego, CA) and the Rapid Analyte Measurement Platform (RAMP) CVD tests by Response Biomedical Corp. (Vancouver, Canada) are two assay instruments that meet the criteria for POCT platform. The Triage Cardio Panel test can determine the concentrations of all three cardiac biomarkers in whole blood by a single use of an FL immunoassay device 25. The RAMP is a lateral flow ICA platform that can provide the concentrations of cardiac biomarkers in whole blood depending on different biomarker cartridges 26. The i‐CHROMA™ FL‐ICA system for cardiac biomarkers incorporates some characteristics from both assay systems—the use of whole blood and serum, similar to Triage, and the analysis of one cardiac marker concentration from a single test, similar to RAMP. In fact, we are under the way to develop an assay system for measurement of three cardiac biomarkers from one single cartridge. Two different calibration curves implanted in a chip in the i‐CHROMA™ FL‐ICA system makes it possible to determine the concentrations of cardiac biomarkers in whole blood and serum. The i‐CHROMA™ cardiac panel system provides several advantages over other methods currently available, including the small amounts of sample required and the fast analysis time of 12 min without sacrificing the dynamic detection range. The ease of performing the whole procedure makes it a suitable system for POCT, and it does not require prior training. As a disposable device with minimum maintenance needed to perform the tests, the i‐CHROMA™ cardiac panel test meets the criteria of POCT.
In conclusion, the i‐CHROMA™ FL‐ICA system for cardiac panel biomarkers is a platform for POCT and is an easy, fast, and reliable method for the quantification of early and late cardiac panel biomarkers that can be used to predict the risk of present and future CVD.
REFERENCES
- 1. Epstein AE, DiMarco JP, Ellenbogen KA, et al. 2012 ACCF/AHA/HRS focused update incorporated into the ACCF/AHA/HRS 2008 guidelines for device‐based therapy of cardiac rhythm abnormalities: A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. Circulation 2013;127(3):e283–e352. [DOI] [PubMed] [Google Scholar]
- 2. European Heart Rhythm A, European Society of C, Heart Rhythm S , et al. 2012 EHRA/HRS expert consensus statement on cardiac resynchronization therapy in heart failure: Implant and follow‐up recommendations and management. Heart Rhythm 2012;9(9):1524–1576. [DOI] [PubMed] [Google Scholar]
- 3. Lefebvre CW, Hoekstra J. Approach to non‐ST‐segment elevation acute coronary syndrome in the emergency department: Risk stratification and treatment strategies. Hosp Pract 2010;38(2):40–49. [PubMed] [Google Scholar]
- 4. Tiwari RP, Jain A, Khan Z, et al. Cardiac troponins I and T: Molecular markers for early diagnosis, prognosis, and accurate triaging of patients with acute myocardial infarction. Mol Diagn Ther 2012;16(6):371–381. [DOI] [PubMed] [Google Scholar]
- 5. Friess U, Stark M. Cardiac markers: A clear cause for point‐of‐care testing. Anal Bioanal Chem 2009;393(5):1453–1462. [DOI] [PubMed] [Google Scholar]
- 6. de Winter RJ, Koster RW, Sturk A, Sanders GT. Value of myoglobin, troponin T, and CK‐MBmass in ruling out an acute myocardial infarction in the emergency room. Circulation 1995;92(12):3401–3407. [DOI] [PubMed] [Google Scholar]
- 7. Gruberg L, Sudarsky D, Kerner A, Hammerman H, Kapeliovich M, Beyar R. Troponin‐positive, CK‐MB‐negative acute myocardial infarction: Clinical, electrocardiographic and angiographic characteristics. J Invasive Cardiol 2008;20(3):125–128. [PubMed] [Google Scholar]
- 8. Yang Z, Yamazaki M, Shen QW, Swartz DR. Differences between cardiac and skeletal troponin interaction with the thin filament probed by troponin exchange in skeletal myofibrils. Biophys J 2009;97(1):183–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Eggers KM, Venge P, Lindahl B, Lind L. Cardiac troponin I levels measured with a high‐sensitive assay increase over time and are strong predictors of mortality in an elderly population. J Am Col Cardiol 2013;61(18):1906–1913. [DOI] [PubMed] [Google Scholar]
- 10. Diercks DB, Peacock WF 4th, Hollander JE, et al. Diagnostic accuracy of a point‐of‐care troponin I assay for acute myocardial infarction within 3 hours after presentation in early presenters to the emergency department with chest pain. Am Heart J 2012;163(1):74–80. [DOI] [PubMed] [Google Scholar]
- 11. Chan D, Ng LL. Biomarkers in acute myocardial infarction. BMC Med 2010;8:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lackner KJ. Laboratory diagnostics of myocardial infarction—Troponins and beyond. Clin Chem Lab Med 2013;51(1):83–89. [DOI] [PubMed] [Google Scholar]
- 13. Ahn JS, Choi S, Jang SH, et al. Development of a point‐of‐care assay system for high‐sensitivity C‐reactive protein in whole blood. Clin Chim Acta 2003;332(1–2):51–59. [DOI] [PubMed] [Google Scholar]
- 14. Kim BC, Jeong JH, Jeong DS, Choi EY, Kim JH, Nahm KB. Simplified laser fluorescence scanner for proteomics studies and early cancer diagnosis. Proc SPIE 2002;4916:103–108. [Google Scholar]
- 15. Bingisser R, Cairns C, Christ M, et al. Cardiac troponin: A critical review of the case for point‐of‐care testing in the ED. Am J Emerg Med 2012;30(8):1639–1649. [DOI] [PubMed] [Google Scholar]
- 16. Wang TJ, Gona P, Larson MG, et al. Multiple biomarkers for the prediction of first major cardiovascular events and death. N Engl J Med 2006;355(25):2631–2639. [DOI] [PubMed] [Google Scholar]
- 17. Zethelius B, Berglund L, Sundstrom J, et al. Use of multiple biomarkers to improve the prediction of death from cardiovascular causes. N Engl J Med 2008;358(20):2107–2116. [DOI] [PubMed] [Google Scholar]
- 18. Apple FS, Collinson PO. Analytical characteristics of high‐sensitivity cardiac troponin assays. Clin Chem 2012;8(1):54–61. [DOI] [PubMed] [Google Scholar]
- 19. Apple FS, Smith SW, Pearce LA, et al. Use of the bioMerieux VIDAS troponin I ultra assay for the diagnosis of myocardial infarction and detection of adverse events in patients presenting with symptoms suggestive of acute coronary syndrome. Clin Chim Acta 2008;390(1–2):72–75. [DOI] [PubMed] [Google Scholar]
- 20. Hofmann D, Buettner M, Rissner F, Wahl M, Sakka SG. Prognostic value of serum myoglobin in patients after cardiac surgery. J Anesth 2007;21(3):304–310. [DOI] [PubMed] [Google Scholar]
- 21. Thygesen K, Mair J, Katus H, et al. Study group on biomarkers in cardiology of the ESCWGoACC. Recommendations for the use of cardiac troponin measurement in acute cardiac care. Eur Heart J 2010;31(18):2197–2204. [DOI] [PubMed] [Google Scholar]
- 22. Yilmaz A, Yalta K, Turgut OO, et al. Clinical importance of elevated CK‐MB and troponin I levels in congestive heart failure. Adv Ther 2006;23(6):1060–1067. [DOI] [PubMed] [Google Scholar]
- 23. Panteghini M. The measurement of cardiac markers: Where should we focus? Am J Clin Path 2002;118(3):354–361. [DOI] [PubMed] [Google Scholar]
- 24. Panteghini M, Pagani F, Yeo KT, et al. Committee on standardization of markers of cardiac damage of the TnI. Evaluation of imprecision for cardiac troponin assays at low‐range concentrations. Clin Chem 2004;50(2):327–332. [DOI] [PubMed] [Google Scholar]
- 25. Calzavacca P, Licari E, Tee A, Bellomo R. Point‐of‐care testing during medical emergency team activations: A pilot study. Resuscitation 2012;83(9):1119–1123. [DOI] [PubMed] [Google Scholar]
- 26. McDonnell B, Hearty S, Leonard P, O'Kennedy R. Cardiac biomarkers and the case for point‐of‐care testing. Clin Biochem 2009;42(7–8):549–561. [DOI] [PubMed] [Google Scholar]


