Abstract
As gene transfer with adeno-associated virus (AAV) vectors is starting to enter clinical practice, this review examines the impact of vector capsid choice in liver-directed gene transfer for hemophilia. Given that there are multiple clinical trials completed and ongoing in this field, it is important to review the clinical evidence, particularly as a range of AAV-vector serotypes including AAV2, AAV5, AAV8, and AAV10 have been tested. Although there have been a number of successful trials, the development of two investigational AAV vectors for hemophilia B has been discontinued because they did not meet efficacy and/or safety expectations. Whether this difference between success and failure of gene transfer approaches reflects capsid choice, vector design, manufacturing system, or other variables is a question of great interest. Here, we examine the body of evidence across trials to determine the possible influences of serotype choice on key clinical outcomes such as safety, vector clearance, treatment eligibility, occurrence of transaminase elevations, activation of capsid-directed cytotoxic T cell responses, and clinical efficacy. In summary, gene transfer requires a balance between achieving sufficient transgene expression and minimizing destructive immune responses, which may be affected by AAV-vector serotype choice.
Main Text
Therapies employing gene transfer using adeno-associated virus (AAV) vectors are being fast-tracked for clinical approval for retinal disease, congestive heart failure, hemophilia A and B, X-linked myotubular myopathy, glioblastoma, glioma, and spinal muscular atrophy.1, 2 The focus of this review will be liver-directed AAV gene therapy for hemophilia, in which there are a number of completed or ongoing phase 1 and 2 trials and phase 3 trials that are recruiting.3 Given that there are multiple clinical trials in this field, it is important to review the clinical evidence, particularly as a range of AAV-vector serotypes including AAV2, AAV5, AAV8, and AAV10 have been tested. In addition, other AAV serotypes such as AAVhu37, a Clade E AAV that is closely related to AAV8,4 have been examined in non-human primate (NHP) models.5 Interestingly, the development of two investigational therapies, DTX101 (rAAV10-hFIX) and BAX335 (AAV8-hFIX), were stopped as they failed to meet manufacturer expectations in terms of efficacy and/or safety. Whether these failures—and the current apparent successes of other programs—reflect capsid choice, vector design, manufacturing system, or other variables is open to question. Although vector design and manufacturing/production systems are beyond the scope of this review, we will examine the impact of capsid choice by exploring AAV serotypes, the basis for serotype distinction, tropism, transduction efficacy, vector shedding, immune responses to AAV, and the impact of pre-existing neutralizing antibodies (NAb) on transduction efficacy to summarize what is known and identify areas that require further investigation.
AAV Capsid Serotypes
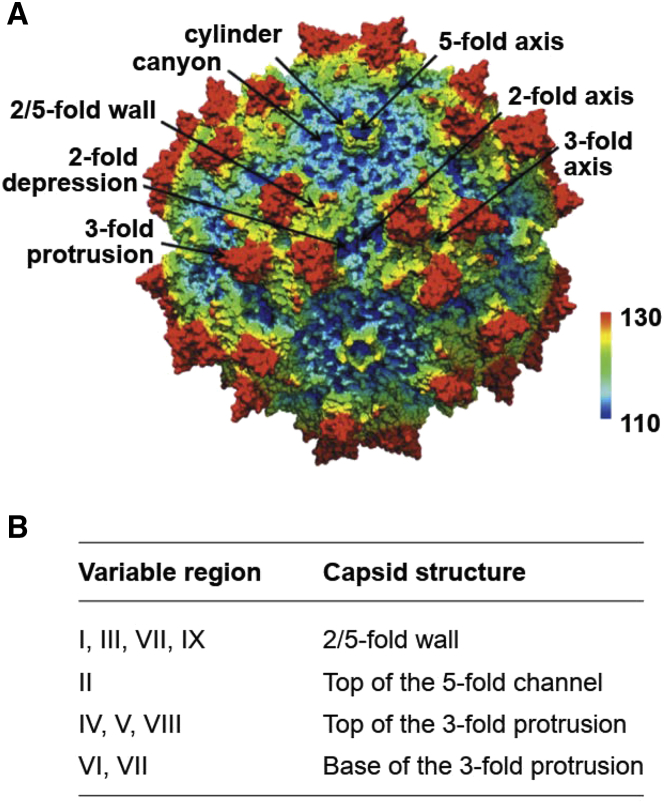
The AAV genome includes rep and cap genes that encode seven proteins.6 The rep gene encodes four non-structural proteins (Rep78, Rep68, Rep 52, and Rep 40), involved with replication, transcriptional control, integration, and encapsidation. The products of the three cap genes (Vp1–3) combine as 50 Vp3, five Vp1, and five Vp2 proteins to form the capsid.6, 7 Capsid assembly is assisted by the assembly-activating protein, a non-structural protein encoded within the cap gene, which promotes capsid stability and interactions between the capsid proteins.8 The AAV capsid includes a core eight-stranded β-barrel motif with large loop insertions between the β strands.9 The common structural features across serotypes are depicted in Figure 1A,9, 10 suggesting that these features may have specific functional activities (e.g., tissue trophism and cellular transduction) although variable regions within these structures between serotypes may confer distinct serotype-specific functional features as vectors for gene transfer and affect immunogenicity.
Figure 1.
AAV Capsids Share Some Common Structural Features across Serotypes
(A) AAV1 showing common capsid structure features shared with other serotypes. The color coding from blue-green-yellow-red represents the surface topology with the darkest blue representing the lowest areas and the red representing the protruding areas of capsid. (B) Location of the nine variable regions (VRs) in the AAV capsid. Figure reproduced from Tseng and Agbandje-McKenna.10
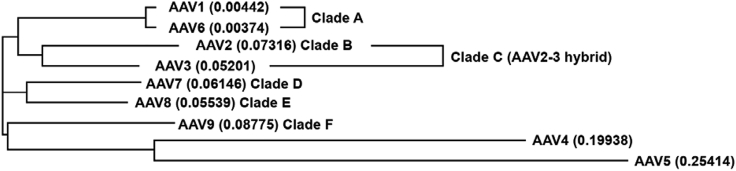
Currently, 13 AAV serotypes have been identified, which are differentiated based on surface antigen expression and amino acid sequence differences.7 AAV have been separated into clades A–F, on the basis of shared serologic and functional attributes, as well as two separate clonal isolates (AAV5 and AAV4) that exhibit greater differences compared with the other serotypes (Figure 2).7 AAV5 is the most phylogenetically distinct as it shares only 58% capsid homology with AAV2 and AAV8 and 57% homology with AAV10 (Figure 2).11 In contrast, the other serotypes commonly used in gene transfer share greater homology (e.g., AAV2 shares 83% homology with AAV8 and 84% homology with AAV10).11 The variance in structure includes conformational differences in regions associated with transduction efficacy and antigenicity, which may be important in terms of differences in tissue tropism, antigenicity, and the likelihood of cross-reactive immunogenicity between serotypes.7, 9, 10, 12
Figure 2.
Phylogenetic Relationships among AAV Serotypes
Figure reproduced Drouin and Agbandje-McKenna.7
Does AAV-Vector Capsid Affect Tissue Tropism?
Tropism can reduce off-target effects by limiting transduction to a particular tissue or cell type and may impact efficacy by concentrating cell transduction in a relevant tissue. Tissue tropism reflects the specific interactions between structures on the AAV-vector capsid that differ between serotypes and glycans (Table 1).13 The initial binding of many AAV serotypes is via primary receptors including glycans and proteoglycans such as heparan sulfate that are widely expressed in different tissues.14 This initial binding is followed by interactions with secondary membrane protein receptors that facilitate internalization.14 A range of secondary receptors has been reported, including the fibroblast growth factor receptor 1 (FGFR1 and alphaV-beta5 integrin) for AAV2,15 hepatocyte growth factor receptor (c-MET) for AAV216 and AAV3,17 and platelet-derived growth factor receptor for AAV5.13 Recently, the protein receptor KIAA0319L (hereafter AAVR) has been identified as critical for the entry of numerous AAV serotypes including AAV1, AAV2, AAV3B, AAV5, AAV6, AAV8, and AAV9.14 Importantly, the interactions between AAV serotypes and their primary and secondary receptors are likely to affect tissue specificity/tropism (Table 1).18 In mice, AAV serotypes 1–9 show overlapping but distinct tissue expression patterns: skeletal muscle (AAV 1–9), liver (AAV 1–3 and 5–9), heart (AAV4 and 6–9), lung (AAV4 and 6–9), brain (AAV 8–9), and testes (AAV9).19 Notably, here are species-specific differences in AAV tropism between mice and nonhuman primates,20, which raises the question of generalizability of findings across species as well as their relevance for humans.
Table 1.
Relationships between AAV Serotype Receptor Usage and Tropism are supported by references13, 14, 18, 20, 59, 60, 61
| Glycan Receptor Serotype Usage | AAV Serotype | Impact on Tropism |
|---|---|---|
| Heparan sulfate proteoglycan | AAV2, AAV3, AAV6, and AAV13 | AAV2 muscle cell interactions |
| α2–3 and α2–6 N-linked sialic acid (SIA) | AAV1, AAV4, AAV5, and AAV6 | AAV5 airway epithelial cell interactions |
| Laminin receptor | AAV8 | widely expressed on tissues targeted by AAV8 including heart, liver, and skeletal muscle |
| Terminal N-linked galactose of SIA | AAV9 | may enable the ability of AAV9 to cross the blood-brain barrier and transduce neural tissues |
| AAV receptor (AAVR, KIAA0319L) | AAV1, AAV2, AAV3B, AAV5, AAV6, AAV8, and AAV9 | AAVR is critical for the entry of numerous AAV serotypes |
Given the difficulties in justifying multiple biopsies in the clinical setting, vector biodistribution has not been reported in currently published trials21, 22, 23, 24, 25 and thus data demonstrating tropism profiles in humans is not available. If less invasive, non-biopsy techniques could be developed to assess AAV-vector tropism, this is an area that could be further investigated in future trials. In the absence of such information in humans, tissue-specific promoters are frequently incorporated into vectors to limit the expression of the transgene to a particular target tissue. For example, recent clinical trials employing systemic administration to target the liver utilize vectors with liver-specific promoters: e.g., AAV5-hFIXWT (AMT-060), AAV5-hFIXPadua (AMT-061), SPK-9001, AAV5-hFVIII-SQ (Valoctocogene Roxaparvovec), and scAAV2/8-LP1-hFIXco.23, 24, 25, 26
Clinical Considerations
AAV-Vector Efficacy
Differences in liver transduction efficacy have been demonstrated in animal models with AAV7 and AAV8-based vectors being approximately 10- to 100-fold more efficient than AAV2- or AAV5-based vectors,27, 28 although AAV tropism in mice is likely to be different from that in NHP and humans. Interestingly, this difference does not appear to reflect the ability of the different serotypes to enter hepatocytes, but instead may reflect more rapid uncoating and conversion of the single-stranded vector DNA into duplex DNA that is transcriptionally active.27, 29 In theory, a more effective vector could be administered at a lower dose compared with a less effective serotype. Administering a lower dose could have potential benefits in terms of reduced immunogenicity. In practical terms, however, a range of factors such as host immunity to the serotype, the quality of vector manufacturing, and transgene activity will also impact the balance between vector dose and the clinical outcomes of gene transfer.
AAV-Vector Clearance
In the field of virology, the term “shedding” is typically used to describe the release of infectious virus from host cells following infection. In the setting of gene therapy, vector shedding is monitored due to the potential risks of vertical transmission to progeny from the presence of AAV vector in semen and from horizontal transmission to close contacts or the wider community via AAV-vector shedding into other body fluids. AAV vectors are designed to be replication deficient, and thus any vector present would represent material from the primary administration. Monitoring of vector shedding, therefore, represents a measure of clearance of the vector from the body rather than active reproduction of the virus, and, as such, may reflect a number of variables including dose, route of administration, target organ,30 and potentially interindividual differences. It is important to note that current shedding assays measure vector DNA, so they are unable to distinguish between vector particles versus different forms of DNA (free, episomal, or integrated). Therefore, the detection of vector DNA in body fluids does not necessarily imply an infectious risk. Indeed, in NHP, AAV-vector DNA was detected in all body fluids for up to 6 days after vector transfer, whereas complete AAV-vector particles were only detected in serum for 48–72 h post transfer.31
In clinical trials, transient vector shedding was observed for AAV5, AAV2/8, and SPK-9001, although shedding was still detectable in some individuals at week 26 in whole blood for AAV5-hFIXWT 2 × 1013 gc/kg, week 52 in whole blood for AAV5-hFIXWT 5 × 1012, and week 52 in feces and whole blood for AAV5-hFVIII-SQ (Table 2). Shedding into semen is transient and germ-line transmission has not been observed in animal studies;30, 32 however, physicians should inform individuals that barrier contraception should be practiced as a precaution until clearance of vector DNA in semen is confirmed. It is of interest that vector shedding was more prolonged for AAV5-hFVIII-SQ than AAV5-hFIXWT, which may reflect the higher doses of AAV5-hFVIII-SQ administered. SPK-9001 and scAAV2/8-LP1-hFIXco were administered at the lowest doses and appeared to have the most transient AAV-vector shedding profile, which also supports a potential dose effect although further investigation is required.
Table 2.
Vector Shedding in Clinical Trials of Liver-Directed Gene Therapy
| Serotype | SPK-9001 (Serotype Unknown) 5 × 1011 gc/kg25 |
scAAV2/8-LP1-hFIXco 2 × 1012 gc/kg22 |
AAV5-hFIXWT (AMT-060) 5 × 1012 gc/kg23 |
AAV5-hFIXWT (AMT-060) 2 × 1013 gc/kg23 |
AAV5-hFVIII-SQ (Valoctocogene Roxaparvovec) 6 × 1013 gc/kg24 |
|---|---|---|---|---|---|
| SPK-100 | AAV2/8 | AAV5 | AAV5 | AAV5 | |
| Period of Vector Shedding, Weeks (Maximum or Range) | |||||
| Nasal secretions | not reported | not reported | 18 | 12 | not reported |
| Saliva | 4–6 | 2 | 20 | 16 | 40–52 |
| Feces | not reported | 2 | 16 | 20 | present to final assessment (week 52) |
| Urine | 2–8 | not reported | 11 | 22 | 6–28 |
| Semen | 4–12 | 2 | 48 | 22 | 36–56 |
| Whole blood | 22–42 (PBMCs) | 2 (plasma) | present to final assessment (week 52) | present to final assessment (week 26) | present to final assessment (week 52) |
Gc, genome copies; PBMCs, peripheral-blood mononuclear cells.
Immune Responses to AAV Vectors
There are two main branches of the adaptive immune system that appear to have the most impact on AAV-vector gene transfer—humoral and cell-mediated immunity. The humoral immune response results in the generation of vector-serotype-specific antibodies, some of which may be neutralizing. Pre-existing neutralizing antibodies to AAV vectors, which may reflect natural exposure to wild-type AAV serotypes or prior AAV-vector exposure, can reduce or prevent successful transduction and thereby impair therapeutic efficacy. Following AAV-vector-based gene transfer, high-titer neutralizing antibodies against the AAV-vector capsid are generated. While these antibodies formed following gene therapy will have no impact on the success of the initial gene transfer, they have implications for readministration of the same serotype and potentially, cross-reactive serotypes. Approaches to circumventing this issue include the use of alternative non-cross-reacting AAV-vector serotypes, which while demonstrated successfully with AAV5 and AAV1 in animal models33 can be challenging as NAb can cross-react across AAV-vector serotypes, and immunoadsorption/plasmapheresis to reduce levels of circulating NAb.34
Cellular immunity includes cytotoxic T cell responses that are usually measured using an enzyme-linked immunosorbent spot (ELISPOT) assay of interferon-γ (IFN-γ) production, which will typically develop 4–12 weeks after gene transfer. One study suggests, however, that memory CD8 cytotoxic T cells in AAV seropositive donor peripheral-blood mononuclear cells (PBMCs) secrete tumor necrosis factor alpha (TNF-α) in response to AAV capsid peptides rather than IFN-γ.35 Therefore, by focusing on IFN-γ responses, trials may be overlooking key CD8 T cell mediated immune responses to AAV-vector capsids. If confirmed, this may explain some of the discrepancies observed between IFN-γ ELISPOT responses, liver transaminase elevations, and loss of factor activity that are described in the next section.
Murine studies indicate that the development of cytotoxic T cell responses against AAV vectors requires innate immune sensing via Toll-like receptor (TLR) 9 on plasmacytoid dendritic cells.36 TLR 9 appears to sense the vector genome, as self-complementary AAV2 vectors induced stronger TLR 9 mediated innate responses than single-stranded AAV2 vectors in mice.37 There is evidence that another TLR (TLR 2) is key for sensing AAV-vector capsid antigens.38 As we discuss in the next section, there appear to be serotype-specific differences in immune responses to AAV vectors in clinical trials. As more information emerges on the interactions between different AAV serotypes and the innate and adaptive immune systems, the reasons for these differences may become clearer. Given the potential for TLRs to recognize differences in AAV-vector genomes, as well as vector capsid, other factors in addition to AAV serotype, such as the use of self-complementary AAV vectors or codon optimization, may affect immunogenicity.
The Impact of AAV-Vector Serotype on Immune Responses and Liver Toxicity/Loss of Efficacy
AAV-vector-mediated liver toxicity indicated by alanine aminotransferase (ALT) elevations and activation of capsid-specific CD8 T cells has been associated with subsequent decline in FIX and FVIII activity in clinical trials with AAV2, AAV8, and AAV10 (Tables 3 and 4).39, 40, 41 In contrast to these findings, with AAV5-based vectors, there was no observed connection between ALT or aspartate aminotransferase (AST) elevations, cytotoxic T cell responses, and reduction of factor activity (Tables 3 and 4) in clinical trials to date (0/10 participants with AAV5hFIX and 1/8 participants with AAV5hFVIII-SQ).23, 24 The lack of T cell responses and maintenance of factor activity in the presence of transaminitis in both AAV5-vector-based trials in hemophilia23, 24 and similar lack of immune response in the porphyria trial42 provide initial indications of serotype-specific differences in the generation of capsid-specific T cell responses, although this will need to be confirmed in further studies (Tables 3 and 4). In addition, this evidence, along with findings of inconsistent relationships between transaminase elevation and T cell responses from the scAAV2/8-LP1-hFIXco trial, suggests that ALT and/or AST elevations may not always signal the destruction of transduced hepatocytes.21, 23
Table 3.
Immunogenicity of Different AAV Gene-Transfer Preparations for Hemophilia B
| Parameter | AAVrh10FIX62, 67 | rAAV2hFIX44 | BAX 33563, 64 | scAAV2/8-LP1-hFIXco21, 22 | SPK-9001 (AAV-FIX)25 | AAV5hFIX23 |
|---|---|---|---|---|---|---|
| Serotype | AAV10 | AAV2 | AAV8 | AAV2/8 | not reported | AAV5 |
| Dose, gc/kg (n) | Co. 1: 1.6 × 1012 (3) | 2 × 1012 (2)a | Co. 1: 2 × 1011 (2) | Co. 1: 2 × 1011 (2) | 5 × 1011 (10) | Co. 1: 5 × 1012 (5) |
| Co. 2: 5.0 × 1012 (3) | Co. 2: 1 × 1012 (3) | Co. 2: 6 × 1011 (2) | Co. 2: 5 × 1013 (5) | |||
| Co. 3: 3 × 1012 (2) | Co. 3: 2 × 1012 (6) | |||||
| Transgene | WT | WT | Padua | WT | Padua | WT |
| Follow up, weeks | 10–52 | 14 | 7-104 | 166 (median) | 49 (mean) | 26–52 |
| Transgene activity, % or IU/dL | 5%–20% (peak) | 3–11 | 0.5-≥25 | 1–6 | 33.7 (mean) | 3–13 |
| ALT elevations | 5/6 | 1/2, same participant experienced AST elevation | 2/2 in Co. 3 | 4/6 in co. 3 | 2/10, same participants experienced AST elevation | 3/10 |
| AST elevations | not reported | 1/2, same patient experienced ALT elevation | not reported | 1/6, participant with the highest ALT elevation | 2/10, same participants experienced ALT elevation | No |
| Capsid-directed T cell activation | 4/6 | yes (only reported for 1 participant in the 4 × 1011 gc/kg group) | 2/2 in Co.3 | yes, Co. 2 and 3 | yes (2/2 with ALT elevation) | 0/3 |
| Immune response steroid responsive | no | not applicable | no, possibly due to delayed start | yes | yes | Yes |
| Loss of FIX expression | yes, 5/6 | yes, 2/2 in participants with ALT elevations | yes, 2/2 in participants with ALT elevations + T cell response | yes, 4/4 participants with ALT elevations + T cell response | yes, 1/2 with ALT/AST elevations + T cell response | No |
ALT, alanine aminotransferase; AST, aspartate aminotransferase; Co., cohort; FIX, factor IX; gc, genome copies; WT, wild type.
Lower doses were tested but did not result in a detectable increase in FIX activity.
Table 4.
Immunogenicity of Different AAV Gene-Transfer Preparations for Hemophilia A and Porphyria
| Parameter | AAV5hFVIII-SQ (Valoctocogene Roxaparvovec)24 | SPK-8011 AAV-VIII41 | GO-8 AAV8-HLP-hFVIII-V3 65 | rAAV2/5-PBGD42 |
|---|---|---|---|---|
| Serotype | AAV5 | Not reported | AAV8 | AAV2/5 |
| Dose, gc/kg (n) | 6 × 1012 (1) | 5 × 1011 (2) | 6 × 1011 (1) | 5 × 1011 (2) |
| 2 × 1013 (1) | 1 × 1012 (3) | 2 × 1012 (2) | 2 × 1012 (2) | |
| 6 × 1013 (6) | 2 × 1012 (7) | 6 × 1012 (2) | ||
| 1.8 × 1013 (2) | ||||
| Transgene | B-domain–deleted hFVIII | B-domain-deleted hFVIII | 17 amino-acid peptide with six N-linked glycosylation motifs from the human FVIII B-domain | WT |
| Follow up, weeks | 52 | 46 | 13–47 | 52 |
| Transgene activity, % or IU/dL | 2–164 | 13-30 | 7–69 | subclinical |
| ALT elevations | 8/9 (low and high) | steroids in 7/12 due to declining FVIII, ALT elevations, or IFN-γ ELISPOT | 2/3 | 1/8 (high) |
| AST elevations | 3/9 | not reported | not reported | not reported |
| Capsid-directed T cell activation | 0/9 | steroids in 7/12 due to declining FVIII, ALT, or IFN-γ ELISPOT | not reported | 0/8 |
| Immune response steroid responsive | no | yes | yes | not reported |
| Loss of FVIII expression | 1/8 with ALT elevations | yes, 7/7 | no | not applicable |
ALT, alanine aminotransferase; AST, aspartate aminotransferase; Co., cohort; FVIII, factor VIII; gc, genome copies; PBGD, porphobilinogen deaminase; WT, wild type.
The best way to control immune responses to AAV vectors remains to be determined. Clinically, the use of prophylactic versus on-demand immunosuppression, high-activity transgenes that enable lower doses of vector to be used, and engineering of the capsid surface to reduce immunogenicity have been proposed and are being investigated. In terms of liver toxicity, there is a need to clarify the relative impact of immune responses, the potential for AAV vectors to induce direct hepatocyte stress responses (whether to AAV-vector capsid or transgene), and the potential for toxicity related to concomitant drug use (e.g., efavirenz for HIV infection).43 Therefore, it should be considered whether it would be advisable to collect liver tissue biopsies in future trials so that this issue can be clarified.
AAV-Vector Serotypes and Neutralizing Antibodies
Anti-AAV NAb have historically been believed to diminish the efficacy of AAV-based therapies delivered systemically in humans, on the basis of results from preclinical and clinical trials utilizing AAV2 and AAV8. In humans, pre-existing NAb at titers as low as 1:17 for AAV244 and 1:1 for the bioengineered capsid AAV-Spark10025 were associated with reduced, or abrogated, therapeutic efficacy (Table 5). These observations have led to the exclusion of subjects with even low levels of anti-AAV NAb from the majority of AAV-vector-based gene therapy trials up until now. In contrast to other AAV-vector serotypes, successful liver transduction was achieved with AAV5 vector in both NHP and humans with pre-existing anti-AAV5 NAb titers up to 1:1,030 for NHP and 1:340 for humans (Table 6).23, 45, 46 Based on data such as this, gene transfer using AAV5 vectors is being investigated in participants with titers of NAbs to the serotype in trials in hemophilia A and B.47, 48
Table 5.
NAb Exclusion Criterion across Trials of Liver-Directed Gene Transfer for Hemophilia
| Developmental Therapeutic | Neutralizing Antibody Definition |
|---|---|
| AAV5-hFIXWT (AMT-060) | 29% inhibition of transduction versus pooled NAb-negative human sera23 |
| SPK-9001 | AAV-Spark100 neutralizing antibody titer >1:525 |
| scAAV2/8-LP1-hFIXco | no AAV8 NAb based on an in vivo transduction inhibition assay22 |
| AAV5-hFVIII-SQ (Valoctocogene Roxaparvovec) | no detectable immunity to AAV5 established with a cell-based transduction inhibition assay and an assay of total AAV5 immunoglobulin24 |
| future studies may focus on total immunoglobulin only as positive results in the transduction inhibition assay did not impact efficacy in a non-human primate study66 | |
| AAVrh10 | > 1.567 |
Table 6.
Pre-existing AAV Immunity and Subsequent T Cell Responses
| AAV555 | rAAV2hFIX44 | AAV5-hFIXWT 23, 33 | SPK-900125 | scAAV2/8- LP1-hFIXco 21, 22 | AAV5-FVIII24 | AAVrh10 62, 67 | |
|---|---|---|---|---|---|---|---|
| Study type | non-human primate | clinical | clinical | clinical | clinical | clinical | clinical |
| Pre-existing immunity | yes | 1/2 (1:2 and 1:17) | 3/10 | 1/10 | no | no | yes (NAb titer < 1:5) |
| T cell response | no | 1 (not in the participant with pre-existing immunity) | no | 2/10 (not participants with pre-existing immunity) | 8/10 (intermediate and high dose group) | no | yes 6/6 |
| Efficacy in participants with pre-existing immunity | no | no | yes | yes, but FIX expression lowest (approx. 10%–15%) | not applicable | not applicable | yes |
| Loss of FIX expression over time in participants with pre-existing immunity | not applicable | yes, peaked at 2 weeks | no | no | not applicable | not applicable | yes, peaked between 3-14 weeks |
NAb, neutralizing antibody.
There is a clear need for a standardized approach for measuring NAbs, so that the cut offs for titers that could cause a clinically relevant impairment of gene transfer can be identified. Importantly, such cut-offs will likely need to be vector serotype and assay specific. As Table 5 indicates, clinical trials include different assays such as inhibition of transduction and direct measurement of antibody titers as well as different cut-offs for demonstrating positivity, so it is impossible to compare findings between studies. An aligned approach to defining clinically relevant titers will be key as gene therapy enters clinical practice.
Due to the high degree of conservation in the amino acid sequence among AAVs,49 anti-AAV antibodies show cross reactivity with a wide range of serotypes.50 AAV2 has the highest seroprevalence of NAb in the general population,50 which may make this serotype most suitable for applications for which NAb are less of a concern, such as gene transfer to the eye51 or brain parenchyma.52 AAV5, which has the least-conserved capsid sequence versus other serotypes,53 and the non-human primate serotype AAV8,49 are among the AAV vectors with the lowest seroprevalence of NAb in humans.50, 54 The seroprevalence of NAb to AAV-vector serotypes also varies by geography, with a higher prevalence of AAV2 NAbs in Africa versus other regions.54 Therefore, in terms of AAV-vector capsid choice, based on rates of pre-existing immunity it makes sense to choose a vector serotype with the lowest prevalence in the general population such as AAV5 or 8.
There does not appear to be an association between pre-existing NAb and subsequent T cell responses. From the re-analysis of the AAV5-hFIXWT trial samples, the three participants with pre-existing NAb did not experience ALT elevations and did not develop T cell responses.45 In agreement with these data, in other human and animal trials, pre-existing immunity does not tend to be associated with subsequent T cell responses, and T cell responses may occur in patients without evidence of pre-existing immunity (Table 6). In addition, there does not appear to be a link between the presence of NAbs and subsequent loss of factor activity as this was observed in some trials but not others.
Pre-existing NAb to AAV-vector serotypes are a major limitation in terms of patient access to gene therapy and so far, these patients have been excluded from gene therapy trials.40 It is possible that sequential administration of non-cross-reacting serotypes may enable re-dosing; as was alluded to earlier in the manuscript, this approach has been successfully demonstrated with AAV5 and AAV1 in NHPs, but has yet to be examined in humans.33 Also in a NHP model, pre-existing immunity following AAV5-human embryonic alkaline phosphatase (SEAP) treatment was bypassed by immune adsorption allowing successful transduction with AAV5-hFIX.55 In a mouse model, co-administration of an AAV vector with tolerogenic nanoparticles blocked anti-AAV immune responses and allowed for effective re-dosing.56 In the future, the use of rational design to alter AAV-vector capsids to avoid pre-existing immunity may be an option.57 Other potential approaches that need further investigation include better immunosuppression regimens, using the lowest possible AAV-vector dose to achieve efficacy while minimizing immune responses, reducing the total capsid exposure by ridding preparations of empty capsids or conversely using empty capsids as decoys, improving manufacturing quality, and reducing potentially immunostimulatory contaminants.40 As therapies enter the clinic, studies examining re-dosing will be a key area for further research.
Discussion
The choice of the most appropriate AAV vector for therapeutic gene delivery depends on a number of factors including the prevalence of NAb to the serotype, tissue tropism, and the risk of immunogenicity. The ideal AAV vector, therefore, would have a low seroprevalence and titer of NAb to allow the widest possible patient access to treatment, would transfect the tissue of choice, and would not elicit immune responses that impact transgene expression. More needs to be elucidated regarding potential AAV-vector serotype-specific differences in the intracellular processing, and transduction of that may affect clinical outcomes in gene therapy.
For liver-directed gene transfer, a number of AAV-vector serotypes have been trialed including AAV2, AAV5, AAV8, and AAV10. In terms of the capsid structure, AAV5 is the most phylogenetically distinct vector serotype, whereas other commonly used serotypes such AAV2 and AAV8 share over 80% homology. This may impact the prevalence of NAb to AAV5 in the general population, which tend to be lower with AAV5 compared with serotypes such as AAV2 and AAV1. Pre-existing NAb to AAV can impair transduction efficacy and for certain AAV-vector serotypes the prevalence of NAb can reach up to 60%,50 which could limit patient access to treatment. Pre-existing NAb to AAV5 at titers commonly observed in the general population do not appear to affect transduction efficacy, and some ongoing trials with AAV5-based vectors will include individuals with NAb.45, 47, 48, 58 Use of diverse AAV-vector serotypes may also permit re-administration in the future due to decreased likelihood of cross-reactive antibodies raised after the first administration. As gene therapy becomes more established, it will be important to standardize NAb assays and define clinically relevant levels to ensure better patient access to gene therapy.
Tropism is key to targeting gene expression to appropriate tissues and to reduce off-target effects. There is increasing evidence elucidating the molecular interactions that underlie tropism, and in animal studies, AAV-vector capsids have been engineered to modify tropism. However, animal models may be poorly predictive of tropism in humans. Additionally, tropism is more difficult to study in humans, so most clinical approaches depend on a tissue-specific promoter to drive expression. This is an area that requires significant further study in humans, although less invasive methods than are currently available are required to enable this.
Vector clearance is relevant due to the risk of horizontal or vertical transmission of infectious AAV vectors, although in reality the infection risk from AAV vectors is low as they are non-pathogenic and replication deficient. Additionally, because current assays assess vector DNA, “shedding” data does not distinguish between vector DNA that is part of an infectious AAV particle and vector DNA fragments that have no infectious risk. Vector shedding was largely transient across clinical trials although in some studies vector shedding was detected in some body fluids up to the last endpoint. There are initial indications that lower-dose AAV vectors may be associated with a shorter duration of vector shedding, but this needs to be confirmed. It is also possible, however, that the duration of shedding is similar but that in some cases the magnitude of vector DNA present is below the limits of detection.
AAV-vector trials have largely demonstrated a modest dose response in terms of transgene expression; however, in some trials, higher doses have been associated with T cell mediated immune responses and associated loss of transgene expression. Therefore, in addition to dose, there may be inherent differences between serotypes in terms of the type of immune responses they elicit and the doses required to do so. For example, with scAAV2/8-LP1-hFIXco, SPK-9001, rAAV2hFIX, and AAVrh10FIX; in some cases, liver damage indicated by ALT elevations is associated with T cell immune responses and subsequent FIX decline. With AAV5-based vectors, in contrast, there did not appear to be an association between ALT elevations, cytotoxic T cell responses, and reduction of FIX activity. Although this provides an initial indication of differences in the immunogenicity of AAV-vector serotypes, our understanding of immune responses to AAV vectors is still at an early stage. Additionally, there is no standardized approach to control immune responses, e.g., by vector dose minimization, vector design, serotype usage, and/or prophylactic or on-demand steroids.
Conclusions
Several factors enter into the consideration of capsid choice for treating patients with gene transfer. A balance must be struck between achieving sufficient transgene expression for clinical benefit and activation of the body’s immune expression. Although dose may be a factor, there are initial indications that there may be inherent differences in immunogenicity between AAV-vector serotypes. Evidence with each serotype is currently limited because only tens of patients have received each construct. As gene transfer becomes more established in the clinic, these gaps in the evidence base should be addressed.
Author Contributions
All of the authors contributed to the development of the review from the initial concept stage, provided critical input during the draft stages, and approved the final version prior to submission.
Conflicts of Interest
S.P. has received a fee paid to his institution from uniQure B.V.; consultant fees from Shire, Novo Nordisk, Bioverativ, CSL Behring, Pfizer, Genentech/Roche, Alnylam, Apcintex, Biomarin, uniQure, Bayer, Freeline, Spark Therapeutics, Catalyst Biosciences, and HEMA Biologics; and a research grant from Shire. F.W.G.L. has received consultancy fees paid to his institution from uniQure B.V., and he has received research grants from CSL Behring, and Baxalta/Shire, outside the submitted work. V.F. and E.K.S. are employees of uniQure. J.P. has received a fee paid to his institution from uniQure B.V. and consultant fees from Shire, Novo Nordisk, Sanofi, Sobi, Pfizer, Genentech/Roche, Alnylam, Apcintex, Biomarin, and Catalyst Biosciences.
Acknowledgments
The authors had full responsibility for development of this review with writing support, funded by uniQure, provided by Mike Lappin of GK Pharmacomm, Ltd.
References
- 1.Morrison C. Landmark gene therapy poised for US approval. Nat. Rev. Drug Discov. 2017;16:739–741. doi: 10.1038/nrd.2017.212. [DOI] [PubMed] [Google Scholar]
- 2.European Medicines Agency PRIME: Priority medicines. 2018. https://www.ema.europa.eu/en/human-regulatory/research-development/prime-priority-medicines
- 3.ClinicalTrials.gov ClinicalTrials.gov. 2018. https://clinicaltrials.gov/
- 4.Wang L., Wang H., Bell P., McCarter R.J., He J., Calcedo R., Vandenberghe L.H., Morizono H., Batshaw M.L., Wilson J.M. Systematic evaluation of AAV vectors for liver directed gene transfer in murine models. Mol. Ther. 2010;18:118–125. doi: 10.1038/mt.2009.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Greig J.A., Nordin J.M.L., White J.W., Wang Q., Bote E., Goode T., Calcedo R., Wadsworth S., Wang L., Wilson J.M. Optimized Adeno-Associated Viral-Mediated Human Factor VIII Gene Therapy in Cynomolgus Macaques. Hum. Gene Ther. 2018 doi: 10.1089/hum.2018.080. Published online December 13, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mitchell A.M., Nicolson S.C., Warischalk J.K., Samulski R.J. AAV’s anatomy: roadmap for optimizing vectors for translational success. Curr. Gene Ther. 2010;10:319–340. doi: 10.2174/156652310793180706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Drouin L.M., Agbandje-McKenna M. Adeno-associated virus structural biology as a tool in vector development. Future Virol. 2013;8:1183–1199. doi: 10.2217/fvl.13.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maurer A.C., Pacouret S., Cepeda Diaz A.K., Blake J., Andres-Mateos E., Vandenberghe L.H. The Assembly-Activating Protein Promotes Stability and Interactions between AAV’s Viral Proteins to Nucleate Capsid Assembly. Cell Rep. 2018;23:1817–1830. doi: 10.1016/j.celrep.2018.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Govindasamy L., DiMattia M.A., Gurda B.L., Halder S., McKenna R., Chiorini J.A., Muzyczka N., Zolotukhin S., Agbandje-McKenna M. Structural insights into adeno-associated virus serotype 5. J. Virol. 2013;87:11187–11199. doi: 10.1128/JVI.00867-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tseng Y.-S., Agbandje-McKenna M. Mapping the AAV capsid host antibody response toward the development of second generation gene delivery vectors. Front. Immunol. 2014;5:9. doi: 10.3389/fimmu.2014.00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vance M.A., Mitchell A., Samulski R.J. AAV biology, infectivity and therapeutic use from bench to clinic. In: Hashad D., editor. Gene Therapy: Principles and Challenges. InTechOpen; 2015. pp. 119–143. [Google Scholar]
- 12.Nam H.J., Lane M.D., Padron E., Gurda B., McKenna R., Kohlbrenner E., Aslanidi G., Byrne B., Muzyczka N., Zolotukhin S., Agbandje-McKenna M. Structure of adeno-associated virus serotype 8, a gene therapy vector. J. Virol. 2007;81:12260–12271. doi: 10.1128/JVI.01304-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Srivastava A. In vivo tissue-tropism of adeno-associated viral vectors. Curr. Opin. Virol. 2016;21:75–80. doi: 10.1016/j.coviro.2016.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pillay S., Zou W., Cheng F., Puschnik A.S., Meyer N.L., Ganaie S.S. AAV serotypes have distinctive interactions with domains of the cellular receptor AAVR. J. Virol. 2017 doi: 10.1128/JVI.00391-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Qing K., Mah C., Hansen J., Zhou S., Dwarki V., Srivastava A. Human fibroblast growth factor receptor 1 is a co-receptor for infection by adeno-associated virus 2. Nat. Med. 1999;5:71–77. doi: 10.1038/4758. [DOI] [PubMed] [Google Scholar]
- 16.Kashiwakura Y., Tamayose K., Iwabuchi K., Hirai Y., Shimada T., Matsumoto K., Nakamura T., Watanabe M., Oshimi K., Daida H. Hepatocyte growth factor receptor is a coreceptor for adeno-associated virus type 2 infection. J. Virol. 2005;79:609–614. doi: 10.1128/JVI.79.1.609-614.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ling C., Lu Y., Kalsi J.K., Jayandharan G.R., Li B., Ma W., Cheng B., Gee S.W., McGoogan K.E., Govindasamy L. Human hepatocyte growth factor receptor is a cellular coreceptor for adeno-associated virus serotype 3. Hum. Gene Ther. 2010;21:1741–1747. doi: 10.1089/hum.2010.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wu Z., Asokan A., Grieger J.C., Govindasamy L., Agbandje-McKenna M., Samulski R.J. Single amino acid changes can influence titer, heparin binding, and tissue tropism in different adeno-associated virus serotypes. J. Virol. 2006;80:11393–11397. doi: 10.1128/JVI.01288-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zincarelli C., Soltys S., Rengo G., Rabinowitz J.E. Analysis of AAV serotypes 1-9 mediated gene expression and tropism in mice after systemic injection. Mol. Ther. 2008;16:1073–1080. doi: 10.1038/mt.2008.76. [DOI] [PubMed] [Google Scholar]
- 20.Watakabe A., Ohtsuka M., Kinoshita M., Takaji M., Isa K., Mizukami H., Ozawa K., Isa T., Yamamori T. Comparative analyses of adeno-associated viral vector serotypes 1, 2, 5, 8 and 9 in marmoset, mouse and macaque cerebral cortex. Neurosci. Res. 2015;93:144–157. doi: 10.1016/j.neures.2014.09.002. [DOI] [PubMed] [Google Scholar]
- 21.Nathwani A.C., Reiss U.M., Tuddenham E.G., Rosales C., Chowdary P., McIntosh J., Della Peruta M., Lheriteau E., Patel N., Raj D. Long-term safety and efficacy of factor IX gene therapy in hemophilia B. N. Engl. J. Med. 2014;371:1994–2004. doi: 10.1056/NEJMoa1407309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nathwani A.C., Tuddenham E.G., Rangarajan S., Rosales C., McIntosh J., Linch D.C., Chowdary P., Riddell A., Pie A.J., Harrington C. Adenovirus-associated virus vector-mediated gene transfer in hemophilia B. N. Engl. J. Med. 2011;365:2357–2365. doi: 10.1056/NEJMoa1108046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miesbach W., Meijer K., Coppens M., Kampmann P., Klamroth R., Schutgens R., Tangelder M., Castaman G., Schwäble J., Bonig H. Gene therapy with adeno-associated virus vector 5-human factor IX in adults with hemophilia B. Blood. 2018;131:1022–1031. doi: 10.1182/blood-2017-09-804419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rangarajan S., Walsh L., Lester W., Perry D., Madan B., Laffan M., Yu H., Vettermann C., Pierce G.F., Wong W.Y., Pasi K.J. AAV5-Factor VIII Gene Transfer in Severe Hemophilia A. N. Engl. J. Med. 2017;377:2519–2530. doi: 10.1056/NEJMoa1708483. [DOI] [PubMed] [Google Scholar]
- 25.George L.A., Sullivan S.K., Giermasz A., Rasko J.E.J., Samelson-Jones B.J., Ducore J., Cuker A., Sullivan L.M., Majumdar S., Teitel J. Hemophilia B Gene Therapy with a High-Specific-Activity Factor IX Variant. N. Engl. J. Med. 2017;377:2215–2227. doi: 10.1056/NEJMoa1708538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nathwani A.C., Gray J.T., Ng C.Y., Zhou J., Spence Y., Waddington S.N., Tuddenham E.G., Kemball-Cook G., McIntosh J., Boon-Spijker M. Self-complementary adeno-associated virus vectors containing a novel liver-specific human factor IX expression cassette enable highly efficient transduction of murine and nonhuman primate liver. Blood. 2006;107:2653–2661. doi: 10.1182/blood-2005-10-4035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Davidoff A.M., Gray J.T., Ng C.Y., Zhang Y., Zhou J., Spence Y., Bakar Y., Nathwani A.C. Comparison of the ability of adeno-associated viral vectors pseudotyped with serotype 2, 5, and 8 capsid proteins to mediate efficient transduction of the liver in murine and nonhuman primate models. Mol. Ther. 2005;11:875–888. doi: 10.1016/j.ymthe.2004.12.022. [DOI] [PubMed] [Google Scholar]
- 28.Gao G., Lu Y., Calcedo R., Grant R.L., Bell P., Wang L., Figueredo J., Lock M., Wilson J.M. Biology of AAV serotype vectors in liver-directed gene transfer to nonhuman primates. Mol. Ther. 2006;13:77–87. doi: 10.1016/j.ymthe.2005.08.017. [DOI] [PubMed] [Google Scholar]
- 29.Thomas C.E., Storm T.A., Huang Z., Kay M.A. Rapid uncoating of vector genomes is the key to efficient liver transduction with pseudotyped adeno-associated virus vectors. J. Virol. 2004;78:3110–3122. doi: 10.1128/JVI.78.6.3110-3122.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Salmon F., Grosios K., Petry H. Safety profile of recombinant adeno-associated viral vectors: focus on alipogene tiparvovec (Glybera®) Expert Rev. Clin. Pharmacol. 2014;7:53–65. doi: 10.1586/17512433.2014.852065. [DOI] [PubMed] [Google Scholar]
- 31.Favre D., Provost N., Blouin V., Blancho G., Chérel Y., Salvetti A., Moullier P. Immediate and long-term safety of recombinant adeno-associated virus injection into the nonhuman primate muscle. Mol. Ther. 2001;4:559–566. doi: 10.1006/mthe.2001.0494. [DOI] [PubMed] [Google Scholar]
- 32.Rajasekaran S., Thatte J., Periasamy J., Javali A., Jayaram M., Sen D., Krishnagopal A., Jayandharan G.R., Sambasivan R. Infectivity of adeno-associated virus serotypes in mouse testis. BMC Biotechnol. 2018;18:70. doi: 10.1186/s12896-018-0479-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Majowicz A., Salas D., Zabaleta N., Rodríguez-Garcia E., González-Aseguinolaza G., Petry H., Ferreira V. Successful Repeated Hepatic Gene Delivery in Mice and Non-human Primates Achieved by Sequential Administration of AAV5ch and AAV1. Mol. Ther. 2017;25:1831–1842. doi: 10.1016/j.ymthe.2017.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mingozzi F., High K.A. Immune responses to AAV vectors: overcoming barriers to successful gene therapy. Blood. 2013;122:23–36. doi: 10.1182/blood-2013-01-306647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kuranda K., Jean-Alphonse P., Leborgne C., Hardet R., Collaud F., Marmier S., Costa Verdera H., Ronzitti G., Veron P., Mingozzi F. Exposure to wild-type AAV drives distinct capsid immunity profiles in humans. J. Clin. Invest. 2018;128:5267–5279. doi: 10.1172/JCI122372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rogers G.L., Shirley J.L., Zolotukhin I., Kumar S.R.P., Sherman A., Perrin G.Q., Hoffman B.E., Srivastava A., Basner-Tschakarjan E., Wallet M.A. Plasmacytoid and conventional dendritic cells cooperate in crosspriming AAV capsid-specific CD8+ T cells. Blood. 2017;129:3184–3195. doi: 10.1182/blood-2016-11-751040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Martino A.T., Suzuki M., Markusic D.M., Zolotukhin I., Ryals R.C., Moghimi B., Ertl H.C., Muruve D.A., Lee B., Herzog R.W. The genome of self-complementary adeno-associated viral vectors increases Toll-like receptor 9-dependent innate immune responses in the liver. Blood. 2011;117:6459–6468. doi: 10.1182/blood-2010-10-314518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hösel M., Broxtermann M., Janicki H., Esser K., Arzberger S., Hartmann P., Gillen S., Kleeff J., Stabenow D., Odenthal M. Toll-like receptor 2-mediated innate immune response in human nonparenchymal liver cells toward adeno-associated viral vectors. Hepatology. 2012;55:287–297. doi: 10.1002/hep.24625. [DOI] [PubMed] [Google Scholar]
- 39.High K.A. Gene therapy for hemophilia: the clot thickens. Hum. Gene Ther. 2014;25:915–922. doi: 10.1089/hum.2014.2541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mingozzi F., Anguela X.M., Pavani G., Chen Y., Davidson R.J., Hui D.J., Yazicioglu M., Elkouby L., Hinderer C.J., Faella A. Overcoming preexisting humoral immunity to AAV using capsid decoys. Sci. Transl. Med. 2013;5:194ra92. doi: 10.1126/scitranslmed.3005795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.High K.A., George L.A., Eyster M.E., Sullivan S.K., Ragni M.V., Croteau S.E. A Phase 1/2 Trial of Investigational Spk-8011 in Hemophilia a Demonstrates Durable Expression and Prevention of Bleeds. Blood. 2018;132:487. [Google Scholar]
- 42.D’Avola D., López-Franco E., Sangro B., Pañeda A., Grossios N., Gil-Farina I., Benito A., Twisk J., Paz M., Ruiz J. Phase I open label liver-directed gene therapy clinical trial for acute intermittent porphyria. J. Hepatol. 2016;65:776–783. doi: 10.1016/j.jhep.2016.05.012. [DOI] [PubMed] [Google Scholar]
- 43.National Hemophilia Foundation’s Medical and Scientific Advisory Council (MASAC) MASAC Document Regarding Risks of Gene Therapy Trials for Hemophilia. 2018. https://www.hemophilia.org/Researchers-Healthcare-Providers/Medical-and-Scientific-Advisory-Council-MASAC/MASAC-Recommendations/MASAC-Document-Regarding-Risks-of-Gene-Therapy-Trials-for-Hemophilia
- 44.Manno C.S., Pierce G.F., Arruda V.R., Glader B., Ragni M., Rasko J.J., Ozelo M.C., Hoots K., Blatt P., Konkle B. Successful transduction of liver in hemophilia by AAV-Factor IX and limitations imposed by the host immune response. Nat. Med. 2006;12:342–347. doi: 10.1038/nm1358. [DOI] [PubMed] [Google Scholar]
- 45.Majowicz A., Nijmeijer B., Lampen M.H., Spronck L., de Haan M., Petry H., van Deventer S.J., Meyer C., Tangelder M., Ferreira V. Therapeutic hFIX Activity Achieved after Single AAV5-hFIX Treatment in Hemophilia B Patients and NHPs with Pre-existing Anti-AAV5 NABs. Mol. Ther. Methods Clin. Dev. 2019;14:27–36. doi: 10.1016/j.omtm.2019.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Majowicz A., Spronck L., de Haan M., Petry H., Nijmeijer B., Ferreira V. Circulating Anti-AAV5 Neutralizing Antibody Titers up to 1:1031 Do Not Affect Liver Transduction Efficacy of AAV5 Vectors in Non-Human Primates. Mol. Ther. 2017;25:94. [Google Scholar]
- 47.ClinicalTrials.gov HOPE-B: Trial of AMT-061 in Severe or Moderately Severe Hemophilia B Patients. 2019. https://clinicaltrials.gov/ct2/show/NCT03569891?term=AMT-061&cond=hemophilia&rank=1
- 48.ClinicalTrials.gov Gene Therapy Study in Severe Haemophilia A Patients With Antibodies Against AAV5 (270-203) 2019. https://clinicaltrials.gov/ct2/show/NCT03520712?term=BMN&cond=hemophilia&rank=4
- 49.Gao G.P., Alvira M.R., Wang L., Calcedo R., Johnston J., Wilson J.M. Novel adeno-associated viruses from rhesus monkeys as vectors for human gene therapy. Proc. Natl. Acad. Sci. USA. 2002;99:11854–11859. doi: 10.1073/pnas.182412299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Boutin S., Monteilhet V., Veron P., Leborgne C., Benveniste O., Montus M.F., Masurier C. Prevalence of serum IgG and neutralizing factors against adeno-associated virus (AAV) types 1, 2, 5, 6, 8, and 9 in the healthy population: implications for gene therapy using AAV vectors. Hum. Gene Ther. 2010;21:704–712. doi: 10.1089/hum.2009.182. [DOI] [PubMed] [Google Scholar]
- 51.Bennett J., Ashtari M., Wellman J., Marshall K.A., Cyckowski L.L., Chung D.C., McCague S., Pierce E.A., Chen Y., Bennicelli J.L. AAV2 gene therapy readministration in three adults with congenital blindness. Sci. Transl. Med. 2012;4:120ra15. doi: 10.1126/scitranslmed.3002865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kaplitt M.G., Feigin A., Tang C., Fitzsimons H.L., Mattis P., Lawlor P.A., Bland R.J., Young D., Strybing K., Eidelberg D., During M.J. Safety and tolerability of gene therapy with an adeno-associated virus (AAV) borne GAD gene for Parkinson’s disease: an open label, phase I trial. Lancet. 2007;369:2097–2105. doi: 10.1016/S0140-6736(07)60982-9. [DOI] [PubMed] [Google Scholar]
- 53.Chiorini J.A., Kim F., Yang L., Kotin R.M. Cloning and characterization of adeno-associated virus type 5. J. Virol. 1999;73:1309–1319. doi: 10.1128/jvi.73.2.1309-1319.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Calcedo R., Vandenberghe L.H., Gao G., Lin J., Wilson J.M. Worldwide epidemiology of neutralizing antibodies to adeno-associated viruses. J. Infect. Dis. 2009;199:381–390. doi: 10.1086/595830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Salas D., Kwikkers K.L., Zabaleta N., Bazo A., Petry H., van Deventer S.J. Immunoadsorption enables successful rAAV5-mediated repeated hepatic gene delivery in nonhuman primates. Blood. Adv. 2019;3:2632–2641. doi: 10.1182/bloodadvances.2019000380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Meliani A., Boisgerault F., Hardet R., Marmier S., Collaud F., Ronzitti G. Antigen-selective modulation of AAV immunogenicity with tolerogenic rapamycin nanoparticles enables successful vector re-administration. Nat. Commun. 2018;9:4098. doi: 10.1038/s41467-018-06621-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bartel M., Schaffer D., Büning H. Enhancing the Clinical Potential of AAV Vectors by Capsid Engineering to Evade Pre-Existing Immunity. Front. Microbiol. 2011;2:204. doi: 10.3389/fmicb.2011.00204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.ClinicalTrials.gov Dose Confirmation Trial of AAV5-hFIXco-Padua. 2019. https://clinicaltrials.gov/ct2/show/NCT03489291?term=AMT-061&cond=hemophilia&rank=2
- 59.Akache B., Grimm D., Pandey K., Yant S.R., Xu H., Kay M.A. The 37/67-kilodalton laminin receptor is a receptor for adeno-associated virus serotypes 8, 2, 3, and 9. J. Virol. 2006;80:9831–9836. doi: 10.1128/JVI.00878-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bell C.L., Gurda B.L., Van Vliet K., Agbandje-McKenna M., Wilson J.M. Identification of the galactose binding domain of the adeno-associated virus serotype 9 capsid. J. Virol. 2012;86:7326–7333. doi: 10.1128/JVI.00448-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Castle M.J., Turunen H.T., Vandenberghe L.H., Wolfe J.H. Controlling AAV Tropism in the Nervous System with Natural and Engineered Capsids. Methods Mol. Biol. 2016;1382:133–149. doi: 10.1007/978-1-4939-3271-9_10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pipe S., Stine K., Rajasekhar A., Everington T., Poma A., Crombez E. 101HEMB01 Is a Phase 1/2 Open-Label, Single Ascending Dose-Finding Trial of DTX101 (AAVrh10FIX) in Patients with Moderate/Severe Hemophilia B That Demonstrated Meaningful but Transient Expression of Human Factor IX (hFIX) Blood. 2017;130:3331. [Google Scholar]
- 63.Monahan P., Walsh C.E., Powell J.S., Konkle B.A., Josephson N.C., Escobar M. Update on a phase 1/2 open-label trial of BAX335, an adeno-associated virus 8 (AAV8) vector-based gene therapy program for hemophilia B. J. Thromb. Haemost. 2015;13:87. [Google Scholar]
- 64.StreetInsider.com Baxalta (BAX) Presents Updated BAX 335 Phase 1/2 Data in Hemophilia B. 2015. https://www.streetinsider.com/Corporate+News/Baxalta+%28BAX%29+Presents+Updated+BAX+335+Phase+12+Data+in+Hemophilia+B/10675560.html
- 65.Nathwani A.C., Tuddenham E., Chowdary P., McIntosh J., Lee D., Rosales C. GO-8: Preliminary Results of a Phase I/II Dose Escalation Trial of Gene Therapy for Haemophilia a Using a Novel Human Factor VIII Variant. Blood. 2018;132:489. [Google Scholar]
- 66.Long B., Sandza K., Holcomb J., Pherarolis J., Crockett L., Falese L. Impact of Pre-Existing Immunogenicity to AAV on Vector Transduction By Bmn 270, an AAV5-Based Gene Therapy Treatment for Hemophilia A. Blood. 2017;130(Suppl. 1):3332. [Google Scholar]
- 67.Calcedo R., Kuri-Cervantes L., Peng H., Qin Q., Boyd S., Schneider M. Immune Responses in 101HEMB01, a Phase 1/2 Open-Label, Single Ascending Dose-Finding Trial of DTX101 (AAVrh10FIX) in Patients with Severe Hemophilia B. Blood. 2017;130(Suppl 1):3333. [Google Scholar]