Abstract
Background
The determination of matrix metalloproteases (MMPs) is relevant in many pathophysiological conditions, especially if associated with extracellular matrix remodeling; however, the results obtained are closely linked to the method used and are not directly comparable. The aim of this study was to perform a reappraisal of quantitative gel zymography technique for MMPs in human plasma, to use for comparison with commercially available ELISA and in those experimental conditions where the MMP active form needs to be revealed.
Methods
The critical methodological parameters of zymography were checked and a comparison with a routinely used ELISA was performed.
Results
Sensitivity and reproducibility levels of zymography are suitable for detection of MMP‐9 in human plasma, providing results closely related to those obtained by ELISA.
Conclusions
Analytical parameters of zymography were suitable for detection of MMPs in human plasma. Quantitative zymography for MMPs is an alternative method for comparing the results of ELISA widely employed for MMP determination, thus reducing the discrepancies between laboratories regarding gelatinase assay.
Keywords: metalloproteases, gel zymography, ELISA, analytical performance
INTRODUCTION
Matrix metalloproteinases (MMPs) are a family of structurally related, zinc‐dependent enzymes mainly involved in the degradation of many components of the extracellular matrix (ECM) during both physiological and pathological processes 1, 2, 3.
On the basis of substrate specificity, sequence similarity, and domain organization, all MMPs can be divided into six groups 4; among these, at the clinical level, a diagnostic and prognostic value has been attributed mainly to gelatinases, MMP‐2 and MMP‐9, and increased expression and activity of these enzymes have been described to play a critical role in a variety of pathological conditions including neoplastic, cardiovascular, and respiratory diseases 5, 6, 7, 8, 9, 10.
MMPs generally consist of a signal peptide that directs its secretion from the cell, a prodomain essential for maintaining the pro‐MMP in a latent form, a catalytic domain containing the highly conserved Zn2+‐binding site, and a proline‐rich hinge region links the catalytic domain to the C‐terminal hemopexin‐like domain, which determines the substrate specificity of the MMP and mediates the interactions with endogenous inhibitors 11. MMPs are initially inactive enzymes containing an autoinhibitory hydrophobic propeptide domain; in fact, there is a cysteine thiol in the propeptide domain that binds to a Zn2+ ion the catalytic site, and this binding prevents MMPs from activating 12. MMPs are activated by many different agents, including reactive oxygen species, such as superoxide, released during inflammation 13. Although the fine regulation of MMP activity is very complex, also due to post‐translational modifications 14, 15, 16, their activity is mainly regulated by natural tissue inhibitors of MMP (TIMPs) found in the ECM and in the serum in the α2‐macroglobulin fraction, which bind tightly to active MMPs in a 1:1 ratio 17.
MMPs contribute to a variety of physiological mechanisms and imbalanced MMP activity is associated with many clinical conditions, including cardiovascular diseases 13, 18, 19, 20, where an excessive MMP activity and ECM turnover contribute to cardiovascular remodeling 20, 21, 22. In addition, MMPs degrade many intracellular substrates that are not components of the ECM. Importantly, activated MMP‐2 and MMP‐9 may degrade many components of cardiac cells and cause cardiac dysfunction 23. MMP‐2 degrades cardiac Troponin I, Myosin light chain 1, Titan, and other sarcomeric and cytoskeletal proteins thus causing myocardial contractile dysfunction 16, 24, 25. It has been demonstrated that during acute pulmonary embolism, the increase in MMP proteolytic activity was associated with increased cardiac Troponin I in serum, suggesting a possible role for MMPs in cardiomyocyte injury 26, 27.
The use of MMP‐2 and MMP‐9 plasma levels as biomarkers in different clinical settings is increasing due to their diagnostic and prognostic value 28, 29. Many different methods have been developed to assess the levels of active and latent forms of proteolitic enzymes, including MMP‐2 and MMP‐9 in peripheral circulation as well as in tissue or cellular extracts. Western blotting and gel zymography are the gold standard for detection of total and active MMP levels, respectively 30. The main limitation of these techniques is the small number of samples that can be processed at once and the poor feasibility for blood and culture medium assays. Immunometric and enzymatic assays for MMP measurement have been developed in the last few decades and are now widely used in experimental and clinical studies; these assays are easier to perform and are generally more sensitive compared to gel zymography and Western blotting, allowing a greater diffusion of MMP determination throughout laboratories. At present, besides the important effect of the biological matrix, the reliability of MMP quantification is affected by further problems, due to the different analytical systems based on MMPs’ different properties (immunometric or enzymatic), as well as the ability to recognize the different forms, pro‐ and active MMPs. Moreover, MMPs bound to their tissue inhibitors (TIMPs) and the MMPs/TIMPs complexes can be found in high concentrations in peripheral circulation 31, 32. These complexes are recognized to different extents by different immunometric and enzymatic assays for MMPs 33; thus, different analytical methods could lead to different results and these methodological differences have to be taken carefully into account when clinical/experimental data are compared. While immunometric systems recognize pro‐ and active MMPs together, the enzymatic assays, exploiting the biological properties of the active form of MMPs, are able to independently evaluate both the pro‐ and the active form of MMPs, after a preanalytical treatment of a biological sample 33. It is noteworthy that zymography is the technique of choice to directly and reliably measure the active form of MMPs 34. The aim of this study was to perform a reappraisal of quantitative gel zymography technique for gelatinases, considering MMP‐9 as a representative example. A comparison was made between the results obtained with zymography and those obtained by a widely used ELISA, whose analytical features had been previously evaluated by us 33.
MATERIALS AND METHODS
Blood Samples
Venous blood samples were obtained in presence of lithium‐heparin 31 from 25 volunteers recruited from the laboratory staff of our institute; blood was centrifuged at 1,000 × g for 15 min and stored in aliquots at −80°C. All determinations were performed within 1 month from blood collection. All subjects gave their written informed consent to be involved in the study, which was approved by the local ethics review committee.
Gel Zymography
Gel zymography is based on sodium dodecyl sulfate‐polyacrylamide gel electrophoresis (SDS‐PAGE) and on the use of gelatin 0.5% (Sigma‐Aldrich, St. Louis, MO) as substrate for detection of MMP enzymatic activity. Three different acrylamide/bisacrylamide ratios (12%, 10%, 7.5%), as well as the incubation time of the enzymatic reaction, were checked. Both recombinant MMP‐9 (R&D Systems, Minneapolis, MN) and molecular weight standards (Broad range marker, Bio‐Rad Laboratories, Hercules, CA) were used to identify the bands obtained by electrophoresed plasma samples. The specificity of MMP determination was checked by incubating the gel at 37°C in Tris‐HCl buffer, 100 mM pH 7.4, containing EDTA (Sigma‐Aldrich) 100 mM, a specific inhibitor of all the MMPs. The use of phenylmethanesulfonyl‐fluoride (PMSF; Sigma‐Aldrich) 100 mM, a serine‐protease inhibitor, allowed us to rule out possible nonspecific interference due to serine‐protease ability to digest gelatin. Within‐assay variability was also obtained by repeated measures of a plasma pool. Sensitivity was achieved by submitting serial dilutions (20 μl/well) of a known amount of a standard MMP‐9 solution (R&D Systems; 1.0 ng/ml) to gel zymography analysis in order to check the smaller detectable activity. The influence of repeated “freeze and thaw” cycles of plasma samples, known to affect MMP activity, was also checked in a small number of plasma samples in order to confirm the stability of MMP‐9 over time (2 and 4 months at −80°C after the first thawing cycle).
Plasma (20 μl/well) diluted (1:30) in Tris‐HCl 100 mM, pH 6.8, SDS 2%, glycerol 10% in presence of bromophenol blue was loaded on gel 7.5% for 1 hr at 20 mA (chosen experimental conditions). After electrophoresis, the gel was incubated for 1 hr at room temperature in Triton X‐100 2% on a rotary shaker. The Triton X‐100 was decanted and an incubation of 16–24 hr at 37°C with Tris‐HCl buffer 100 mM, pH 7.4 containing CaCl2 10 mM was performed. The gel was stained in Coomassie Brilliant Blue R‐250 0.5% (Bio‐Rad Laboratories) in methanol 40%, acetic acid 10% for 2 hr and then destained in methanol/acetic acid two times. Gelatinolytic activities were detected as unstained bands against the background of Coomassie Blue stained gelatin. In order to quantify the concentrations of the analyzed protein in the unknown samples, a dose–response curve was built in each run, by adding 20 μl/well of scalar solutions of MMP standards (R&D Systems) to the gel (20, 15, 10, and 5 pg/well, corresponding to 1.0–0.125 ng/ml). The degree of MMP digestion was quantified using a backlit scanner (scanner Epson expression 1680 pro) and the image density was determined by a specific image program (BioRad QuantityOne Software).
MMP‐9 Immunometric Assay
Total MMP‐9 was measured in 1:40 diluted plasma by specific immunometric assay (R&D Systems), previously standardized in our laboratory 33. In our conditions, the working range (analyte measured with a imprecision of less than 10%) was 0.19–16 ng/ml, the sensitivity was 0.05 ± 0.01 ng/ml, the within‐assay variability resulted 414.3 ± 23.5 ng/ml (5.7%) while the between‐assay variability was < 15%. The concentration of total MMP‐9 was measured in parallel using the zymography and ELISA for comparison purposes.
Statistical Analysis
All values of sample concentrations and the quality control parameters for the immunometric assay were calculated using a four‐parameter logistic function to interpolate the dose–response curve. The results of the two assay systems were compared by Pearson regression and paired t‐test. In the method comparison, because a high correlation between two methods does not mean that these methods have high degree of agreement, Bland–Altman analysis (MedCalc software) was also performed, where the differences of two competing measurements on the vertical axis were plotted against the mean of both on the horizontal axis 35. The mean difference is a measure for constant bias.
RESULTS
The results obtained by gel zymography analysis of human blood samples using different acrylamide/bis acrylamide ratios indicated that 7.5% gels are the more suitable for visualizing MMP‐9 forms (Fig. 1). In all conditions, four bands with gelatinolytic activity are found: MMP‐9 dimers, MMP‐9–TIMP‐1 complex, MMP‐9, and MMP‐2, respectively, as expected 31, 32, 36. The time course of the proteolytic activity of MMPs (Fig. 2A) indicated 16–24 hr as optimal incubation time 37. A 7.5% gel and an incubation time of 16–24 hr were used for routine assay. The complete inhibition of gelatinolytic activity by EDTA, 100 mM, confirmed that intensity of the bands was due only to MMP activity and no serine proteases are present in the samples, as demonstrated by the PMSF treatment (Fig. 2B). Figure 2C shows that repeated freeze and thaw cycles, during −80°C sample storage, induce a significant progressive decrease in MMP‐9 activity; a 50% decrease in the MMP‐9 activity was observed after the first freeze and thaw cycle while subsequent freeze and thaw cycles affected the activity value to a lesser extent. Figure 3 reports a representative example of within‐assay variability (22.1 ± 1.1 ng/ml, mean ± SEM, CV = 11.7%) (a) and dose–response curve (b).
Figure 1.
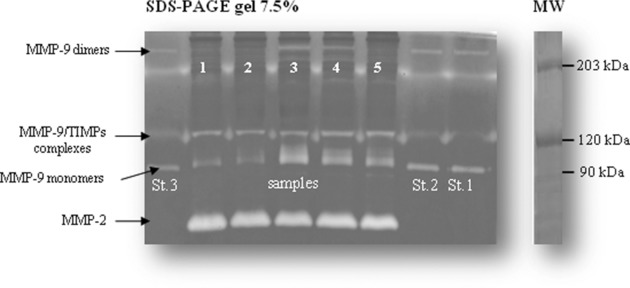
A representative separation of MMPs on a 7.5% polyacrylamide gel. St. 1–3 lanes correspond to standard MMP‐9; MW Marker lane is also depicted.
Figure 2.
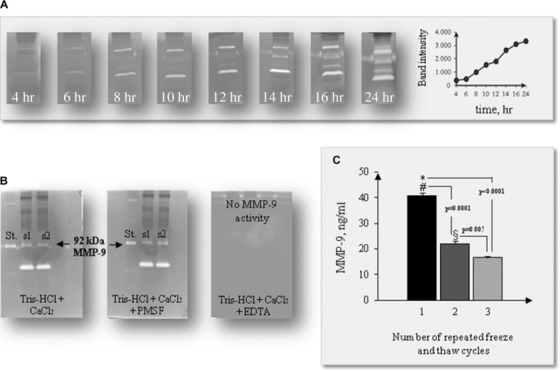
Time course of the proteolytic activity of MMPs (A); zymography specificity checked by EDTA and PMSF treatment (B); effects of freeze and thaw cycles on MMP‐9 activity (C).
Figure 3.
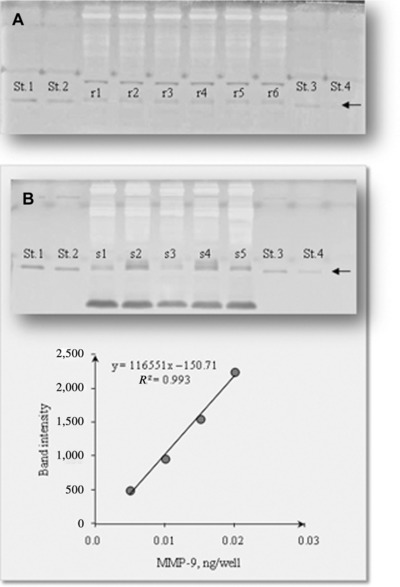
Within‐assay variability of a pool plasma sample (r1–r6) and MMP‐9 calibration curve (St. 1–St. 4) (A); a representative example of dose–response curve for MMP‐9 and the respective gel (B).
As to detection limit evaluation, gel band intensity obtained by serial dilutions of MMP‐9 standard protein are reported in Figure 4, allowing us to determine the detection limit of zymography of MMP‐9, which resulted about 2.5 pg/well, corresponding to 0.12 ng/ml.
Figure 4.
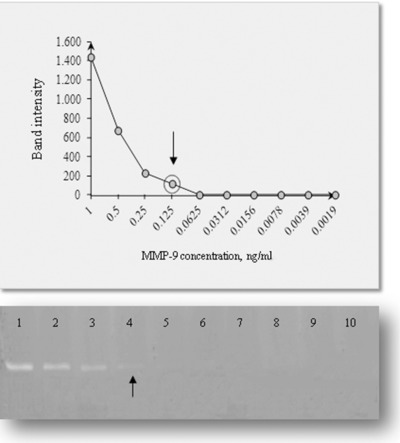
Detection limit determined by serial dilutions of a known amount of MMP‐9 standard. Lane density values and the corresponding gel zymography are depicted.
Finally, Figure 5 depicts the Pearson correlation and the Bland–Altman plot obtained for the blood MMP‐9 assay with the two procedures; a linear positive correlation was found between the MMP‐9 values of zymography and ELISA with the ELISA values significantly lower than those obtained with zymography (P = 0.0028). The Bland–Altman plot suggests the presence of an absolute systematic error (mean bias 6.3 ng/ml), probably due to intrinsic differences in assay methods.
Figure 5.
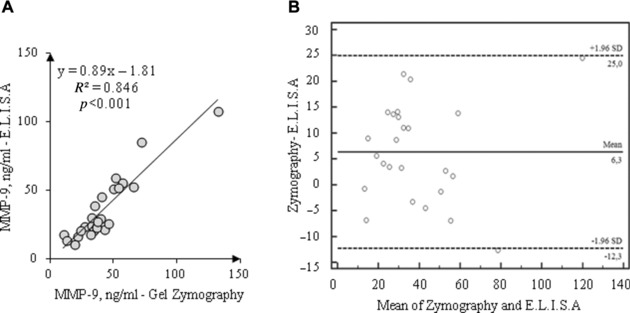
Comparison between ELISA and gel zymography for MMP‐9 (n = 25): Pearson correlation (A); Bland–Altman plot (B).
DISCUSSION
In this study, the main analytical features of gel zymography for gelatinases have been reported. Accuracy, reproducibility, and sensitivity have been checked and compared with an ELISA method routinely used in our laboratory 33. In our conditions, gel zymography allowed us to identify four bands with gelatinolytic activity, corresponding to MMP‐9 dimers, MMP‐9‐TIMP‐1 complex, MMP‐9, and MMP‐2 monomer forms, as derived by respective molecular weights (about 225, 130, 92 kDa for MMP‐9) 36. The specificity of the bands was verified first by addition of EDTA, known to specifically inhibit gelatinolytic activity; moreover, the presence of protease inhibitor PMSF ruled out any possible interference due to serine proteases. As to the preanalytical treatment, the time of sample storage at −80°C and the freeze and thaw cycles remain critical points 32, 37; for this, our samples were analyzed within 1 month from collection.
Due to calibration curve built in each run using MMP‐9 standard, the amount of monomer was quantified in the unknown samples. Variability and sensitivity levels resulted comparable to those of ELISA, as previously determined in our laboratory 33.
A positive relationship was found between the plasma concentrations of MMP‐9, measured by gel zymography, and those obtained by ELISA, although a small absolute systematic error (probably due to intrinsic differences in assay methods) has been revealed by Bland–Altman analysis.
Gel zymography, by the preliminary electrophoretic separation, is able to minimize possible interference such as that of TIMP/MMP complexes, known to be present in human blood and measured to different extents by ELISAs, although the use of diluted samples contributes to reducing preanalytical misinterpretations in immunometric assays. The preanalytical phase of the MMP assay is an issue of pivotal importance, strongly influencing the experimental values 37. Specially, the effect of the specimen collection in preanalytical variation of MMP in blood has been widely investigated: since 1996 Jung 38, for the assay of MMP‐1 by ELISA, already suggested using plasma heparin samples instead of serum or EDTA plasma in order to avoid nonspecific effects, probably due to interfering substances released during platelet aggregation. Jung also concluded in 1998 that “the clinician should be aware that the commutability of MMP values measured by ELISAs are impossible if different kinds of specimens are used” 39. This is true as confirmed again by Gerlach 40. At present, many studies on the role of MMPs in different clinical settings are performed using both plasma or serum samples 41, 42, 43, thus introducing a possible bias in MMP determination in blood, although a positive correlation between the results obtained in serum and plasma was described 44.
The main advantage of gel zymography is that it allows visualization of both the latent and active forms of gelatinases; thus, it is preferred in those experimental conditions that evaluate the activity of MMPs 34, 45. It is noteworthy that the presence of MMP dimers and MMP/TIMP complexes in the plasma samples, revealed by gel zymography, could thoroughly characterize the role of MMP in associated diseases, with important implications for future drug design 46. Moreover, gel zymography allows separation and visualization of MMP‐2 bands, so both MMP‐2 and MMP‐9 can be determined in the same run from a single biological sample. Due to its analytical features, gel zymography could be dedicated first to specific research studies targeting MMP function or as a reference method for evaluating the reliability of ELISA, as it is easy to use (less time‐consuming and able to measure a larger number of samples).
ACKNOWLEDGMENTS
The authors thank Dr. Ettore Balestreri, CNR Institute of Biophysics, Pisa, Italy. This work was financially supported by Italian Ministry of University and Research PRIN‐2008 grant (bioartificial stem cell niches for cardiac tissue engineering, 2010–2012).
Grant sponsor: Italian Ministry of University and Research PRIN‐2008.
REFERENCES
- 1. Woessner JF Jr. The family of matrix metalloproteinases. Ann NY Acad Sci 1994;732:11–21. [DOI] [PubMed] [Google Scholar]
- 2. Egeblad M, Werb Z. New functions for the matrix metallopreoteases in cancer progression. Nat Rev Cancer 2002;2:161–174. [DOI] [PubMed] [Google Scholar]
- 3. Chakraborti S, Mandal M, Das S, Mandal A, Chakraborti T. Regulation of matrix metalloproteases: An overview. Mol Cell Biochem 2003;253:269–285. [DOI] [PubMed] [Google Scholar]
- 4. Visse R, Nagase H. Structure, function, and biochemistry of matrix metalloproteinases and tissue inhibitors of metalloproteinases. Circ Res 2003;92:827–839. [DOI] [PubMed] [Google Scholar]
- 5. Farias E, Ranuncolo S, Cresta C, et al. Plasma metalloproteinase activity is enhanced in the euglobulin fraction of breast and lung cancer patients. Int J Cancer 2000;89:389–394. [DOI] [PubMed] [Google Scholar]
- 6. Ranuncolo SM, Armanasco E, Cresta C, Bal De Jier Joffe E, Puricelli L. Plasma MMP‐9 (92 KDa‐MMP) activity is useful in the follow‐up and in assessment of prognosis in breast cancer patients. Int J Cancer 2003;106:745–751. [DOI] [PubMed] [Google Scholar]
- 7. Blankenberg S, Rupprecht HJ, Poirier O, et al. Plasma concentrations and genetic variation of matrix metalloproteinase 9 and prognosis of patients with cardiovascular disease. Circulation 2003;107:1579–1585. [DOI] [PubMed] [Google Scholar]
- 8. Altieri P, Brunelli C, Garibaldi S, et al. Metalloproteinases 2 and 9 are increased in plasma of patients with heart failure. Eur J Clin Invest 2004;33:648–656. [DOI] [PubMed] [Google Scholar]
- 9. Lynch JR, Blessing R, White WD, Grocott HP, Newman MF, Laskowitz DT. Novel diagnostic test for acute stroke. Stroke 2004;35:57–59. [DOI] [PubMed] [Google Scholar]
- 10. Ohbayashi H, Matrix metalloproteinases in lung diseases. Curr Protein Pept Sci 2002;3:409–421. [DOI] [PubMed] [Google Scholar]
- 11. Overall CM, Molecular determinants of metalloproteinase substrate specificity: Matrix metallo‐proteinase substrate binding domains, modules, and exosites. Mol Biotechnol 2002;22:51–86. [DOI] [PubMed] [Google Scholar]
- 12. Nagase H, Visse R, Murphy G. Structure and function of matrix metalloproteinases and TIMPs. Cardiovasc Res 2006;69:562–573. [DOI] [PubMed] [Google Scholar]
- 13. Galis ZS, Khatri JJ. Matrix metalloproteinases in vascular remodeling and atherogenesis: The good, the bad, and the ugly. Circ Res 2002;90:251–262. [PubMed] [Google Scholar]
- 14. Hadler‐Olsen E, Fadnes B, Sylte I, Uhlin‐Hansen L, Winberg JO. Regulation of matrix metalloproteinase activity in health and disease. Febs J 2011;278:28–45. [DOI] [PubMed] [Google Scholar]
- 15. Castro MM, Kandasamy AD, Youssef N, Schulz R. Matrix metalloproteinase inhibitor properties of tetracyclines: Therapeutic potential in cardiovascular diseases. Pharmacol Res 2011; 64:551–560. [DOI] [PubMed] [Google Scholar]
- 16. Kandasamy AD, Chow AK, Ali MA, Schulz R. Matrix metalloproteinase‐2 and myocardial oxidative stress injury: Beyond the matrix. Cardiovasc Res 2009;85:413–423. [DOI] [PubMed] [Google Scholar]
- 17. Brew K, Dinakarpandian D, Nagase H. Tissue inhibitors of metalloproteinases: Evolution, structure and function. Biochim Biophys Acta 2000;1477:267–283. Review. [DOI] [PubMed] [Google Scholar]
- 18. Spinale FG, Myocardial matrix remodeling and the matrix metalloproteinases: Influence on cardiac form and function. Physiol Rev 2007;87:1285–1342. [DOI] [PubMed] [Google Scholar]
- 19. Castro MM, Tanus‐Santos JE, Gerlach RF. Matrix metalloproteinases: Targets for doxycycline to prevent the vascular alterations of hypertension. Pharmacol Res 2011;64:567–572. [DOI] [PubMed] [Google Scholar]
- 20. Caruso R, Caselli C, Boroni C, et al. Relationship between myocardial redox state and matrix metalloproteinase activity in patients on left ventricular assist device support. Circ J 2011;75:2387–2396. [DOI] [PubMed] [Google Scholar]
- 21. Castro MM, Rizzi E, Prado CM, Rossi MA, Tanus‐Santos JE, Gerlach RF. Imbalance between matrix metalloproteinases and tissue inhibitor of metalloproteinases in hypertensive vascular remodeling. Matrix Biol 2010;29:194–201. [DOI] [PubMed] [Google Scholar]
- 22. Rizzi E, Castro MM, Prado CM, et al. Matrix metalloproteinase inhibition improves cardiac dysfunction and remodeling in 2‐ kidney, 1‐clip hypertension. J Card Fail 2010;16:599–608. [DOI] [PubMed] [Google Scholar]
- 23. Chow AK, Cena J, Schulz R. Acute actions and novel targets of matrix metalloproteinases in the heart and vasculature. Br J Pharmacol 2007;152:189–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Schulz R. Intracellular targets of matrix metalloproteinase‐2 in cardiac disease: Rationale and therapeutic approaches. Annu Rev Pharmacol Toxicol 2007;47:211–242. [DOI] [PubMed] [Google Scholar]
- 25. Uzuelli JA, Dias‐Junior CA, Tanus‐Santos JE. Severity dependent increases in circulating cardiac troponin I and MMP‐9 concentrations after experimental acute pulmonary thromboembolism. Clin Chim Acta 2008;388:184–188. [DOI] [PubMed] [Google Scholar]
- 26. Neto‐Neves EM, Kiss T, Muhl D, Tanus‐Santos JE. Matrix metalloproteinases as drug targets in acute pulmonary embolism. Curr Drug Targets 2013;14:344–352. [DOI] [PubMed] [Google Scholar]
- 27. Neto‐Neves EM, Dias‐Junior CA, Rizzi E, et al. Metalloproteinase inhibition protects against cardiomyocyte injury during experimental acute pulmonary thromboembolism. Crit Care Med 2011;39:349–356. [DOI] [PubMed] [Google Scholar]
- 28. Blankenberg S, Rupprecht HJ, Poirier O, et al. Plasma concentrations and genetic variation of matrix metalloproteinase 9 and prognosis of patients with cardiovascular disease. Circulation 2003;107:1579–1585. [DOI] [PubMed] [Google Scholar]
- 29. Marchesi C, Dentali F, Nicolini E, et al. Plasma levels of matrix metalloproteinases and their inhibitors in hypertension: A systematic review and meta‐analysis. J Hypertens 2012;30:3–16. [DOI] [PubMed] [Google Scholar]
- 30. Kleiner DE, Stetler‐Stevenson WG. Quantitative zymography: Detection of picogram quantities of gelatinases. Anal Biochem 1994;218:325–329. [DOI] [PubMed] [Google Scholar]
- 31. Lein M, Nowak L, Jung K, et al. Analytical aspect regarding the measurement of matrix metalloproteinases and their inhibitors in blood. Clin Biochem 1997;30:491–496. [DOI] [PubMed] [Google Scholar]
- 32. Rouy D, Ernens I, Jeanty C. Wagner DR. Plasma storage at −80°C does not protect matrix metalloproteinases from degradation. Anal Biochem 2005;338:294–298. [DOI] [PubMed] [Google Scholar]
- 33. Colotti C, Angeli V, Del Ry S, Maltinti M, Vittorini S, Giannessi D. Matrix metalloproteinases‐2 and ‐9 concentration and activity in serum and culture medium samples: A methodological reappraisal. Clin Chem Lab Med 2007;45:1292–1298. [DOI] [PubMed] [Google Scholar]
- 34. Cristallini C, Gagliardi M, Barbani N, Giannessi D, Guerra GD. Novel biodegradable, biomimetic and functionalised polymer scaffolds to prevent expansion of post‐infarct left ventricular remodeling. J Mater Sci Mater Med 2012;23:205–216. [DOI] [PubMed] [Google Scholar]
- 35. Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;1:307–310. [PubMed] [Google Scholar]
- 36. Mannello F, Tonti GA, Tanus‐Santos JE, Gerlach RF. Silicate increases the release of MMP‐9 forms in peripheral blood: Why gelatin zymography differs significantly in citrate plasma and serum obtained with or without clot activators. Clin Chem. 2007;53:1981–1982. [DOI] [PubMed] [Google Scholar]
- 37. Souza‐Tarla C, Uzuelli JA, Machado AA, Tanus‐Santos JE. Methodological issues affecting the determination of plasma matrix metalloproteinase (MMP)‐2 and MMP‐9 activities. Clin Biochem 2005;38:410–414. [DOI] [PubMed] [Google Scholar]
- 38. Jung K, Nowak L, Lein M, Henke W, Schnorr D, Loening SA. Role of specimen collection in preanalytical variation of metalloproteinases and their inhibitors. Clin Chem 1996;42:2043–2044. [PubMed] [Google Scholar]
- 39. Jung K, Laube C, Lein M, et al. Kind of sample as preanalytical determinant of matrix metalloproteinases 2 and 9 (MMP2; MMP9) and tissue inhibitor of metalloproteinase 2 TIMP2 in blood. Clin Chem 1998;44:1060–1062. [PubMed] [Google Scholar]
- 40. Gerlach RF, Uzuelli JA, Souza‐Tarla CD, Tanus‐Santos JE. Effect of anticoagulants on the determination of plasma matrix metalloproteinase (MMP)‐2 and MMP‐9 activities. Anal Biochem. 2005;344:147–149. [DOI] [PubMed] [Google Scholar]
- 41. Abaci O, Kocas C, Kilickesmez KO, Uner S, Kucukoglu S. Matrix metalloproteinase‐2 and ‐9 levels in patients with dilated ascending aorta and bicuspid aortic valve. Echocardiography 2013;30:121–126. [DOI] [PubMed] [Google Scholar]
- 42. Tanaka K, Essick EE, Doros G, et al. Circulating matrix metalloproteinases and tissue inhibitors of metalloproteinases in cardiac amyloidosis. J Am Heart Assoc 2013;2:e005868:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Cojocarui IM, Cojocaru M, Sapira V, Socoliuc G, Hertea C, Paveliu S. Changes in plasma matrix metalloproteinase‐9 levels in patients with acute ischemic stroke. Rom J Intern Med 2012;50:155–158. [PubMed] [Google Scholar]
- 44. Gerlach RF, Meschiari CA, Marcaccini AM, et al. Positive correlations between serum and plasma matrix metalloproteinase (MMP)‐2 or MMP‐9 levels in disease conditions. Clin Chem Lab Med 2009;47:888–891. [DOI] [PubMed] [Google Scholar]
- 45. Snoek‐van Beurden P, Von de Hoff JH. Zymographic technique for the analysis of matrix metalloproteinases and their inhibitors. Biotechniques 2005;38:73–83. [DOI] [PubMed] [Google Scholar]
- 46. Dufour A, Zucker S, Sampson NS, Kuscu C, Cao J. Role of matrix metalloproteinase‐9 dimers in cell migration: Design of inhibitory peptides. J Biol Chem. 2010;285:35944–35956. [DOI] [PMC free article] [PubMed] [Google Scholar]


