Abstract
Background
Diarrheagenic Escherichia coli (DEC) strains are important causes of diarrhea. However, they cannot be distinguished from E. coli of the intestinal microbiota by conventional microbiological tests.
Methods
This work presents a two‐system multiplex PCR for detection of DEC. Primers for 16S rRNA gene were added as internal amplification control to validate negative reactions. The multiplex‐PCR system 1 contains primers for detection of Shiga toxin producing E. coli (STEC; stx1, stx2), enteropathogenic E. coli (EPEC; eae, bfpA), atypical enteropathogenic E. coli (aEPEc; eae), enteroinvasive E. coli (ETEC; lt, st), enteroinvasive E. coli (EIEC; ial), and the internal amplification control 16S rRNA. The system 2 contains primers for EIEC (ipaH), enteroaggregative E. coli (CVD432), diffusely adherent E. coli (daaE), and 16S rRNA. The protocol was tested with E. coli reference strains, and also with cultures of fecal specimens of people with diarrhea and healthy controls.
Results
The protocol correctly identified the DEC reference strains. No DEC marker was amplified for negative controls; these results were validated by the amplification of a fragment of the 16S rRNA gene. The frequency of DEC was 7.6% for both patients and healthy controls; two Shigella sonnei strains were detected in the group with diarrhea. The identity of the amplicons was confirmed by DNA sequencing.
Conclusion
The protocol is specific for DEC Shigella and is suitable for clinical laboratories. J. Clin. Lab. Anal. 27:155–161, 2013. © 2013 Wiley Periodicals, Inc.
Keywords: diarrhea, enteropathogens, molecular diagnosis, internal PCR control, molecular method
INTRODUCTION
Escherichia coli is the most abundant facultative anaerobe of the human intestinal microflora. However, several diarrheagenic pathotypes of E. coli are recognized: enteropathogenic E. coli (EPEC), atypical enteropathogenic E. coli (aEPEC), Shiga toxin producing E. coli (STEC), enterotoxigenic E. coli (ETEC), enteroaggregative E. coli (EAEC), enteroinvasive E. coli (EIEC), and diffusely adherent E. coli (DAEC; 1, 2. Recently, strains with mixed characteristics of STEC and EAEC have also been described and associated with severe disease 3. The diarrheagenic strains are distinguished by their specific virulence factors 1. EPEC contain the pathogenicity island LEE, associated with the attaching and effacing (A/E) lesions on intestinal cells, and the EAF virulence plasmid (EPEC adherence factor) that encodes the bundle forming pilus (BFP), associated with the localized adherence pattern of EPEC in HeLa and HEp‐2 cells 1, 4. aEPEC contain the LEE island but lack the EAF plasmid 1, 2, 5. ETEC is distinguished by the production of the heat‐labile enterotoxin (LT) and/or the heat‐stable enterotoxins (ST; 4. In Shigella, which are now considered as forms of E. coli 6, and EIEC virulence is largely due to a 220 kb virulence plasmid that encodes a T3SS on the Mxi‐Spa locus that is required for invasion, cell survival, and apoptosis of macrophages 7. STECs are characterized by the production of Shiga toxins Stx1 and/or Stx2, which have the ability to inhibit protein synthesis in eukaryotic cells, can be detected by the cytotoxic effect on Vero cells 1, 8, 9. Some STEC strains may also contain the LEE pathogenicity island 1, 9. EAECs are defined as E. coli that do not secrete LT or ST and adhere to HEp‐2 cells in a pattern known as autoaggregative that is mediated by aggregative adherence fimbriae (AAFs), related to the Dr family of adhesins, encoded in virulence plasmids called pAA. EAEC adherence to intestinal mucosa is characterized as a biofilm composed of aggregates of bacteria in association with a thick mucus layer that may promote persistent infection 4, 7. Dispersin is a protein produced by EAEC that decreases bacterial autoaggregation, allowing its dispersion along the intestinal mucosa. Dispersin is secreted by an ABC protein transport system coded by a cluster of genes designated aat‐PABCD, present in plasmid pAA. Transcription of the dispersin gene and the aat cluster is dependent on AggR, a regulator of virulence genes in EAEC 10. It was shown that the sequence of the probe CVD432, originally used to detect EAEC in hybridization assays, corresponds to a region in the pAA coding for the dispersin secretion apparatus 10, 11.
DAEC are characterized by the presence of a diffuse pattern of adherence to HEp‐2 cells 4, which is mediated by fimbrial (F1845, Dr) and afimbrial (Afa) adhesins. Unlike other pathogenic E. coli, the pathogenesis of DAEC seems to be predominately mediated through Afa–Dr adhesin interactions with host cells 7.
The diagnosis of diarrheagenic E. coli (DEC) is hampered by the fact that they are indistinguishable from commensal strains based on biochemical tests and serotypic markers are rarely sufficient to reliably identify a strain as diarrhoeagenic. Identification of DEC requires the use of immunological assays, cell culture, or molecular techniques 4, 12, 13. We have developed a two‐system multiplex assay containing primers targeting markers of each DEC category and an internal amplification control to validate the negative results. The assay was tested with E. coli reference strains, and also with cultures of fecal specimens of people with diarrhea and healthy controls.
MATERIALS AND METHODS
Bacterial Strains
Reference strains used as positive controls were IAL 307 (O124:K72, EIEC), IAL 2391 (EAEC), C1845 (DAEC), E2348/69 (EPEC), H10407 (ETEC), and EDL 933 (STEC). Strains E. coli ATCC 25922 and E. coli DH10B were used as negative controls. Clinical isolates of STEC, M03, and J307 14, and of Klebsiella pneumoniae, Shigella sonnei, Salmonella ser. Thyphimurium, Enterobacter aerogenes, Enterobacter cloacae, Providencia rettgeri, Citrobacter freundii, Serratia marcescens, Pseudomonas aeruginosa, and Staphylococcus aureus were also tested.
Feces Specimens
We analyzed the fecal samples of 250 sequential outpatients (children and adults) with diarrhea, which were submitted to feces culture in a clinical laboratory at Curitiba‐PR, Brazil. The 250 controls (children and adults) were selected among healthy subjects, without diarrhea and disease complaints, submitted to routine checkup. Feces samples were collected in Cary Blair transport medium and maintained under refrigeration.
Preparation of DNA Samples
Fecal samples were inoculated on MacConkey agar (Oxoid; Basingstoke, UK) plates and, after incubation overnight at 36°C, a loopful of the confluent region of growth was resuspended in 500 μl of sterile water and used for DNA extraction by the boiling method. The extracts were centrifuged at 14,000 rpm for 2 min and 3 μl of the supernatants were used in each one of the multiplex‐PCR systems. Screening for DEC was performed using a pool of colonies. For samples positive in the screening step, that is, presenting bands of DNA amplification according to Table 1, a second step was performed to identify the colonies containing the DEC markers. In this step up to 100 isolated colonies of each sample, including lactose positive and lactose negative, were selected, used for DNA extraction, and tested individually by single PCR for the specific DEC marker (Table 1), according to the result in the screening step. The colonies confirmed as DEC were then stored at −20°C for additional testing.
Table 1.
Primers Used for Detection of Diarrheagenic E. coli and Internal Amplification Control
| DEC | Primer | Sequence | Amplicon | |
|---|---|---|---|---|
| group | (concentration μM) | (5′–3′) | size (pb) | Reference |
| STEC | stx1 | F‐CTGGATTTAATGTCGCATAGTG | 150 | 15, 16 |
| (0.4) | R‐AGAACGCCCACTGAGATCATC | |||
| stx2 | F‐GGCACTGTCTGAAACTGCTCC | 255 | 15 | |
| (0.48) | R‐TCGCCAGTTATCTGACATTCTG | |||
| EPEC | bfpA | F‐AATGGTGCTTGCGCTTGCTGC | 324 | 17 |
| (0.48) | R‐GCCGCTTTATCCAACCTGGTA | |||
| eae | F‐GACCCGGCACAAGCATAAGC | 384 | 15 | |
| (0.48) | R‐CCACCTGCAGCAACAAGAGG | |||
| ETEC | lt | F‐GGCGACAGATTATACCGTGC | 450 | 16 |
| (0.48) | R‐CGGTCTCTATATTCCCTGTT | |||
| st | F‐ATTTTTMTTTCTGTATTRTCTT | 190 | 17 | |
| (0.48) | R‐CACCCGGTACARGCAGGATT | |||
| EIEC | ial | F‐GGTATGATGATGATGAGTCCA | 650 | 16 |
| (0.6) | R‐GGAGGCCAACAATTATTTCC | |||
| ipaH | F‐GTTCCTTGACCGCCTTTCCGATACCGTC | 600 | 18 | |
| (0.48) | R‐GCCGGTCAGCCACCCTCTGAGAGTAC | |||
| EAEC | CVD432 | F‐CTGGCGAAAGACTGTATCAT | 630 | 18 |
| (0.48) | R‐CAATGTATAGAAATCCGCTGTT | |||
| DAEC | daaE | F‐GAACGTTGGTTAATGTGGGGTAA | 542 | 19 |
| (0.48) | R‐TATTCACCGGTCGGTTATCAGT | |||
| Internal | 16S | F‐CCAGCAGCCGCGGTAATACG | 996 | 20 |
| Control | (0.48) | R‐ATCGGYTACCTTGTTACGACTTC |
Multiplex PCR
Primers for DEC strains described in literature were analyzed and selected to compose the two PCR systems according to the ΔG value calculated by OligoAnalyzer (www.idtdna.com) to avoid heterodimer formation. The sequences of the primers selected are indicated in Table 1. PCR reactions were performed in a final volume of 25 μl, containing Taq DNA Polymerase buffer 1× (Invitrogen, Foster City, CA), 1.5 mM MgCl2, 0.2 mM dNTP, 1 U of Taq DNA polymerase platinum (Invitrogen), and 3 μL of template DNA prepared as above. The concentration of each pair of primers (Table 1) was adjusted empirically to obtain DNA bands of similar intensities with controls. The multiplex‐PCR system 1 contained primers for the detection of STEC (stx1, stx2), EPEC (eae, bfpA), aEPEc (eae), ETEC (lt, st), EIEC (ial, which corresponds to the spa 9 gene, a component of the Mxi‐Spa secretion machinery), and the internal control 16S rRNA. The system 2 contained primers for EIEC (ipaH, invasion plasmid antigen), EAEC (CVD432, aatA gene), DAEC (daaE, gene required for expression of the F1845 fimbriae), and 16S rRNA. The cycling programs used were 1 cycle at 94°C (4 min), 35 cycles at 94°C (1 min), 55°C (1 min), 72°C (1 min), and a final cycle at 72°C (5 min) for multiplex‐PCR system 1; for system 2, the conditions were the same except the annealing temperature that was 58°C (1 min). The reactions were performed in a Biocycler MJ96G thermocycler. Detection of PCR products was by electrophoresis in 2% agarose gel stained with ethidium bromide and visualized under UV light. The sensitivity of the multiplex‐PCR protocol was tested with serial dilutions of reference DEC strains in the range of 108–10 CFU/ml.
Biochemical Identification of the Strains
Strains harboring virulence markers of DEC were identified using the API‐20E strips and APIWEB (bioMérieux, Marcy‐l'Etoile, France) according to the manufacturer's instructions.
DNA Sequencing
For each strain, amplified DNA samples containing DEC markers were sequenced using the DYEnamic ET Dye Terminator Cycle Sequencing kit (GE Healthcare, Piscataway, NJ) and the automatic DNA sequencer ABI Prism 377 (Applied Biosystems, Foster City, CA) to confirm the identity of the fragment. Sequences were analyzed using BioEdit Sequence Alignment Editor 21, BLASTn 22 and CLUSTAL W (http://www.ebi.ac.uk/Tools/msa/clustalw2).
Serotyping
Escherichia coli strains were serotyped using O (O1–O181) and H (H1–H56) antisera prepared at the Adolfo Lutz Institute (São Paulo, Brazil).
Cell Cytotoxicity Assay
stx production was determined by the Vero cell cytotoxicity assay 23. Briefly, the strains were grown overnight in Penassay broth (Antibiotic Medium no. 3, Difco, Sparks, NV), and then centrifuged (14,000 rpm, 2 min). The resulting cell‐free supernatants were filtered with a 0.22‐μm membrane and stored at −20°C. Vero cells were seeded (1 × 103) into 96‐well plates and maintained in RPMI medium (Gibco, Paisley, UK) containing 2 mM glutamine, 40 μg/ml of gentamicin, 2.5 μg/ml amphotericin B, 10 μg/ml of ciprofloxacin, and 10% fetal bovine serum. The cells were grown at 37°C in a 5% CO2 atmosphere for 24 hr. Sterile supernatants (25 μl) were added to the wells and incubated under the same cell growth conditions described above for 72 hr. Cytotoxic effects such as cell disruption and detachment were observed under the microscope. STEC EDL 933 and E. coli DH10B strains were used as positive and negative control, respectively. This study was approved by the Ethical Committee of our institution.
RESULTS
Sensitivity and Specificity of the Method
The multiplex‐PCR protocol was able to detect all the E. coli diarrheagenic pathotypes (Fig. 1). The detection limit was 104 CFU/ml for both PCR systems. STEC clinical control strains M03 and J307, and the S. sonnei strain were also correctly identified. As expected, the protocol did not distinguish between EIEC and Shigella, since both are positive for ipaH and ial. No amplification for DEC gene markers was seen for other clinical control strains tested, showing the specificity of the method. The negative results were validated by the amplification of the internal control.
Figure 1.
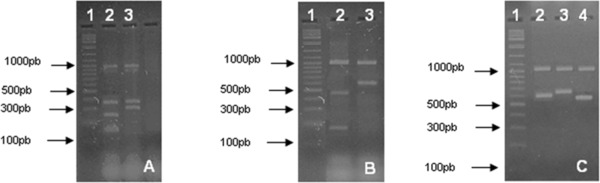
Multiplex‐PCR amplification pattern for DEC positive controls. (A) (1) 100 pb DNA ladder (Fermentas), (2) STEC (stx1 stx2 eae), (3) EPEC (eae, bfpA). (B) (1) 100 pb DNA ladder (Fermentas), (2) ETEC (lt, st), (3) EIEC (ial). (C) (1) 100 pb DNA ladder (Fermentas), (2) EIEC (ipaH), (3) EAEC (CVD432), (4) DAEC (daaE). A band of approximately 1,000 pb seen in all gels correspond to the internal amplification control 16S rRNA gene. (A) and (B) correspond to the multiplex‐PCR system 1 target genes, and (C) to system 2 target genes.
Rate of Detection of DEC Among Fecal Specimens
Among diarrheal samples a total of 19 DEC (7.6%), belonging to aEPEC (ten strains), EAEC (six strains), STEC, DAEC, and EIEC (one strain each), and two S. sonnei strains (0.8%) were recovered. Nineteen DEC strains (7.6%) were also isolated from the control samples, which corresponded to aEPEC (seven strains), EAEC (eight strains), STEC (three strains), and DAEC (one strain). The same rate of isolation of DEC was seen in both groups. Figure 2 shows the DNA amplification pattern of some of these strains.
Figure 2.
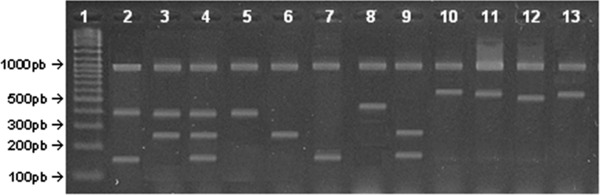
Multiplex‐PCR amplification pattern observed for some DEC strains detected. (1) 100 pb DNA ladder (Fermentas). (2–4), (6 and 7), and (9), respectively, STEC strains 107 CS, 44 CS, 62 CS, 150 CS, 149 D, and 10 D. (5) Strain 49 D (aEPEC). (8) Strain 116 D (ETEC). (10 and 11) Strain 29 D (EIEC, systems 1 and 2, respectively). (12) Strain 133 D (DAEC). (13) Strain 239 D (EAEC).
Characterization of the DEC Strains
DNA sequences of the amplified fragments confirmed the presence of virulence genes in these bacteria. Sequences were submitted to GenBank under accession numbers for aEPEC, JQ638605–JQ638623; EAEC, JQ638624–JQ638637; EIEC, JQ638638 and JQ638641; and Shigella, JQ638639, JQ638640, JQ638642, and JQ638643. Sterile supernatants of all but one STEC strains (150 CS, where CS indicates the isolates from healthy controls) were cytotoxic to Vero cells (detachment/disruption). Virulence markers and serotype are indicated in Table 2.
Table 2.
Characteristics of Diarrheagenic E. coli Isolates
| Straina | DEC pathotype | Serotype |
|---|---|---|
| 3 CS | eae/aEPEC | ONT:H‐ |
| 18 CS | CVD432/EAEC | ONT:H2 |
| 21 CS, 56 CS, 66 CS | CVD432/EAEC | OR:H‐ |
| 65 CS | eae/aEPEC | ONT:H8 |
| 77 CS, 133 D | daaE/DAEC | O25:H4 |
| 78 CS | eae/aEPEC | OR:H4 |
| 88 CS | stx2/STEC | ONT:H16 |
| 94 CS | eae/aEPEC | ONT:H6 |
| 107 CS | stx1, eae/STEC | O177:H‐ |
| 128 CS | eae/aEPEC | O177:H11 |
| 132 CS | eae/aEPEC | O51:H40 |
| 150 CS | stx2/STEC | ONT:H9 |
| 173 CS, 141 D | CVD432/EAEC | O3:H2 |
| 179 CS, 245 D | CVD432/EAEC | ONT:H4 |
| 191 CS | CVD432, EAEC | OR:H2 |
| 225 CS | eae/aEPEC | ONT:H7 |
| 226 CS, 75 D | CVD432/EAEC | O25:H4 |
| 10 D | stx1, stx2/STEC | ONT:H16 |
| 28 D | eae/aEPEC | O157:H16 |
| 29 D | ipaH, ial/EIEC | O144:H‐ |
| 49 D | eae/aEPEC | O177:H‐ |
| 85 D | eae/aEPEC | O91:H14 |
| 86 D | eae/aEPEC | O145:H28 |
| 171 D | CVD432/EAEC | ONT:H25 |
| 172 D | eae/aEPEC | O25:H4 |
| 190 D | eae/aEPEC | O127:H40 |
| 200 D | eae/aEPEC | ONT:HNT |
| 213 D | eae/aEPEC | O108:H21 |
| 216 D | eae/aEPEC | O137:H6 |
| 217 D | CVD432/EAEC | O3:H‐ |
| 237 D | eae/aEPEC | OR:H40 |
| 239 D | CVD432/EAEC | ONT:H28 |
CS indicates the isolates from healthy controls; D indicates isolates from person with diarrhea; aEPEC, atypical enteropathogenic E. coli; EAEC, enteroaggregative E. coli; DAEC, diffusely adherent E. coli; STEC, Shiga toxin producing E. coli; EIEC, enteroinvasive E. coli (EIEC).
Two additional strains (25 D and 27 D) were isolated from people with diarrhea, both were positive for ipaH and ial but were identified as Shigella sonnei by biochemical tests.
DISCUSSION
DECs are important causes of diarrhea 24, 25, 26, 27, 28, 29, 30, however their detection depends on the use of methods able to distinguish them from the intestinal commensal E. coli. Several strategies have been described for detection and characterization of DEC based mainly on the detection of the virulence‐associated characteristics 4, 13. There are several multiplex‐PCR protocols described, some of them are able to detect most of DEC 16, 18, 25, 31, 32, 33, 34 but do not include primers for identification of EAEC and/or DAEC. Other systems detect all DEC 19, 35 but do not include an internal PCR control.
This work describes a two‐system multiplex PCR for detection of DEC, which includes an internal PCR control. Together the two PCR systems contain ten pairs of primers targeting markers of the seven DEC pathotypes. In both systems, the internal amplification control consists of a pair of primers for the 16S rRNA gene 20, a nontarget DNA sequence present in the same sample tube, which is co‐amplified simultaneously with the target sequence. The presence of primers for nontarget DNA sequences is essential to validate a negative result, which may be due to inhibition of the PCR by malfunction of the thermocycler, incorrect PCR mixture, poor DNA polymerase activity, or the presence of inhibitors in the sample. When a PCR containing an internal amplification control is used, the corresponding control band should always be produced, and its absence reveals failure in the PCR 36. Persson et al. 37 developed a multiplex PCR for DEC including the 16S rRNA gene as an internal PCR control, however their protocol does not detect EAEC and DAEC, which is possible in the system presented in this study.
The multiplex‐PCR system used in the present work correctly identified the DEC reference strains (Fig. 1), and reactions without DNA amplification for DEC markers were observed in the negative controls. The correct functioning of the PCR in these negative reactions was monitored by the amplification of a DNA band of 996 bp corresponding to a fragment of the bacterial 16S rRNA gene 20. Besides, the multiplex‐PCR protocol was used to test cultures of 250 feces specimens of people with diarrhea and 250 healthy controls. The frequency of DEC was 7.6% for both patient and healthy control groups (Table 2). Two strains of S. sonnei were also found in the group with diarrhea. DNA sequencing confirmed the identity of the amplicons indicating that the multiplex‐PCR protocol is specific for DEC markers. The similar frequencies of DEC found between the two groups analyzed is in contrast with other studies that found higher DEC frequencies in patients with diarrhea 29, 38, 39. This is probably due to differences in the sample characteristics, since in those studies restrictive criteria, such as 3–4 emissions per day, blood presence in feces, among others, were used to include a sample in the diarrheal group. The DEC frequency found in the diarrheal group (7.6%) was lower than those observed in some studies 24, 25, 29, 38, 39, which reported frequencies ranging from 16% to 33%. This may also be due to the criteria used to define diarrhea and to the sample composition since those studies included only children, the main age group affected by enteropathogens. The most frequent pathotypes recovered were aEPEC (4% and 2.8%, respectively, for diarrheal and nondiarrheal samples) and EAEC (2.4% and 3.2%, respectively, for diarrheal and nondiarrheal samples). These results are in agreement with others indicating that aEPEC and EAEC are the most prevalent pathotypes 25, 26, 27, 29, 39. Other pathotypes were found in low frequencies. STEC strains were found in 0.4% and 1.2% of diarrheal and nondiarrheal samples, respectively. Interestingly, STEC strain 150 CS that contained a stx2 type gene did not show cytotoxic effects in the Vero cells assay suggesting that it does not express stx2. This was also observed in other studies 40, 41, 42, 43, some of which detected the presence of the insertion sequence IS1203 interrupting the coding region of the stx2 genes resulting in the inactivation of the genes and absence of cytotoxic effects 40, 41, 43. DAEC frequency was 0.4% in both groups and EIEC was found only in the diarrheal samples with a frequency of 0.4% (Table 2). ETEC and typical EPEC were not found. The absence of typical EPEC is in agreement with other studies undertaken in Brazil, which found low frequencies or absence of this DEC pathotype 24, 26. Most of the DEC isolates belonged to distinct serogroups (Table 2). Interestingly, among the 19 strains isolated from the control group 13 were ONT (nontypeable) or OR (rough), while among those recovered from diarrheal group, 13 had their somatic antigens identified (Table 2) and only six were ONT (nontypeable) or OR (rough). The most common serotype identified was O25:H4 that was found in five strains isolated from control and diarrheal groups, which belonged to DAEC, EAEC, and aEPEC categories (Table 2).
CONCLUSION
In summary, the protocol described here allowed the detection of DEC pathotypes and the use of an internal amplification control allows validation of negative reactions. The use of multiplex‐PCR systems allows the detection of several targets simultaneously and is an alternative for DEC screening and identification in microbiology laboratories, saving time, cost, and effort to diagnose these bacteria.
ACKNOWLEDGMENTS
We wish to thank Dr Isabel Scaletsky and Dr Katia Aranda who kindly send us EPEC, ETEC, and DAEC reference strains, and Dr M.G. Yates for reading the manuscript and for suggestions. We thank CAPES/REUNI for scholarships.
Grant sponsor: Fundação Araucária and Brazilian Program of National Institutes of Science and Technology ‐ INCT/Brazilian Research Council ‐ CNPq/MCT.
REFERENCES
- 1. Kaper JB, Nataro JP, Mobley HLT. Pathogenic Escherichia coli .Nature Rev Microbiol 2004;2:123–140. [DOI] [PubMed] [Google Scholar]
- 2. Schmidt MA. LEEways: Tales of EPEC, ATEC and EHEC. Cell Microbiol 2010;12:1544–1552. [DOI] [PubMed] [Google Scholar]
- 3. Bielaszewska M, Mellmann A, Zhang W, et al. Characterisation of the Escherichia coli strain associated with an outbreak of haemolytic uraemic syndrome in Germany, 2011: A microbiological study. Lancet Infect Dis 2011;11:671–676. [DOI] [PubMed] [Google Scholar]
- 4. Nataro JP, Kaper JB. Diarrheagenic Escherichia coli . Clin Microbiol Rev 1998;11:142–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Trabulsi LR, Keller R, Gomes TAT. Typical and atypical enteropathogenic Escherichia coli . Emerg Infect Dis 2002;8:508–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Pupo GM, Lan R, Reeves PR. Multiple independent origins of Shigella clones of Escherichia coli and convergent evolution of many of their characteristics. PNAS 2000;97:10567–10572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Croxen MA, Finlay BB. Molecular mechanisms of Escherichia coli pathogenicity. Nature Rev Microbiol 2010;8:26–38. [DOI] [PubMed] [Google Scholar]
- 8. Konowalchuk J, Speirs JI, Stavric S. Vero response to a cytotoxin of Escherichia coli . Infect Immun 1977;18:775–779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Guth BEC, Prado V, Rivas M. Shiga toxin‐producing Escherichia coli In: Torres AG, editor. Pathogenic Escherichia coli in Latin America. Bentham Science Publishers Ltd., Chicago, IL, 2010. p 65–83. [Google Scholar]
- 10. Nishi J, Sheikh J, Mizuguchi K, et al. The export of coat protein from Enteroaggregative Escherichia coli by a specific ATP‐binding cassette transporter system. J Biol Chem 2003;278:45680–45689. [DOI] [PubMed] [Google Scholar]
- 11. Navarro‐Garcia F, Elias WP, Flores J, Okhuysen PC. Enteroaggregative Escherichia coli In: Torres AG, editor. Pathogenic Escherichia coli in Latin America. Bentham Science Publishers Ltd., Chicago, IL, 2010. p 48–64. [Google Scholar]
- 12. Yang JR, Wu FT, Tsai JL, et al. Comparison between O serotyping method and multiplex real‐time PCR to identify diarrheagenic Escherichia coli in Taiwan. J Clin Micro 2007;45:3620–3625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Piazza RMF, Abe CM, Horton DSPQ, Miliwebsky E, Chinen I, Vaz TMI, Irino K. Detection and subtyping methods of diarrheagenic Escherichia coli strains In: Torres AG, editor. Pathogenic Escherichia coli in Latin America. Bentham Science Publishers Ltd, Chicago, IL, 2010. p 95–115. [Google Scholar]
- 14. De Toni F, Souza EM, Pedrosa FO, et al. A prospective study on Shiga toxin‐producing Escherichia coli in children with diarrhoea in Paraná State, Brazil. Lett Appl Microbiol 2009;48:645–647. [DOI] [PubMed] [Google Scholar]
- 15. Paton AW, Paton JC. Detection and characterization of Shiga toxigenic Escherichia coli by using multiplex PCR assays for stx1, stx2, eaeA, Enterohemorrhagic E. coli hlyA, rfbO111, and rfbO157. J Clin Microbiol 1998;36:598–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. López‐Saucedo C, Cerna JF, Villegas‐Sepulveda N, et al. Single multiplex polymerase chain reaction to detect diverse loci associated with diarrheagenic Escherichia coli . Emerg Infect Dis 2003;9:27–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Stacy‐Phipps S, Mecca JJ, Weiss JB. Multiplex PCR assay and simple preparation method for stool specimens detect enterotoxaigenic Escherichia coli DNA during course of infection. J Clin Microbiol 1995;33:1054–1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Aranda KRS, Fagundes‐Neto U, Scaletsky ICA. Evaluation of multiplex PCRs for diagnosis of infection with diarrheagenic Escherichia coli and Shigella spp. J Clin Microbiol 2004;42:5849–5853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Vidal M, Kruger E, Durán C, et al. Single multiplex PCR assay to identify simultaneously the six categories of diarrheagenic Escherichia coli associated with enteric infections. J Clin Microbiol 2005;43:5362–5365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lu JJ, Perng CL, Lee SY, Wan CC. Use of PCR with universal primers and restriction endonuclease digestions for detection and identification of common bacterial pathogens in cerebrospinal fluid. J Clin Microbiol 2000;38:2076–2080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hall TA. BioEdit: A user‐friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp Ser 1999;41:95–98. [Google Scholar]
- 22. Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tool. J Mol Biol 1990;215:403–410. [DOI] [PubMed] [Google Scholar]
- 23. Gentry MK, Dalrymple JM. Quantitative microtiter cytotoxicity assay for Shigella toxin. J Clin Microbiol 1980;12:361–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Franzolin MR, Alves RCB, Keller R, et al. Prevalence of diarrheagenic Escherichia coli in children with diarrhea in Salvador, Bahia, Brazil. Mem Inst Oswaldo Cruz 2005;100:359–363. [DOI] [PubMed] [Google Scholar]
- 25. Aranda KRS, Fabbricotti SH, Fagundes‐Neto U, Scaletsky ICA. Single multiplex assay to identify simultaneously enteropathogenic, enteroaggregative, enterotoxigenic, enteroinvasive and Shiga toxin‐producing Escherichia coli strains in Brazilian children. FEMS Microbiol Lett 2007;267:145–150. [DOI] [PubMed] [Google Scholar]
- 26. Araujo JM, Tabarelli GF, Aranda KRS, et al. Typical enteroaggregative and atypical enteropathogenic types of Escherichia coli are the most prevalent diarrhea‐associated pathotypes among Brazilian children. J Clin Microbiol 2007;45:3396–3399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Spano LC, Sadovsky ADI, Segui PN, et al. Age‐specific prevalence of diffusely adherent Escherichia coli in Brazilian children with acute diarrhoea. J Med Microbiol 2008;57:359–363. [DOI] [PubMed] [Google Scholar]
- 28. Estrada‐Garcia T, Lopez‐Saucedo C, Thompson‐Bonilla R, et al. Association of diarrheagenic Escherichia coli pathotypes with infection and diarrhea among Mexican children and association of atypical enteropathogenic E. coli with acute diarrhea. J Clin Microbiol 2009;47:93–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Moreno ACR, Fernandes Filho A, Gomes TAT, et al. Etiology of childhood diarrhea in the northeast of Brazil: Significant emergent diarrheal pathogens. Diagn Microbiol Infect Dis 2010;66:50–57. [DOI] [PubMed] [Google Scholar]
- 30. Liebchen A, Benz I, Mellmann A, et al. Characterization of Escherichia coli strains isolated from patients with diarrhea in São Paulo, Brazil: Identification of intermediate virulence factor profiles by multiplex PCR. J Clin Microbiol 2011;49:2274–2278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Toma C, Lu Y, Higa N, et al. Multiplex PCR assay for identification of human diarrheagenic Escherichia coli . J Clin Microbiol 2003;41:2669–2671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Müller D, Greune L, Heusipp G, et al. Identification of unconventional intestinal pathogenic Escherichia coli isolates expressing intermediate virulence factor profiles by using a novel single‐step multiplex PCR. Appl Environ Microbiol 2007;73:3380–3390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Fujioka M, Kasai K, Miura T, Sato T, Otomo Y. Rapid diagnostic method for the detection of diarrheagenic Escherichia coli by multiplex PCR. Jpn J Infect Dis 2009;62:476–480. [PubMed] [Google Scholar]
- 34. Antikainen J, Tarkka E, Haukka K, Siitonen A, Vaara M, Kirveskari J. New 16‐plex PCR method for rapid detection of diarrheagenic Escherichia coli directly from stool samples. Eur J Clin Microbiol Infect Dis 2009;28:899–908. [DOI] [PubMed] [Google Scholar]
- 35. Gómez‐Duarte OG, Bai J, Newell E. Detection of Escherichia coli, Salmonella spp., Shigella spp., Yersinia enterocolitica, Vibrio cholerae, and Campylobacter spp. enteropathogens by 3‐reaction multiplex polymerase chain reaction. Diagn Microbiol Infect Dis 2009;63:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hoorfar J, Malorny B, Abdulmawjood A, Cook N, Wagner M, Fach P. Practical considerations in design of internal amplification controls for diagnostic PCR assays. J Clin Microbiol 2004;42:1863–1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Persson S, Olsen KEP, Scheutz F, Krogfelt KA, Gerner‐Smidt P. 2007. A method for fast and simple detection of major diarrhoeagenic Escherichia coli in the routine diagnostic laboratory. Clin Microbiol Infect 13:516–524. [DOI] [PubMed] [Google Scholar]
- 38. Orlandi PP, Magalhães GF, Matos NB, et al. Etiology of diarrheal infections in children of Porto Velho (Rondonia, Western Amazon region, Brazil). Braz J Med Biol Res 2006;39:507–517. [DOI] [PubMed] [Google Scholar]
- 39. Bueris V, Sircili MP, Taddei CR, et al. Detection of diarrheagenic Escherichia coli from children with and without diarrhea in Salvador, Bahia, Brazil. Mem Inst Oswaldo Cruz 2007;102:839–844. [DOI] [PubMed] [Google Scholar]
- 40. Kusumoto M, Nishiya Y, Kawamura Y, Shinagawa K. Identification of an insertion sequence, IS1203 variant, in a Shiga toxin 2 gene of Escherichia coli 0157:H7. J Biosci Bioeng 1999;87:93–96. [DOI] [PubMed] [Google Scholar]
- 41. Jinneman KC, Weagant SD, Johnson JM, et al. A large insertion in the Shiga‐like toxin 2 gene (stx2) of an Escherichia coli O157:H7 clinical isolate. Int J Food Microbiol 2000;57:115–124. [Google Scholar]
- 42. Rodolpho D, Marin JM. Isolation of Shiga toxigenic Escherichia coli from butcheries in Taquaritinga city, State of São Paulo, Brazil. Braz J Microbiol 2007;38:599–602. [Google Scholar]
- 43. Ding H, Huang L, Mao X, Zou Q. Characterization of stx2 and its variants in Escherichia coli O157:H7 isolated from patients and animals. Afr J Biotechnol 2011;10:2991–2998. [Google Scholar]


