Abstract
It has been 10 years since machine learning was first applied to neuroimaging data in psychiatric disorders to identify diagnostic and prognostic markers at the level of the individual. Proof of concept findings in major depression have since been extended in international samples and are beginning to include hundreds of samples from multisite data. Neuroimaging provides the unique capability to detect an acute depressive state in major depression, while we would not expect perfect classification with current diagnostic criteria which are based solely on clinical features. We review developments and the potential impact of heterogeneity, as well as homogeneity, on classification for diagnosis and prediction of clinical outcome. It is likely that there are distinct biotypes which comprise the disorder and which predict clinical outcome. Neuroimaging-based biotypes could aid in identifying the illness in individuals who are unable to recognise their illness and perhaps to identify the treatment resistant form early in the course of the illness. We propose that heterogeneous symptom profiles can arise from a limited number of neural biotypes and that apparently heterogeneous clinical outcomes include a common baseline predictor and common mechanism of treatment. Baseline predictors of clinical outcome reflect factors which indicate the general likelihood of response as well as those which are selective for a particular form of treatment. Irrespective of the mechanism, the capacity for response will moderate the outcome, which includes inherent models of interpersonal relationships that could be associated with genetic risk load and represented by patterns of functional and structural neural correlates as a predictive biomarker. We propose that methods which directly address heterogeneity are essential and that a synergistic combination could bring together data-driven inductive and symptom-based deductive approaches. Through this iterative process, major depression can develop from being syndrome characterized by a collection of symptoms to a disease with an identifiable pathophysiology.
Keywords: Major depression, Machine learning, Psychotherapy, Antidepressant medication, Prediction, Biomarkers
Highlights
-
•
Multitudinous heterogeneous symptom profiles can arise from a limited number of neural patterns.
-
•
Heterogeneous clinical outcomes can include a common baseline predictor of response and common mechanism of treatment.
-
•
Addressing heterogeneity is essential in combining data-driven inductive and symptom-based deductive approaches.
-
•
Integrating neuroimaging-based biotypes with genetic studies could aid in the specificity in identifying variants.
1. Introduction
Major depression is characterized by a persistent low mood or inability to experience usual feelings of pleasure, which is associated with impairments in neurovegetative, psychomotor and cognitive functioning (American Psychiatric Association, 2013; World Health Organization, 2004). Although melancholia has been recognised throughout millennia, current diagnostic criteria are the result of attempts in the past decades to develop reliable, ontological classifications (Kendler, 2017). However, the criteria are based only on observable clinical features because no diagnostic pathophysiology has yet been identified. At the present time, major depression is thus a syndrome, which is characterized by a collection of symptoms, rather than a disease with an identifiable pathophysiology.
There is significant heterogeneity in the symptom profiles that can make up a diagnosis, in the clinical outcomes for a given treatment, and in the longitudinal course for individual patients. Addressing heterogeneity is essential, which includes taking into account potential homogeneous factors. Within the heterogeneity in our current classification criteria, we support a core concept of the illness as a primary disorder in mood, in particular a lowering in mood that is embodied in the individual, though we would not expect perfect classification with current diagnostic criteria (Fu and Costafreda, 2013).
We propose that neuroimaging can aid in identifying potential biotypes by quantifying the heterogeneity, as well as homogeneity, that comprise major depression and the biotypes that can predict clinical outcome. We review developments from the initial studies 10 years ago, which had applied machine learning to neuroimaging data to classify major depression and to predict clinical outcome (Fu and Costafreda, 2013; Fu et al., 2008a; Costafreda et al., 2009), to replication and progression in international independent datasets (Drysdale et al., 2017; Kambeitz et al., 2017; Sankar et al., 2016), and how heterogeneity is being addressed in recent studies (Drysdale et al., 2017; Davatzikos, 2019; Davatzikos, 2018). We review homogeneous mechanisms in psychological and pharmacological therapies, and we discuss the potential to identify biomarkers to predict clinical response. We propose that multitudinous heterogeneous symptom profiles can arise from a limited number of neural patterns as well as how apparently heterogeneous clinical outcomes can include a common baseline predictor of response and common mechanism of treatment.
We conclude with a proposal for the necessary steps and recent developments to identify neuroimaging-based biotypes: 1) harmonization of longitudinal multisite datasets acquired in different scanners and with different image acquisition protocols (Erus et al., 2018); 2) modelling the pathological process by multiple regularized transformations from the healthy to the patient population to identify the multiple neuroanatomical patterns that characterize disease heterogeneity (CHIMERA) (Dong et al., 2016); and 3) characterisation of the neuroanatomical heterogeneity through delineation of the multiple hyperplanes within disease populations (HYDRA) (Varol et al., 2017).
2. Heterogeneity in major depression phenotypes
Johannsen (Johannsen, 1911; reprinted in Johnansen, 2014) introduced the terms: “genotype”, which describes the sum of genes in a gamete or zygote; “phenotype”, referring what can be readily observed; and “biotype”, which represents a given genotype in which biotypes can evolve from each other through small changes in genotype. Our current diagnosis of major depression is a phenotype, and many phenotypic combinations are possible within present diagnostic systems (American Psychiatric Association, 2013; World Health Organization, 2004). In terms of symptoms, there are many potential combinations of clinical profiles, and there is significant clinical comorbidity with anxiety disorders (American Psychiatric Association, 2013; World Health Organization, 2004; Kendler, 2017). In terms of the longitudinal course of the illness, it is unknown whether the initial episode will be a single episode, or points to a course of unipolar depression, or is an acute depressive episode of a bipolar disorder (in the present review, ‘major depression’ refers to ‘unipolar depression’ in distinction to bipolar disorder). Heterogeneous phenotypes can arise from a common genotype, and seemingly homogeneous phenotypes can arise from different genotypes (Johannsen, 1911).
Major depression is the leading mental health disorder worldwide, affecting over 350 million people (Vos et al., 2015). Yet, heritability estimates are around 37% (Ripke et al., 2013), which is relatively low in contrast to bipolar disorder and schizophrenia which have heritability estimates that are consistently up to 90% from twin and molecular genetic studies (Geschwind and Flint, 2015). The heritability of major depression is polygenic, consisting of hundreds of variants and genes, each providing a small component to the genetic contribution, and samples have generally consisted of people of European ancestry (Howard et al., 2019). Genetic risk variants are not clinically useful at the level of the individual, and how genetic risk progresses into an acute depressive episode is unknown (McIntosh et al., 2019).
The genetic and environmental factors that lead to major depression are expressed in subtle and widespread alterations in brain structure and brain function. Magnetic resonance imaging (MRI) has revealed effects in multiple networks, including in frontolimbic circuits and default mode network (Wise et al., 2017), which are evident in the first episode (Wang et al., 2017; Cole et al., 2011) and supported by neuropathological abnormalities (Boldrini et al., 2013). Multivariate pattern analysis integrates the subtle and spatially distributed alterations into models, learning to categorise the patterns and then to identify them in new data. Applying machine learning, proof of concept data demonstrated the ability to identify major depression at the level of the individual with high sensitivity and specificity from structural MRI and task-based functional MRI data (Fu et al., 2008a; Costafreda et al., 2009; Marquand et al., 2008), and distinct models predicted clinical response at baseline before the start of treatment with measures of confidence of the accuracy of the predictions (Nouretdinov et al., 2011).
Kambeitz et al. (Kambeitz et al., 2017) meta-analysis of 33 international samples of major depression (n = 912) and healthy (n = 864) participants demonstrated an overall classification with 77% sensitivity and 78% specificity, based on structural MRI, resting state functional MRI, task-based functional MRI and diffusion tensor imaging. Most studies to date have generated dichotomous classification labels for diagnosis, either major depression or healthy control, primarily due to limited sample sizes of participants with major depression (n = 15–57) (Kambeitz et al., 2017; Sankar et al., 2016). In the largest multisite sample to date, Drysdale et al. (Drysdale et al., 2017) were able to identify a number of subtype models based on resting state functional MRI data, which were then validated in independent samples. The models were developed from what would be considered a more treatment-resistant form of depression, that is from participants with major depression (n = 220) with active symptoms which had failed to respond to at least two antidepressant treatment trials and while taking medications. The models were then trained in the full sample of depression (n = 333) and healthy (n = 378) participants and further validated in independent samples of depression (n = 125) and healthy (n = 352) participants.
A common pattern of altered connectivity was evident, which encompassed the ventromedial prefrontal, orbitofrontal and posterior cingulate cortices, insula, and subcortical regions, and distinct patterns of functional connectivity and clinical symptom profiles were revealed in four subtypes with high sensitivity and specificity (82–92%). Subtype 1 was associated with anxiety, early and middle insomnia, and anergia; subtype 2 was primarily associated with anergia; subtype 3 with anhedonia and psychomotor retardation; and subtype 4 with the highest levels of anxiety, early and middle insomnia, as well as anhedonia. The subtypes were not accounted for by depressive severity only as there were no significant differences in depressive severity scores in subtypes 1, 3 and 4, although there was a modest decrease in subtype 2. Increased thalamic and frontostriatal connectivity associated with anhedonia and psychomotor retardation was most pronounced in subtypes 3 and 4. Reduced fronto-amygdala connectivity associated with anxiety was most severe in subtypes 1 and 4. Reduced connectivity in anterior cingulate and orbitofrontal regions involved in motivation associated with symptoms of anergia and fatigue were most evident in subtypes 1 and 2. By addressing heterogeneity, Drysdale et al. (Drysdale et al., 2017) represent an important step in identifying potential biotypes that comprise major depression.
While the highest levels of sensitivity and specificity in classification have been achieved in the treatment-resistant form of depression (Kambeitz et al., 2017; Sankar et al., 2016), these could be confounded by biological effects of treatment resistance and antidepressant medication on brain structure and function (Lui et al., 2011; Ferri et al., 2017). From a methodological perspective, Dinga et al. (Dinga et al., 2019) suggest that the clustering algorithm had led to overfitting as their replication analysis could not generate comparable statistically significant subtypes. However, the samples also differed significantly in their clinical characteristics in terms of treatment resistance and depressive state, which are associated with distinct neural correlates (Lui et al., 2011; Ferri et al., 2017). Drysdale et al. (Drysdale et al., 2017) training sample had been based on treatment resistant depression in an acute episode, while Dinga et al. (Dinga et al., 2019) sample (n = 178) was comprised of major depression, anxiety disorders, as well as comorbid depression and anxiety disorders, in which about half the sample were in remission or had mild symptoms. The training samples, though larger than the majority of studies to date, could have been sufficiently non-overlapping, were underpowered, and might reflect homogeneity in the neural biotypes of less treatment resistant forms of the illness that are in remission or with few symptoms and which present as heterogeneous clinical phenotypes (Drysdale et al., 2017; Dinga et al., 2019).
As a corollary, treatment-resistant depression is currently a clinical diagnosis that refers to a depressive episode that does not improve despite a series of treatments. A predictor model of clinical and sociodemographic data has demonstrated an accuracy of 75% in identifying treatment resistant depression (Kautzky et al., 2018). Clinical features in the model though included duration of illness, lifetime duration of hospitalizations and number of depressive episodes, which precludes identification early in the course of the illness. If the treatment resistant biotype is present at the onset of the episode, rather than as a consequence of subsequent treatments, then the biotype might be identifiable early in the illness. Low rates of remission, which is less than one third of treatment trials irrespective of the form of treatment (Cuijpers et al., 2008; Gartlehner et al., 2017), indicate that current treatments are insufficient and highlight a subgroup which consistently shows a limited or lack of clinical improvement, suggesting that the pathophysiology of treatment-resistant depression could already be present early in the course of illness.
Common and distinct neural patterns between unipolar major depression and other disorders are another source of heterogeneity. Conjunction analysis of grey matter volumes has observed common reductions in anterior cingulate, dorsomedial and ventromedial prefrontal cortices and insula in unipolar depression and bipolar disorder relative to healthy controls with additional reductions which included right middle frontal and left hippocampus in unipolar depression relative to bipolar disorder (Wise et al., 2017). Classification results have demonstrated a contribution of reduced grey matter volumes in the anterior cingulate in unipolar depression relative to bipolar disorder (Redlich et al., 2014), although the datasets were based on different samples (Redlich et al., 2014; Wise et al., 2017).
In addition to the inherent heterogeneity in the disorder, demographic-related factors, such as age, sex and ethnicity, are potential variables which can be controlled for. Most studies have consisted of ethnically homogeneous samples, either predominantly Caucasian or Chinese, raising the issue of whether the models can be generalised to the wider patient population, although we have found proof of principle from a small community sample of African, Asian and Caucasian patients based on structural MRI (Sankar et al., 2016). Furthermore, applying neuroimaging-based biotypes as a model of major depression for genetic studies could improve the specificity of identifying variants for major depression as well as from a greater ethnic diversity, addressing limitations associated with broader definitions of depression and current genetic studies which have most often been assessed in people of European ancestry (McIntosh et al., 2019).
Heterogeneity in methodology is a controllable variable. Task-based functional MRI protocols focused on a cognitive task (Marquand et al., 2008) show lower levels of classification accuracy relative to an emotion-based task (Fu et al., 2008a), which is not unexpected in major depression. Overlooking task effects adds variability that is not random, which degrades the quality of the signal and introduces confounds in meta-analyses of classification biomarkers (Kambeitz et al., 2017; Lee et al., 2018).
3. Heterogeneity (and homogeneity) in treatment mechanisms
Selective serotonin reuptake inhibitor (SSRI) antidepressants enhance synaptic plasticity, in which the effects on mood might be moderated by environmental stressors (Harmer et al., 2017; Kraus et al., 2017). Pharmacological treatment is associated with increases in regional cerebral volumes, such as in the hippocampus (Arnone et al., 2013). Hippocampal volume is reduced in major depression, which is evident in the first episode (Cole et al., 2011). SSRI treatment is associated with increased hippocampal volume (Arnone et al., 2013), and an early increase in hippocampal volume following one week of treatment with a serotonin noradrenaline reuptake inhibitor (SNRI) antidepressant was predictive of clinical response (Fu et al., 2015), which could reflect enhancement in synaptic plasticity and neurogenesis (Harmer et al., 2017; Kraus et al., 2017).
Psychotherapy is an effective treatment for an acute depressive episode, is the preferred form of treatment for many individuals with depression, and demonstrates a sustained benefit in preventing a subsequent depressive relapse (Hollon et al., 2005; Cuijpers et al., 2013; McHugh et al., 2013). The most common forms of short term psychotherapy are cognitive behavior therapy, behavioral activation, interpersonal therapy, and psychodynamic psychotherapy. Distinct formulations and mechanisms have been proposed, however efficacy has either been comparable or no significant differences have been observed between treatments (Weissman et al., 1979; Dimidjian et al., 2006; de Maat et al., 2008; Barth et al., 2013; Palpacuer et al., 2017). Moreover, our understanding of the neural mechanisms is limited, and there have been remarkably few longitudinal studies of the neural correlates of psychotherapy in depression, about a quarter of the number of pharmacological studies (Fu et al., 2013; Sankar et al., 2018).
The theoretical formulation of cognitive behavior therapy (CBT) proposes a cognitive triad of biased negative views of onself, one's future and experiences in the outside world which leads to characteristic affective and behavioral symptoms (Beck et al., 1979). CBT attempts to intervene in this cycle by addressing negative cognitions: automatic thoughts (eg. “I'm a failure”) and dysfunctional attitudes (eg. “I should be happy all the time”) that in turn reflect schemas and core beliefs which organize new experiences. Cognitive change is a key mechanism in which modifying maladaptive cognitions leads to an improvement in mood (Beck et al., 1979; Jacobson et al., 1996). Behavioral activation therapy focuses on behavioral change to increase engagement in constructive reinforcing activities and to reduce engagement in avoidance and withdrawal behaviours which maintain depression. Behavioral activation refers to the process of changing behaviours in order to engage in positively reinforcing and adaptive activities, which has demonstrated efficacy as a stand-alone component of CBT (Dimidjian et al., 2006; Ekers et al., 2014).
Interpersonal therapy (IPT) is based on the premise that maladaptive communication processes impact negatively on mood. IPT seeks to address interpersonal difficulties common in depression, focusing on four main themes: bereavement related to the grief and loss of a significant other; role transition due to a life change which affects relationships, such as a new job or loss of functioning; interpersonal disputes in expectations in relationships with significant others; or interpersonal deficits which could be reflected in social isolation or difficulties in maintaining relationships (Cuijpers et al., 2016). Short term psychodynamic psychotherapy applies support as well as insight to discuss internalized past relationships, intrapersonal patterns and current relationships. Psychodynamic psychotherapy considers how internalized past relationships and unconscious processes could impact on interpersonal relationships and day to day functioning to improve awareness of such processes which in turn aids in the ability to modify responses and behaviours in current behaviours and relationships (de Maat et al., 2008; Driessen et al., 2010).
If a particular process is a mechanism, it is necessary to establish the temporal sequence such it is the specific process which leads to improvements in depressive symptoms (Kraemer et al., 2002). As a potential mechanism in CBT, a change in cognition would be expected to precede any improvements in depressive symptoms. In support, early changes in cognition during CBT and “sudden gains” (Tang and DeRubeis, 1999; Tang et al., 2005) as well as therapist adherence to techniques and competence (Strunk et al., 2010) are associated with symptom improvements. However, when changes in mood symptoms were taken into account, early changes in cognitive content no longer predicted subsequent improvements (Jarrett et al., 2007). Moreover, improvements in depressive symptoms have also been observed to precede changes in cognitions (Furlong and Oei, 2002). Component analysis suggests that the effectiveness of CBT can be largely ascribed to behavioral activation (Jacobson et al., 1996; Dimidjian et al., 2006), while acknowledging a predictive component of cognitive change, though assessed as a nominal contribution (Vittengl et al., 2014).
Although heterogeneous mechanisms have been proposed, the effects are small. It is well established that common factors contribute to the efficacy of the different forms of psychotherapy. Therapeutic alliance refers to the relationship between the patient and therapist and has consistently demonstrated a mediating effect in clinical outcome to psychotherapy (Grencavage and Norcross, 1990), as well as to pharmacological and placebo treatments (Krell et al., 2004; Leuchter et al., 2014). Patient outcome expectations describe the prognostic beliefs about effects of engaging in treatment that could be positive, negative or ambivalent, which show a small association with treatment outcome, which may further be mediated by the therapeutic alliance (Constantino et al., 2011). A common role of the therapist in short term psychotherapy is as an advocate for the patient. The CBT therapist is proposed to represent an active, authoritative advocate for change who supports patients to engage in activities and thoughts, which in turn leads to improvements in depressive symptoms (Vittengl et al., 2014). The goal of the IPT therapist is to be the patient's ally who reinforces beneficial interpersonal skills and actively reviews adverse outcomes including through role play and rehearsal (Cuijpers et al., 2016). A range of outcomes has been observed between therapists, in which adherence and competence seem to have a limited effect (Webb et al., 2010), while therapist adaptiveness and empathy are correlated with clinical outcome (Elliott et al., 2018). How, when and what a therapist responds to in a session reflects their adaptiveness and empathy.
Enhanced synaptic plasticity is a potential common mechanism of antidepressant medication (Harmer et al., 2017; Kraus et al., 2017) and psychotherapy. Synaptic plasticity is a fundamental mechanism in learning and memory (Takeuchi et al., 2014; Bocchio et al., 2017), and learning is an important mechanism in psychotherapy, in which the therapist provides an essential component with whom skills are learned. Moreover, we propose that the patient therapist relationship builds upon inherent models of relationships. Even in the absence of a therapist who is physically present, we would expect that inherent models would have an impact on the perceived relationship, such as in internet-based treatments. We propose that encoding of the relationship is episodic with the repeated sessions of the therapy and that recall of the relationship may be initially explicit.
Furthermore, treatment with CBT (Fu et al., 2008b) as well as treatment with a selective serotonin reuptake inhibitor antidepressant (Fu et al., 2004; Arnone et al., 2012) has been associated with normalisation of amygdala responses to sad emotional expressions. Encouragement and validation from another person is associated with a reduction in pain (Che et al., 2018), in which the emotional-affective dimensions are encoded in the amygdala (Neugebauer, 2015). While support is an inherent component of the patient therapist relationship in both psychological and pharmacological treatments, learning in the context of the patient therapist relationship is potential mechanism, which would have a greater contribution in psychotherapy. Normalisation of amygdala responses could reflect common effects on mood following psychological and pharmacological treatments, in which learning in the context of the patient therapist relationship is a contributing mechanism.
4. Heterogeneity in treatment responsiveness and outcomes
Baseline predictors of clinical outcome reflect factors which indicate the general likelihood of response to a variety of forms of treatments and those which are selective for a particular form of treatment. Irrespective of the mechanism of treatment, the capacity for response will moderate the outcome. If there is an inherent capacity, then there would be general predictor of clinical responsivity to current first line treatments (Fu et al., 2013; Gartlehner et al., 2017; Harmer et al., 2017; Kraus et al., 2017). The absence of such marker/s would indicate a reduced likelihood of response.
Short term psychological treatments require the therapist to be an active advocate for the patient (Beck et al., 1979; Driessen et al., 2010; Lutz et al., 2013; Amick et al., 2015; Cuijpers et al., 2016). This could be explicit in the physical presence of the therapist or an inherent implicit presence. Therapeutic alliance is a mediator of treatment outcomes to both pharmacological and psychological treatments (Grencavage and Norcross, 1990; Constantino et al., 2011; Krell et al., 2004; Leuchter et al., 2014), but how an individual with depression experiences the interaction with the therapist would be affected in part by their inherent patterns in interactions in close relationships (Bowlby, 1969; Reis and Grenyer, 2004). Predisposing patterns in interpersonal relationships would moderate treatment outcome as a general predictor of clinical outcome.
If impairments in inherent models of interpersonal relationships moderate clinical outcome to psychological treatments, then factors which impact on their development would contribute to clinical outcome, such as attachment patterns (Bowlby, 1969; Reis and Grenyer, 2004). Spatial and temporal patterns of interactions between an individual and their attachment figures frame how the individual then perceives and responds in other interpersonal relationships, termed ‘internal working models’ (Bowlby, 1969). For example, attachment theory describes how interpersonal relationships evolve from relationships with early caregivers to diverse adult relationships, which are malleable and can continue to develop. It is estimated that 55% of healthy adults have a secure attachment pattern, but it is the insecure attachment profile which predominates in depression and specific subtypes have been associated with poorer clinical outcomes (Bowlby, 1969; Reis and Grenyer, 2004).
Limited development of inherent models of interpersonal relationships could also be associated with genetic risk load, for example it might be expected that patients with depression with a high genetic risk load for autistic spectrum disorder would prefer an internet based CBT, however, if they have an impaired inherent model of interpersonal relationships, then their ability to interact with and utilise the CBT format would be limited (Bowlby, 1969), as potentially for any short term therapy which could be transdiagnostic.
Shared features in the forms of therapy include treatment durations, which have mostly been short term in the order of weeks to months, low rates of clinical effectiveness, and the role of the therapist as an active advocate who interacts directly with the patient (Amick et al., 2015; Gartlehner et al., 2017; Swift et al., 2017). However, if the ability of the patient to engage with the therapist depends in part on their inherent models of interpersonal relationships, then impairments in the models would impact on how their relationship develops with the therapist, to which some therapists may be better able to respond and adapt their treatment. This heterogeneity could be evident in part in amygdala responsivity, for example an insecure anxious attachment pattern is associated with increased amygdala response to emotional stimuli (Ran and Zhang, 2018) and attachment patterns modulate experimentally induced pain ratings in the presence of an observer (Sambo et al., 2010).
5. Addressing heterogeneity
Being able to identify an acute depressive episode in major depression that is not treatment-resistant, to predict treatment outcome and to predict the course of the illness are key clinical challenges. Model development requires the appropriate data and methodology, which can be summarised, but the quality of the data and methodology are paramount (Fu and Costafreda, 2013; Davatzikos, 2019). In order to reflect clinical practice and to develop models that are not confounded by treatment, the data would be from a community based medication-free population in an acute depressive episode with first episode or recurrent depression that is not treatment-resistant. Well characterized large datasets are required with consideration of ethical and data sharing issues. We would not expect perfect classification with current diagnostic criteria because clinical presentations do not necessarily reflect specific biological measures and do not identify specific pathophysiologies. We would expect an iterative process in which the neuroimaging-based biomarkers are applied to specify potential pathophysiologies (Fu and Costafreda, 2013).
Methods that directly address heterogeneity are essential. Several studies have begun to use high-dimensional clustering methods to dissect imaging heterogeneity in various diseases (Dong et al., 2017; Davatzikos, 2018; Kim et al., 2019). While direct clustering of patient data can be informative, it is confounded by variations related to demographics and other factors that are not related to disease pathophysiology. Two semi-supervised learning methods are proposed in an attempt to address this limitation: CHIMERA (Dong et al., 2016) and HYDRA (Varol et al., 2017).
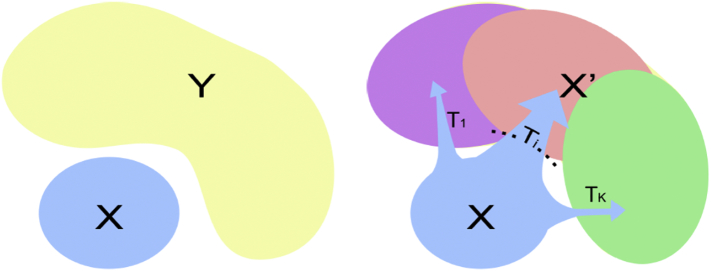
CHIMERA is primarily generative, and it assumes that the statistical distribution of imaging features of the (heterogeneous) patient cohort is derived from the statistical distribution of the healthy cohort via a number of transformations that reflect the effects of underlying (heterogeneous) pathophysiologies (Fig. 1). Covariates are taken into account explicitly. CHIMERA is probabilistic clustering approach that models the pathological process by a combination of multiple regularized transformations from the healthy control population to the patient population. The populations are considered as point distributions which are matched by a variant of the coherent point drift algorithm. For example, a 40 year old woman with depression would have been a 40 year old healthy woman had she been spared the disorder. This is directly modelled in CHIMERA which seeks to identify the multiple imaging patterns that relate to disease effects in order to characterize disease heterogeneity.
Fig. 1.
CHIMERA is a primarily generative method, which assumes that the distribution of measurements from patients (Y, in the figure) is derived from the distribution of controls (X, in the figure), after some (unknown) transformations (T_i) are applied. The latter represent (heterogeneous) disease effects.
HYDRA takes a similar approach, but from the discriminative angle: it uses a number of support vector machine hyperplanes to separate patients from health controls in which each hyperplane reflects one subtype. Covariates are first regressed out of the data. The subtypes are captured by multiple linear hyperplanes which form a convex polytope that separates two populations in which each face of the polytope defines a disease subtype (Fig. 2). Both methods use cross-validation and split-sample analyses to find the optimal number of subtypes.
Fig. 2.
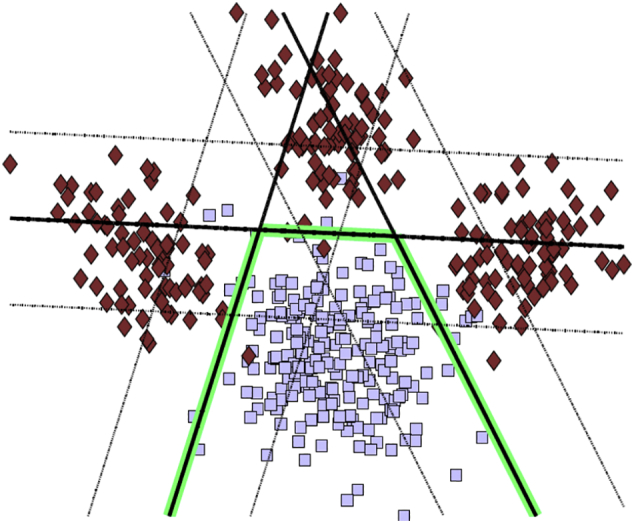
The HYDRA method is mostly discriminative, in that it attempts to separate patients and controls as well as possible, using multiple hyperplanes, one for each subtype.
The potential of these methods to capture neuroanatomical heterogeneity from MRI has been demonstrated in schizophrenia, mild cognitive impairment and Alzheimer's disease (Dong et al., 2017; Honnorat et al., 2017; Davatzikos, 2018; Kim et al., 2019). Data-driven, inductive modelling strategies model the neuroanatomical patterns that make up major depression as a collection of directions of deviation from normal neuroanatomical patterns. These approaches model the pathological processes associated with depression by a combination of multiple regularized transformations from the healthy control population to the patient population, thereby seeking to identify multiple neuroanatomical patterns that relate to disease effects and to characterize disease heterogeneity. A complementary deductive approach, such as reported clinical symptoms, would apply prior knowledge to identify the linear combinations of neuroanatomical features that correlate with clinical clusters. A synergistic combination could also bring together data-driven inductive and symptom-based deductive approaches such that the clinical measures will be used in CHIMERA and HYDRA to inform the clustering.
Psychological and pharmacological treatments propose distinct heterogeneous mechanisms. Yet, short term psychotherapies advocate an active therapist as a common homogeneous component. If outcomes to short term psychotherapy as well as to antidepressant medication depend in part on inherent models of interpersonal interactions, then this is measurable prior to the initiation of treatment. Measures to date though have been subjective or clinician-rated without a gold standard. Neuroimaging markers offer the potential to characterize the homogeneous as well as heterogeneous mechanisms of clinical effectiveness at the level of the individual with the potential to lead to patient-specific treatments.
In summary, neuroimaging-based biomarkers offer the strongest potential to date to identify the biotypes that comprise our current symptom-based diagnosis of major depression. Increasing sample sizes from multisite collaborations will provide increased power to delineate the neural biotypes that comprise the diagnosis. Among the purported heterogeneous treatment mechanisms, a common factor is the patient clinician relationship. Learning in the context of this relationship is a potential common mechanism which could modulate amygdala responsivity. The inherent model of interpersonal relationships would moderate clinical outcome, which could be associated with genetic risk load and represented by patterns of functional and structural neural correlates. A predictive biomarker would aid in the stratification of the illness, indicating the most appropriate treatment or combination of treatments, which would improve recovery and disability, as well as increase statistical power in treatment studies by reducing heterogeneity in samples. Integrating neuroimaging-based biotypes with genetic studies could aid the specificity in identifying variants. It is essential to directly address heterogeneity in developing biomarkers, in which a synergistic combination could bring together data-driven inductive and symptom-based deductive approaches.
Disclosures
CF, YF and CD have nothing to disclose.
Acknowledgements
YF, CD are partially supported by National Institute of Health, USA grants: EB022573, MH112070, and AG054409. CF acknowledged support from Medical Research Council, UK grant: G0802594.
References
- Diagnostic and Statistical Manual of Mental Disorders (DSM-5) 2013. [DOI] [PubMed] [Google Scholar]
- Amick H.R., Gartlehner G., Gaynes B.N., Forneris C., Asher G.N., Morgan L.C. Comparative benefits and harms of second generation antidepressants and cognitive behavioral therapies in initial treatment of major depressive disorder: systematic review and meta-analysis. BMJ. 2015;351 doi: 10.1136/bmj.h6019. Dec 8. (h6019) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnone D., McKie S., Elliott R., Thomas E.J., Downey D., Juhasz G. Increased amygdala responses to sad but not fearful faces in major depression: relation to mood state and pharmacological treatment. Am. J. Psychiatry. 2012;169(8):841–850. doi: 10.1176/appi.ajp.2012.11121774. Aug. [DOI] [PubMed] [Google Scholar]
- Arnone D., McKie S., Elliott R., Juhasz G., Thomas E.J., Downey D. State-dependent changes in hippocampal grey matter in depression. Mol. Psychiatry. 2013;18(12):1265–1272. doi: 10.1038/mp.2012.150. Dec. [DOI] [PubMed] [Google Scholar]
- Barth J., Munder T., Gerger H., Nüesch E., Trelle S., Znoj H. Comparative efficacy of seven psychotherapeutic interventions for patients with depression: a network meta-analysis. PLoS Med. 2013;10(5) doi: 10.1371/journal.pmed.1001454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck A.T., Rush A.J., Shaw B.F., Emery G. Guildford Press; New York, NY: 1979. Cognitive Therapy of Depression. [Google Scholar]
- Bocchio M., Nabavi S., Capogna M. Synaptic plasticity, engrams, and network oscillations in amygdala circuits for storage and retrieval of emotional memories. Neuron. 2017;94(4):731–743. doi: 10.1016/j.neuron.2017.03.022. May 17. [DOI] [PubMed] [Google Scholar]
- Boldrini M., Santiago A.N., Hen R., Dwork A.J., Rosoklija G.B., Tamir H. Hippocampal granule neuron number and dentate gyrus volume in antidepressant-treated and untreated major depression. Neuropsychopharmacology. 2013;38(6):1068–1077. doi: 10.1038/npp.2013.5. May. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowlby J. Hogarth Press and the Institute of Psychoanalysis; London, UK: 1969. Attachment and Loss. [Google Scholar]
- Che X., Cash R., Chung S., Fitzgerald P.B., Fitzgibbon B.M. Investigating the influence of social support on experimental pain and related physiological arousal: A systematic review and meta-analysis. Neurosci. Biobehav. Rev. 2018;92:437–452. doi: 10.1016/j.neubiorev.2018.07.005. [DOI] [PubMed] [Google Scholar]
- Cole J., Costafreda S.G., McGuffin P., Fu C.H.Y. Hippocampal atrophy in first episode depression: a meta-analysis of magnetic resonance imaging studies. J. Affect. Disord. 2011;134(1–3):483–487. doi: 10.1016/j.jad.2011.05.057. Nov. [DOI] [PubMed] [Google Scholar]
- Constantino M.J., Arnkoff D.B., Glass C.R., Ametrano R.M., Smith J.Z. Expectations. J. Clin. Psychol. 2011;67(2):184–192. doi: 10.1002/jclp.20754. Feb. [DOI] [PubMed] [Google Scholar]
- Costafreda S.G., Chu C., Ashburner J., Fu C.H.Y. Prognostic and diagnostic potential of the structural neuroanatomy of depression.Domschke K. PLoS One. 2009;4(7) doi: 10.1371/journal.pone.0006353. Jul 27. e6353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuijpers P., van Straten A., Andersson G., van Oppen P. Psychotherapy for depression in adults: a meta-analysis of comparative outcome studies. J. Consult. Clin. Psychol. 2008;76(6):909–922. doi: 10.1037/a0013075. Dec. [DOI] [PubMed] [Google Scholar]
- Cuijpers P., Hollon S.D., van Straten A., Bockting C., Berking M., Andersson G. Does cognitive behaviour therapy have an enduring effect that is superior to keeping patients on continuation pharmacotherapy? A meta-analysis. BMJ Open. 2013;3(4) doi: 10.1136/bmjopen-2012-002542. e002542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuijpers P., Donker T., Weissman M.M., Ravitz P., Cristea I.A. Interpersonal psychotherapy for mental health problems: a comprehensive meta-analysis. Am. J. Psychiatry. 2016;173(7):680–687. doi: 10.1176/appi.ajp.2015.15091141. Jul. [DOI] [PubMed] [Google Scholar]
- Davatzikos C. Quantifying anatomical and functional heterogeneity in big datasets, using machine learning methods towards a dimensional neuroimaging framework. Biol. Psychiatry. 2018;83(9):S14–S15. May. [Google Scholar]
- Davatzikos C. Machine learning in neuroimaging: progress and challenges. NeuroImage. 2019;197:652–656. doi: 10.1016/j.neuroimage.2018.10.003. Aug. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Maat S., Dekker J., Schoevers R., van Aalst G., Gijsbers-van Wijk C., Hendriksen M. Short psychodynamic supportive psychotherapy, antidepressants, and their combination in the treatment of major depression: a mega-analysis based on three randomized clinical trials. Depress. Anxiety. 2008;25(7):565–574. doi: 10.1002/da.20305. [DOI] [PubMed] [Google Scholar]
- Dimidjian S., Hollon S.D., Dobson K.S., Schmaling K.B., Kohlenberg R.J., Addis M.E. Randomized trial of behavioral activation, cognitive therapy, and antidepressant medication in the acute treatment of adults with major depression. J. Consult. Clin. Psychol. 2006;74(4):658–670. doi: 10.1037/0022-006X.74.4.658. Aug. [DOI] [PubMed] [Google Scholar]
- Dinga R., Schmaal L., Penninx B.W.J.H., van Tol M.J., Veltman D.J., van Velzen L. Evaluating the evidence for biotypes of depression: Methodological replication and extension of. NeuroImage Clin. 2019;22 doi: 10.1016/j.nicl.2019.101796. 101796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong A., Honnorat N., Gaonkar B., Davatzikos C. CHIMERA: Clustering of heterogeneous disease effects via distribution matching of imaging patterns. IEEE Trans. Med. Imaging. 2016;35(2):612–621. doi: 10.1109/TMI.2015.2487423. Feb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong A., Toledo J.B., Honnorat N., Doshi J., Varol E., Sotiras A. Heterogeneity of neuroanatomical patterns in prodromal Alzheimer's disease: links to cognition, progression and biomarkers. Brain. 2017;140(3):735–747. doi: 10.1093/brain/aww319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driessen E., Cuijpers P., de Maat S.C.M., Abbass A.A., de Jonghe F., Dekker J.J.M. The efficacy of short-term psychodynamic psychotherapy for depression: a meta-analysis. Clin. Psychol. Rev. 2010;30(1):25–36. doi: 10.1016/j.cpr.2009.08.010. Feb. [DOI] [PubMed] [Google Scholar]
- Drysdale A.T., Grosenick L., Downar J., Dunlop K., Mansouri F., Meng Y. Resting-state connectivity biomarkers define neurophysiological subtypes of depression. Nat. Med. 2017;23(1):28–38. doi: 10.1038/nm.4246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekers D., Webster L., Van Straten A., Cuijpers P., Richards D., Gilbody S. Behavioural activation for depression; an update of meta-analysis of effectiveness and sub group analysis. PLoS One. 2014;9(6) doi: 10.1371/journal.pone.0100100. e100100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott R., Bohart A.C., Watson J.C., Murphy D. Therapist empathy and client outcome: an updated meta-analysis. Psychotherapy. 2018;55(4):399–410. doi: 10.1037/pst0000175. [DOI] [PubMed] [Google Scholar]
- Erus G., Doshi J., An Y., Verganelakis D., Resnick S.M., Davatzikos C. Longitudinally and inter-site consistent multi-atlas based parcellation of brain anatomy using harmonized atlases. NeuroImage. 2018;166:71–78. doi: 10.1016/j.neuroimage.2017.10.026. 01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferri J., Eisendrath S.J., Fryer S.L., Gillung E., Roach B.J., Mathalon D.H. Blunted amygdala activity is associated with depression severity in treatment-resistant depression. Cogn. Affect. Behav. Neurosci. 2017;17(6):1221–1231. doi: 10.3758/s13415-017-0544-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu C.H.Y., Costafreda S.G. Neuroimaging-based biomarkers in psychiatry: clinical opportunities of a paradigm shift. Can. J. Psychiatr. 2013;58(9):499–508. doi: 10.1177/070674371305800904. Sep. [DOI] [PubMed] [Google Scholar]
- Fu C.H.Y., Mourao-Miranda J., Costafreda S.G., Khanna A., Marquand A.F., Williams S.C.R. Pattern classification of sad facial processing: toward the development of neurobiological markers in depression. Biol. Psychiatry. 2008;63(7):656–662. doi: 10.1016/j.biopsych.2007.08.020. Apr 1. [DOI] [PubMed] [Google Scholar]
- Fu C.H.Y., Williams S.C.R., Cleare A.J., Scott J., Mitterschiffthaler M.T., Walsh N.D. Neural responses to sad facial expressions in major depression following cognitive behavioral therapy. Biol. Psychiatry. 2008;64(6):505–512. doi: 10.1016/j.biopsych.2008.04.033. Sep 15. [DOI] [PubMed] [Google Scholar]
- Fu C.H.Y., Steiner H., Costafreda S.G. Predictive neural biomarkers of clinical response in depression: A meta-analysis of functional and structural neuroimaging studies of pharmacological and psychological therapies. Neurobiol. Dis. 2013;52:75–83. doi: 10.1016/j.nbd.2012.05.008. Apr. [DOI] [PubMed] [Google Scholar]
- Fu C.H., Williams S.C., Cleare A.J., Brammer M.J., Walsh N.D., Kim J. Attenuation of the neural response to sad faces in major depression by antidepressant treatment: a prospective, event-related functional magnetic resonance imaging study.Arch. Gen Psychiatry. 2004;61(9):877–889. doi: 10.1001/archpsyc.61.9.877. Sep. [DOI] [PubMed] [Google Scholar]
- Fu C.H.Y., Costafreda S.G., Sankar A., Adams T.M., Rasenick M.M., Liu P. Multimodal functional and structural neuroimaging investigation of major depressive disorder following treatment with duloxetine. BMC Psychiatry. 2015;15(1):82. doi: 10.1186/s12888-015-0457-2. Dec. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furlong M., Oei T.P.S. Changes to automatic thoughts and dysfunctional attitudes in group CBT for depression. Behav. Cogn. Psychother. 2002;30(3):351–360. Jul. [Google Scholar]
- Gartlehner G., Wagner G., Matyas N., Titscher V., Greimel J., Lux L. Pharmacological and non-pharmacological treatments for major depressive disorder: review of systematic reviews. BMJ Open. 2017;7(6) doi: 10.1136/bmjopen-2016-014912. Jun. e014912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geschwind D.H., Flint J. Genetics and genomics of psychiatric disease. Science. 2015;349(6255):1489–1494. doi: 10.1126/science.aaa8954. Sep 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grencavage L.M., Norcross J.C. Where are the commonalities among the therapeutic common factors? Prof. Psychol. Res. Pract. 1990;21(5):372–378. [Google Scholar]
- Harmer C.J., Duman R.S., Cowen P.J. How do antidepressants work? New perspectives for refining future treatment approaches. Lancet Psychiatry. 2017;4(5):409–418. doi: 10.1016/S2215-0366(17)30015-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollon S.D., DeRubeis R.J., Shelton R.C., Amsterdam J.D., Salomon R.M., O'Reardon J.P. Prevention of relapse following cognitive therapy vs medications in moderate to severe depression. Arch. Gen. Psychiatry. 2005;62(4):417–422. doi: 10.1001/archpsyc.62.4.417. Apr. [DOI] [PubMed] [Google Scholar]
- Honnorat N., Dong A., Meisenzal-Lechner E., Koutsouleris N., Davatzikos C. Neuroanatomical heterogeneity of schizophrenia revealed by semi-supervised machine learning methods. Shizophr. Res. 2017 doi: 10.1016/j.schres.2017.12.008. Dec, pii:S0920-9964(17), 30760-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard D.M., Adams M.J., Clarke T.-K., Hafferty J.D., Gibson J., Shirali M. Genome-wide meta-analysis of depression identifies 102 independent variants and highlights the importance of the prefrontal brain regions. Nat. Neurosci. 2019;22(3):343–352. doi: 10.1038/s41593-018-0326-7. Mar. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson N.S., Dobson K.S., Truax P.A., Addis M.E., Koerner K., Gollan J.K. A component analysis of cognitive-behavioral treatment for depression. J. Consult. Clin. Psychol. 1996;64(2):295–304. doi: 10.1037//0022-006x.64.2.295. Apr. [DOI] [PubMed] [Google Scholar]
- Jarrett R.B., Vittengl J.R., Doyle K., Clark L.A. Changes in cognitive content during and following cognitive therapy for recurrent depression: substantial and enduring, but not predictive of change in depressive symptoms. J. Consult. Clin. Psychol. 2007;75(3):432–446. doi: 10.1037/0022-006X.75.3.432. Jun. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johannsen W. The genotype conception of heredity. Am Nat Repr Int J Epidemiol. 1911;45:129–159. doi: 10.1093/ije/dyu063. Reprinted in 2014;4:989–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kambeitz J., Cabral C., Sacchet M.D., Gotlib I.H., Zahn R., Serpa M.H. Detecting neuroimaging biomarkers for depression: a meta-analysis of multivariate pattern recognition studies. Biol. Psychiatry. 2017;82(5):330–338. doi: 10.1016/j.biopsych.2016.10.028. 01. [DOI] [PubMed] [Google Scholar]
- Kautzky A., Dold M., Bartova L., Spies M., Vanicek T., Souery D. Refining prediction in treatment-resistant depression: results of machine learning analyses in the TRD III sample. J Clin Psychiatry. 2018;79(1) doi: 10.4088/JCP.16m11385. Feb, pii: 16m11385. [DOI] [PubMed] [Google Scholar]
- Kendler K.S. The genealogy of major depression: symptoms and signs of melancholia from 1880 to 1900. Mol. Psychiatry. 2017;22(11):1539–1553. doi: 10.1038/mp.2017.148. [DOI] [PubMed] [Google Scholar]
- Kim H.J., Park J.-Y., Seo S.W., Jung Y.H., Kim Y., Jang H. Cortical atrophy pattern-based subtyping predicts prognosis of amnestic MCI: an individual-level analysis. Neurobiol. Aging. 2019;74:38–45. doi: 10.1016/j.neurobiolaging.2018.10.010. Feb. [DOI] [PubMed] [Google Scholar]
- Kraemer H.C., Wilson G.T., Fairburn C.G., Agras W.S. Mediators and moderators of treatment effects in randomized clinical trials. Arch. Gen. Psychiatry. 2002;59(10):877–883. doi: 10.1001/archpsyc.59.10.877. Oct. [DOI] [PubMed] [Google Scholar]
- Kraus C., Castrén E., Kasper S., Lanzenberger R. Serotonin and neuroplasticity - links between molecular, functional and structural pathophysiology in depression. Neurosci. Biobehav. Rev. 2017;77:317–326. doi: 10.1016/j.neubiorev.2017.03.007. [DOI] [PubMed] [Google Scholar]
- Krell H.V., Leuchter A.F., Morgan M., Cook I.A., Abrams M. Subject expectations of treatment effectiveness and outcome of treatment with an experimental antidepressant. J Clin Psychiatry. 2004;65(9):1174–1179. doi: 10.4088/jcp.v65n0904. Sep. [DOI] [PubMed] [Google Scholar]
- Lee Y., Ragguett R.-M., Mansur R.B., Boutilier J.J., Rosenblat J.D., Trevizol A. Applications of machine learning algorithms to predict therapeutic outcomes in depression: A meta-analysis and systematic review. J. Affect. Disord. 2018;241:519–532. doi: 10.1016/j.jad.2018.08.073. 01. [DOI] [PubMed] [Google Scholar]
- Leuchter A.F., Hunter A.M., Tartter M., Cook I.A. Role of pill-taking, expectation and therapeutic alliance in the placebo response in clinical trials for major depression. Br. J. Psychiatry J. Ment. Sci. 2014;205(6):443–449. doi: 10.1192/bjp.bp.113.140343. Dec. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lui S., Wu Q., Qiu L., Yang X., Kuang W., Chan R.C.K. Resting-state functional connectivity in treatment-resistant depression. Am. J. Psychiatry. 2011;168(6):642–648. doi: 10.1176/appi.ajp.2010.10101419. Jun. [DOI] [PubMed] [Google Scholar]
- Lutz W., Ehrlich T., Rubel J., Hallwachs N., Röttger M.-A., Jorasz C. The ups and downs of psychotherapy: sudden gains and sudden losses identified with session reports. Psychother Res J Soc Psychother Res. 2013;23(1):14–24. doi: 10.1080/10503307.2012.693837. [DOI] [PubMed] [Google Scholar]
- Marquand A.F., Mourão-Miranda J., Brammer M.J., Cleare A.J., CHY Fu. Neuroanatomy of verbal working memory as a diagnostic biomarker for depression. NeuroReport. 2008;19(15):1507–1511. doi: 10.1097/WNR.0b013e328310425e. Oct. [DOI] [PubMed] [Google Scholar]
- McHugh R.K., Whitton S.W., Peckham A.D., Welge J.A., Otto M.W. Patient preference for psychological vs pharmacologic treatment of psychiatric disorders: a meta-analytic review. J Clin Psychiatry. 2013;74(6):595–602. doi: 10.4088/JCP.12r07757. Jun. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntosh A.M., Sullivan P.F., Lewis C.M. Uncovering the genetic architecture of major depression. Neuron. 2019;102(1):91–103. doi: 10.1016/j.neuron.2019.03.022. Apr 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neugebauer V. Amygdala pain mechanisms. Handb. Exp. Pharmacol. 2015;227:261–284. doi: 10.1007/978-3-662-46450-2_13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nouretdinov I., Costafreda S.G., Gammerman A., Chervonenkis A., Vovk V., Vapnik V. Machine learning classification with confidence: Application of transductive conformal predictors to MRI-based diagnostic and prognostic markers in depression. NeuroImage. 2011;56(2):809–813. doi: 10.1016/j.neuroimage.2010.05.023. May. [DOI] [PubMed] [Google Scholar]
- Palpacuer C., Gallet L., Drapier D., Reymann J.-M., Falissard B., Naudet F. Specific and non-specific effects of psychotherapeutic interventions for depression: Results from a meta-analysis of 84 studies. J. Psychiatr. Res. 2017;87:95–104. doi: 10.1016/j.jpsychires.2016.12.015. [DOI] [PubMed] [Google Scholar]
- Ran G., Zhang Q. The neural correlates of attachment style during emotional processing: an activation likelihood estimation meta-analysis. Attach Hum. Dev. 2018;20(6):626–633. doi: 10.1080/14616734.2018.1465105. Nov 2. [DOI] [PubMed] [Google Scholar]
- Redlich R., Almeida J.J.R., Grotegerd D., Opel N., Kugel H., Heindel W. Brain morphometric biomarkers distinguishing unipolar and bipolar depression. A voxel-based morphometry-pattern classification approach. JAMA Psychiatry. 2014;71(11):1222–1230. doi: 10.1001/jamapsychiatry.2014.1100. Nov. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reis S., Grenyer B.F.S. Fearful attachment, working alliance and treatment response for individuals with major depression. Clin Psychol Psychother. 2004;11(6):414–424. Nov. [Google Scholar]
- Ripke S., Wray N.R., Lewis C.M., Hamilton S.P., Weissman M.M., Breen G. A mega-analysis of genome-wide association studies for major depressive disorder. Mol. Psychiatry. 2013;18(4):497–511. doi: 10.1038/mp.2012.21. Apr. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambo C.F., Howard M., Kopelman M., Williams S., Fotopoulou A. Knowing you care: effects of perceived empathy and attachment style on pain perception. Pain. 2010;151(3):687–693. doi: 10.1016/j.pain.2010.08.035. Dec. [DOI] [PubMed] [Google Scholar]
- Sankar A., Zhang T., Gaonkar B., Doshi J., Erus G., Costafreda S.G. Diagnostic potential of structural neuroimaging for depression from a multi-ethnic community sample. BJPsych Open. 2016;2(04):247–254. doi: 10.1192/bjpo.bp.115.002493. Jul. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sankar A., Melin A., Lorenzetti V., Horton P., Costafreda S.G., Fu C.H.Y. A systematic review and meta-analysis of the neural correlates of psychological therapies in major depression. Psychiatry Res. Neuroimaging. 2018;279:31–39. doi: 10.1016/j.pscychresns.2018.07.002. Sep. [DOI] [PubMed] [Google Scholar]
- Strunk D.R., Brotman M.A., DeRubeis R.J., Hollon S.D. Therapist competence in cognitive therapy for depression: predicting subsequent symptom change. J. Consult. Clin. Psychol. 2010;78(3):429–437. doi: 10.1037/a0019631. Jun. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swift J.K., Greenberg R.P., Tompkins K.A., Parkin S.R. Treatment refusal and premature termination in psychotherapy, pharmacotherapy, and their combination: A meta-analysis of head-to-head comparisons. Psychotherapy. 2017;54(1):47–57. doi: 10.1037/pst0000104. Mar. [DOI] [PubMed] [Google Scholar]
- Takeuchi T., Duszkiewicz A.J., Morris R.G.M. The synaptic plasticity and memory hypothesis: encoding, storage and persistence. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 2014;369(1633) doi: 10.1098/rstb.2013.0288. Jan 5. 20130288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang T.Z., DeRubeis R.J. Sudden gains and critical sessions in cognitive-behavioral therapy for depression. J. Consult. Clin. Psychol. 1999;67(6):894–904. doi: 10.1037//0022-006x.67.6.894. Dec. [DOI] [PubMed] [Google Scholar]
- Tang T.Z., DeRubeis R.J., Beberman R., Pham T. Cognitive changes, critical sessions, and sudden gains in cognitive-behavioral therapy for depression. J. Consult. Clin. Psychol. 2005;73(1):168–172. doi: 10.1037/0022-006X.73.1.168. Feb. [DOI] [PubMed] [Google Scholar]
- Vos T., Barber R.M., Bell B., Bertozzi-Villa A., Biryokov S., Global Burden of Disease Study 2013 Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 301 acute and chronic diseases and injuries in 188 countries, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2015;386(9995):743–800. doi: 10.1016/S0140-6736(15)60692-4. Aug 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varol E., Sotiras A., Davatzikos C. Alzheimer's Disease Neuroimaging Initiative. HYDRA: Revealing heterogeneity of imaging and genetic patterns through a multiple max-margin discriminative analysis framework. NeuroImage. 2017;145(Pt B):346–364. doi: 10.1016/j.neuroimage.2016.02.041. 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vittengl J.R., Clark L.A., Thase M.E., Jarrett R.B. Are Improvements in Cognitive Content and Depressive Symptoms Correlates or Mediators during Acute-Phase Cognitive Therapy for Recurrent Major Depressive Disorder? Int. J. Cogn. Ther. 2014;7(3):251–271. doi: 10.1521/ijct.2014.7.3.251. Sep. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W., Zhao Y., Hu X., Huang X., Kuang W., Lui S. Conjoint and dissociated structural and functional abnormalities in first-episode drug-naive patients with major depressive disorder: a multimodal meta-analysis. Sci. Rep. 2017;7(1):10401. doi: 10.1038/s41598-017-08944-5. Dec. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb C.A., Derubeis R.J., Barber J.P. Therapist adherence/competence and treatment outcome: a meta-analytic review. J. Consult. Clin. Psychol. 2010;78(2):200–211. doi: 10.1037/a0018912. Apr. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissman M.M., Prusoff B.A., Dimascio A., Neu C., Goklaney M., Klerman G.L. The efficacy of drugs and psychotherapy in the treatment of acute depressive episodes. Am. J. Psychiatry. 1979;136(4B):555–558. Apr. [PubMed] [Google Scholar]
- Wise T., Radua J., Via E., Cardoner N., Abe O., Adams T.M. Common and distinct patterns of grey-matter volume alteration in major depression and bipolar disorder: evidence from voxel-based meta-analysis. Mol. Psychiatry. 2017;22(10):1455–1463. doi: 10.1038/mp.2016.72. Oct. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization . 2nd edition. World Health Organization; Geneva: 2004. International Statistical Classification of Diseases. 10th revision. [Google Scholar]