Abstract
Objectives
Serum creatinine is an important clinical marker for renal clearance. However, the Jaffe method had much interference and the accuracy had not been tested in patients under hemodialysis (HD) with standard isotope dilution‐liquid chromatography‐mass spectrometry (IDLCMS) method. The validity of enzymatic method is also unknown.
Methods
The predialysis serum creatinine levels of 126 patients under regular HD for 3 months were checked by Jaffe, enzymatic, and IDLCMS methods. We compared the value of the Jaffe and enzymatic to that of IDLCMS in linear regression model. And we also tried to find the clinical parameters that influence the difference between Jaffe vs. IDLCMS and enzymatic vs. IDLCMS method.
Results
We found significant underestimate serum creatinine in uremic patients by Jaffe and enzymatic methods. Serum glucose and globulin are positive biases, whereas albumin, potassium, and phosphorus are negative biases. Enzymatic method is less affected by serum glucose and serum protein. Albumin acts differently in uremic serum compared to the results of mixing them with normal serum.
Conclusions
For uremic patients, in whom creatinine level is high and many of them suffered from diabetes mellitus, serum creatinine can be either under‐ or overestimated by Jaffe method. Enzymatic method is less affected and may be a better method. J. Clin. Lab. Anal. 26:206‐214, 2012. © 2012 Wiley Periodicals, Inc.
Keywords: creatinine, Jaffe method, enzymatic method, isotope dilution‐liquid chromatography‐mass spectrometry (IDLCMS), hemodialysis (HD), uremia
INTRODUCTION
The renal function was most accurately determined by the clearance of inulin 1, 2. However, it is impossible to be performed on every person with kidney disease on a regular basis. So, we generally measure serum creatinine instead 3. The measurement of creatinine is the most common way to determine the renal function of a patient, so it is of great clinical importance. But the value of serum creatinine can be influenced by the method and other confounding factors 4. Currently, there are three kinds of methods available for clinical use. One is the most popular method, the Jaffe method 5. The second is the enzymatic method and the last is the isotope dilution‐liquid chromatography‐mass spectrometry (IDLCMS) method 6, 7.
The Jaffe method is popular; however, this method is easily confounded with hydrogen carbon compound such as nitromethane and other noncreatinine chromatogen 8, 9, 10, 11. The enzymatic method is less affected due to is specificity 6. But it can also be confounded by certain carbonyl compound. IDLCMS is the most accurate method, but it is expensive and the cost limits its application 12, 13, 14, 15. In normal people, these three methods showed good linear correlation, but the linear correlation of these three different methods has not been proved in uremic patients, in whom serum creatinine level is much higher than normal population 16. Uremic patients suffer from renal failure and are subjected to accumulation of toxic waste 17. Because there is no such study concerning measurement error of serum creatinine in uremic people in Jaffe and enzymatic methods compared to IDLCMS method, so we try to determine whether Jaffe or enzymatic method is confounded in uremic patients.
MATERIALS AND METHODS
Inclusion and Exclusion Criteria
This is a cross‐sectional study approved by the institutional review board. We included patients who have received 4 hr of maintenance HD therapy three times weekly for at least 3 months. Patients who had peritoneal dialysis and transplantation are excluded. Patients who had intravenous lipid nutrition supplement, propofol, dopamine, methotrexate, fluoruracil, vancomycin, predisolone, furosemide, or cyclosporine are also excluded. A total of 126 patients are included.
Clinical and Blood Result Data Collection
Demographic and clinical data such as age, gender, body weight, duration of dialysis, cause of renal failure (diabetes, or chronic glomerulonephritis), and duration of receiving hemodialysis (HD) were obtained from the medical records. Laboratory parameters are gathered at the beginning of the month prior to HD.
Creatinine and Other Biochemical Parameter Measurement
We use IDLCMS as gold standard to evaluate the other two methods. First, we check their correlation. Then, we checked the association of their difference to the clinical and biochemical profile.
The IDLCMS method is determined as following steps. A 10‐μl aliquot of each serum sample was diluted with 740 μl of distilled water and fortified with 500 μl of 1 μg/ml creatinine‐D3 solution (C/D/N Isotopes Inc., Quebec, Canada). Three volumes of methanol were added to the above mixture to precipitate out proteins. After vortexing for 1 min and centrifugation at 12,000g for 10 min at 4°C, 500 μl of the supernatant was collected for further analysis. The quantitative LC‐MS/MS analysis of creatinine was performed on an Agilent 1200 series SL RRLC system coupled to an Agilent 6410 triple quadruple mass spectrometer equipped with an ESI source in positive mode (Agilent Tech., Santa Clara, CA). One microliter of the sample was injected onto a 2.1 mm × 100 mm Kinetex 2.6‐μm HILIC column (Phenomenex Inc., Torrance, CA) with a mobile phase consisting of 0.1% formic acid in water (A) and in acetonitrile (B) at a constant flow rate of 0.3 ml/min. The following linear gradient program for elution was applied: 0–1 min held on 90% B, 1–5 min linearly decreased from 90% to 40% B, and held for 1 min before returning to initial condition. Optimized MS parameters were as follows: capillary voltage of 4,000 V, drying gas temperature of 350°C, drying gas flow rate of 8 l/min, nitrogen nebulizer pressure of 30 psi, and dwell time of 200 msec. The detection was carried out by the multiple reaction monitoring (MRM) modes. The fragmentor voltage (V), collision energy (V), and MRM transitions monitored were as follows: 102, 15, m/z 117→47 for creatinine‐D3; 100, 20, m/z 114→44 for creatinine. The monitored precursor and product ions were acquired by the Agilent MassHunter Qualitative software and quantified with the Agilent MassHunter Quantitative software. The standard at concentrations ranging from 0.0025 to 0.05 mg/dl was used to establish the standard curve constructed by plotting the ratio of peak area for creatinine to that for creatinine‐D3 vs. analyte concentration for six concentrations (0.0025, 0.005, 0.0075, 0.01, 0.025, and 0.05 mg/dl).
The hemogram auto‐analyzer was SYSMEX XE2100 (Sysmex, Kobe, Japan). The Jaffe method and other biochemical parameters are determined by ADVIA® 1800 Chemistry System, Siemens, Germany. Jaffe's colorimetric method is made by injecting patient's serum into an alkaline picrate solution. There are two reagents used in this method with components and concentrations listed as following: Reagent 1(R1) : Sodium hydroxide 0.2 mol/l, Reagent 2 (R2) : Picric acid 20 mmol/l. Mix four parts of R1 and one part of R2 to form a monoreagent. The stability of monoreagent: 5 hr at 15–25 °C. We incubated 50 μl of distill water with 1,000 μl monoreagent for 60 sec as blank and 50 μl of patient serum with 1,000 μl monoreagent for 180 sec as sample. Creatinine in the serum sample combines with alkaline picrate to form a red‐colored complex or chromophore, the light absorbance of which can then be measured in the 490–510 nm range. The rate of absorbance is directly proportional to the creatinine concentration in the serum as compared to the blank.
The enzymatic method is determined by Beckman AU640 with amidohydrolase procedure that uses the reaction sequence:
The hydrogen peroxide generated in the above reaction sequence can be measured spectrophotometrically using a Trinder's reaction acceptor, producing a quinoneimine with high molar absorptivity.
Statistical Analysis
Data statistical analysis was done using the SPSS statistical software (version 18.0). Distributions of continuous variables in groups were expressed as mean ± SD and compared by Student's t‐test. A statistically significant value was P less than 0.05.
RESULTS
The Demographic and Laboratory Profile
We list the profile in Table 1. It is representative of the general uremic patients under regular dialysis in Taiwan and the sex distribution is even. The creatinine level is high above normal limit due to uremia.
Table 1.
Demographic Data, Hemogram, and Biochemical Profile
| Item | Normal range | Mean | SD | |
|---|---|---|---|---|
| Demographic profile | Age (year) | 62.55 | 14.28 | |
| Male n(%) | 68 (53.5%) | |||
| DM n(%) | 63 (50.0%) | |||
| CGN n(%) | 30 (23.6%) | |||
| HD duration (year) | 4.15 | 5.07 | ||
| Hemogram | WBC (×1,000/μl) | 3.5—10 | 7.14 | 2.01 |
| R.B.C. (×106/μl) | M/F:4.2–6.2/3.7–5.5 | 3.49 | 0.51 | |
| Hb (g/dl) | M/F:12.3–18.3 /11.3–15.3 | 10.46 | 1.36 | |
| Hct (%) | M/F:39–53/ 33–47 | 32.01 | 4.14 | |
| MCV (fl) | 80–99 | 92.21 | 6.21 | |
| Platelet (×1,000/μl) | 150–400 | 211.26 | 87.60 | |
| Biochemical profile | Cholesterol (mg/dl) | <200 | 162.46 | 32.58 |
| Triglyceride (mg/dl) | <150 | 142.78 | 86.97 | |
| Glucose (mg/dl) | 70–100 | 152.49 | 79.49 | |
| Total protein (gm/dl) | 6.0–8.3 | 6.53 | 0.61 | |
| Albumin (gm/dl) | 3.5–5.3 | 3.89 | 0.45 | |
| AST (IU/l) | 10–42 | 23.34 | 10.68 | |
| ALT (IU/l) | 10–49 | 21.14 | 16.02 | |
| Alk‐P (IU/l) | 37–108 | 101.87 | 51.93 | |
| Total bilirubin (mg/dl) | 0.2–1.2 | 0.20 | 0.14 | |
| Uric acid (mg/dl) | M/F:4.0–7.5 /3.0–6.0 | 7.10 | 1.29 | |
| Sodium (meq/l) | 132–146 | 139.23 | 3.26 | |
| Potassium (meq/l) | 3.5–5.5 | 4.73 | 0.71 | |
| Calcium (mg/dl) | 8.3–10.6 | 9.29 | 0.84 | |
| Phosphorus (mg/dl) | 2.4–5.1 | 4.72 | 1.33 | |
| Creatinine (mg/dl) | By Jaffe method | M/F: 0.7–1.3/0.5–1.1 | 9.01 | 2.42 |
| By enzymatic method | 8.82 | 2.44 | ||
| By IDLCMS method | 9.51 | 2.61 |
DM, diabetes mellitus; CGN, chronic glomerulonephritis; HD, hemodialysis; WBC, white blood cell, RBC, red blood cell; Hb, hemoglobulin; Hct, hematocrit; MCV, mean corpuscular volume; AST, aspartate aminotransferase; ALT, Alanine transaminase; Alk‐P, alkaline phosphatase; IDLCMS, isotope dilution‐liquid chromatography‐mass spectrometry, M/F: male/ female.
The Linear Correlation of Creatinine Values Determined by Three Methods
We try to verify whether the linear correlation still exist in the uremic group, when using Jaffe and enzymatic method compared to IDLCMS method. Figure 1 showed that both methods (Jaffe and enzymatic methods) showed good correlation as compared to the IDLCMS method. The linear regression statistics for Jaffe vs. IDLCMS is y = 0.9152x + 0.3088 (R 2 = 0.9712), while for enzymatic vs. IDLCMS is y = 0.9228x + 0.0453 (R 2 = 0.9716). In Both cases, numbers are 126.
Figure 1.
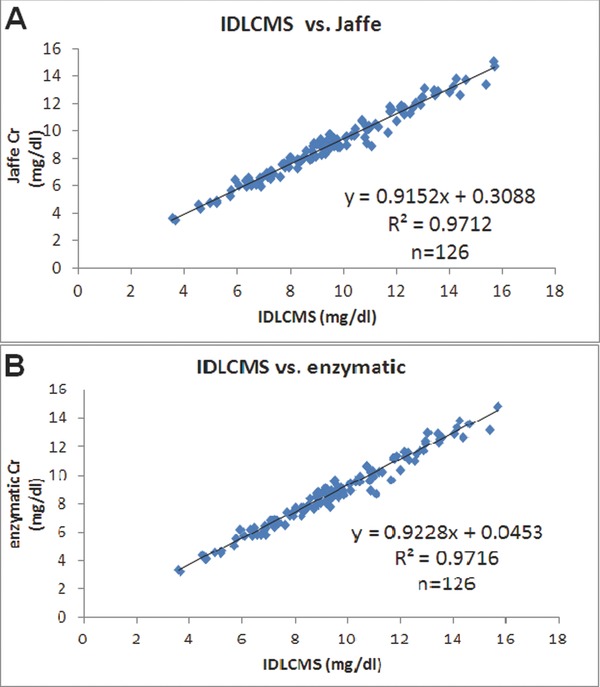
The linear correlations of creatinine values determined by three methods. (A) IDLCMS vs. Jaffe. (B) IDLCMS vs. enzymatic.
The Linear Correlation of Creatinine Measurement Differences Determined by Three Methods and Clinical Parameters
We try to verify the association between the difference of Jaffe and enzymatic methods (compared to IDLCMS) and the clinical parameters, which include age, sex, cause of renal failure (diabetes mellitus [DM], chronic glomerulonephritis), hemogram, biochemical parameters (triglyceride, cholesterol, glucose, total protein, albumin, liver function, total bilirubin, uric acid, electrolyte, BUN, Cr) (Table 1).
If we define a substance can falsely increase the serum creatinine value by certain method as a positive bias, and substance can decrease creatinine value as a negative bias, we can get the results by using univariate linear regression model (Table 2). As renal function deteriorated with increased serum creatinine level, the negative deviation of serum creatinine also increased. There could be a substance accumulated in patient's serum, which acts as a negative bias. Glucose is a positive bias for both, with stronger effect to Jaffe method (Fig. 2). Serum protein is unrelated to serum creatinine difference. However, albumin is a strong negative bias, while globulin is a strong positive bias for Jaffe method. This effect is less significant in enzymatic method (Fig. 3). Potassium and phosphorus are strong negative biases for both methods (Fig. 4). The multivariate linear regression model was presented in Table 3. It showed that none of the factors (glucose, albumin, globulin, Cr determined by IDLCMS) are independent factors to determine the deviation of serum measured by either Jaffe or enzymatic methods, except for the real serum creatinine value. Although it may be contributed by the calibration procedure, the possibility of certain substance that acts as a negative bias still cannot be ruled out 18.
Table 2.
The Univariate Linear Regression Model of Clinical Factors Associated With Creatinine Difference Measured by Jaffe vs. IDLCMS and Enzymatic vs. IDLCMS
| Jaffe–IDLCMS (P/coefficient) | Enzymatic–IDLCMS (P/coefficient) | |||
|---|---|---|---|---|
| Cr by IDLCMS (mg/dl)*, † | 2.07 × 10−8 | –0.085 | 2.61 × 10−7 | –0.077 |
| Glucose (mg/dl)* | 0.019 | 0.001 | 0.051 | 0.001 |
| Total protein (g/dl) | 0.472 | 0.050 | 0.598 | 0.036 |
| Albumin (g/dl)* | 0.041 | –0.233 | 0.068 | –0.204 |
| Globulin (g/dl)* | 0.022 | 0.167 | 0.057 | 0.123 |
| Potassium (meq/l)*, † | 0.035 | –0.125 | 0.040 | –0.120 |
| Phosphorus (mg/dl)*, † | 0.052 | –0.061 | 0.043 | –0.062 |
*P < 0.05 in Jaffe‐ IDLCMS. † P < 0.05 in enzymatic‐IDLCMS.
IDLCMS, isotope dilution‐liquid chromatography‐mass spectrometry; Jaffe–IDLCMS, difference between Jaffe and IDLCMS; Enzymatic–IDLCMS, difference between enzymatic and IDLCMS.
Figure 2.
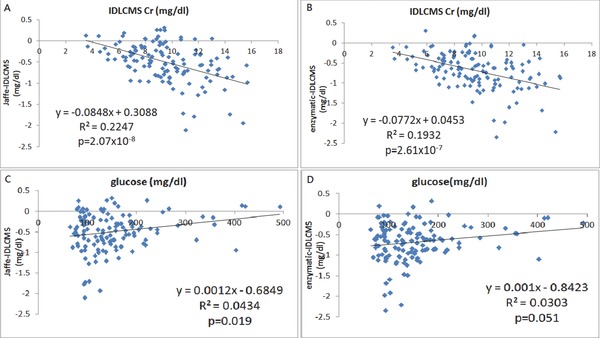
The linear correlation of creatinine differences determined Jaffe vs. IDLCMS and enzymatic vs. IDLCMS with serum Cr concentrations (by IDLCMS) (A and B), and glucose levels (C and D), respectively.
Figure 3.
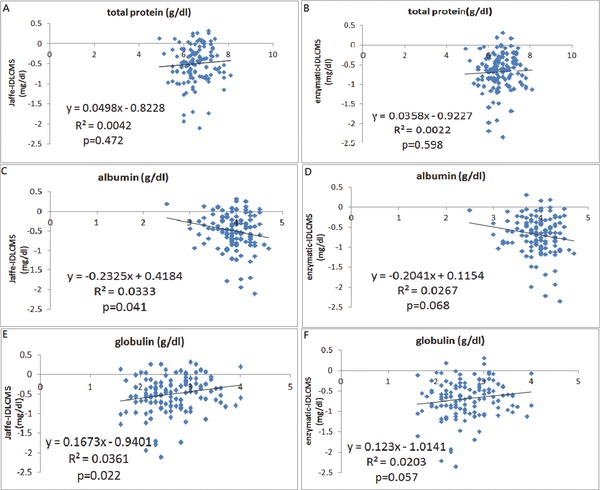
The linear correlation of creatinine differences determined by Jaffe vs. IDLCMS and enzymatic vs. IDLCMS with total protein (A and B), albumin (C and D), globulin (total protein‐albumin) (E and F), respectively.
Figure 4.
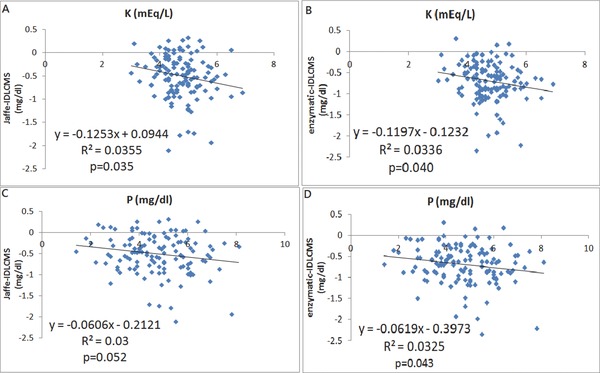
The linear correlation of creatinine differences determined Jaffe vs. IDLCMS and enzymatic vs. IDLCMS with potassium (A and B) and phosphorus (C and D), respectively.
Table 3.
The Multivariate Linear Regression Model of Clinical Factors Associated With Creatinine Difference Measured by Different Methods Respectively
| 95% CI for B | |||||
|---|---|---|---|---|---|
| Cr difference between Jaffe and IDLCMS | B estimate | P‐value | Lower | Upper | r = 0.526, r 2 = 0.277 |
| Glucose (for each 1 mg/dl increase) | 0.001 | 0.298 | 0.000 | 0.002 | |
| Albumin (for each 1g/dl increase) | 0.175 | 0.146 | –0.062 | 0.412 | |
| Globulin (for each 1g/dl increase) | 0.126 | 0.080 | –0.015 | 0.268 | |
| Cr by IDLCMS (for each 1 mg/dl increase)* | –0.086 | 1.15×10−6 | –1.119 | –0.053 | |
| 95% CI for B | |||||
|---|---|---|---|---|---|
| Cr difference between enzymatic and IDLCMS | B estimate | P‐value | Lower | Upper | r = 0.491, r 2 = 0.241 |
| Glucose (for each 1 mg/dl increase) | 0.000 | 0.484 | –0.001 | 0.001 | |
| Albumin (for each 1g/dl increase) | 0.170 | 0.159 | –0.068 | 0.409 | |
| Globulin (for each 1g/dl increase) | 0.092 | 0.205 | –0.051 | 0.234 | |
| Cr by IDLCMS (for each 1 mg/dl increase)* | –0.080 | 5.34 × 10−6 | –0.114 | –0.047 | |
*P < 0.05.
B, unstandardized regression beta coefficient; CI, confidence intervals; IDLCMS, isotope dilution‐liquid chromatography‐mass spectrometry.
As for comparison in different groups, we found that there are no significant difference by both methods between different sex groups (male vs. female), or age groups (age ≤ 65 vs. age > 65). However, there are significant differences between DM and non‐DM groups (Table 4). Serum creatinine value measured by Jaffe method is higher in DM group than that of non‐DM group. If we compare the glucose (AC) level between DM and non‐DM groups, we found that the average glucose (AC) level is much higher in the DM group (mean and SD of glucose between DM and non‐DM group: 185 ± 95 vs. 118 ± 33 mg/dl, n = 62:64, P < 0.0001). Although this may be related to other factors such as oral medications including sulfonylurea or antibiotic, serum glucose certainly played a role in the elevation of measured serum creatinine in the Jaffe method as a positive bias 19.
Table 4.
The Comparison With Differences of Creatinine‐Measuring Methods in Different Groups
| Jaffe–IDLCMS | Enzymatic–IDLCMS | ||||||
|---|---|---|---|---|---|---|---|
| Groups | Number | Mean | SD | P | Mean | SD | P |
| Age > 65 | 64 | –0.45 | 0.51 | 0.21 | –0.66 | 0.50 | 0.41 |
| Age ≤ 65 | 62 | –0.55 | 0.43 | –0.73 | 0.43 | ||
| Male | 64 | –0.51 | 0.44 | 0.83 | –0.71 | 0.45 | 0.66 |
| Female | 62 | –0.50 | 0.49 | –0.68 | 0.47 | ||
| DM | 62 | –0.41 | 0.45 | 0.04* | –0.62 | 0.43 | 0.11 |
| Non‐DM | 64 | –0.58 | 0.47 | –0.75 | 0.48 | ||
*P < 0.05.
IDLCMS, isotope dilution‐liquid chromatography‐mass spectrometry; Jaffe–IDLCMS, difference between Jaffe and IDLCMS; Enzymatic–IDLCMS, difference between enzymatic and IDLCMS.
DISCUSSION
We try to find if uremia possesses any influence on Jaffe method and enzymatic method and try to determine the influence factors. We found serum creatinine had a significant negative bias from real value as the real creatinine concentration increased (Fig. 2). We speculate that certain substance is accumulated in uremic patients’ serum and exerts its effect as a negative bias after binding with albumin. Thus, the higher the albumin, the more the negative‐bias substance can bind with albumin and exert its negative‐bias effect. This hypothesis can explain why albumin became a strong negative bias in uremic serum instead of positive bias in normal serum. This substance should be a positively charged substance because albumin carries a negative charge. Uremic patients have less renal function and are prone to accumulate toxic waste in their body. However, it needs further investigation 17.
Also serum glucose can falsely increase creatinine measured by Jaffe or enzymatic methods, which is compatible with previous study results 6, 20, 21. However, it influences the value measured by Jaffe method more than that measured by enzymatic method.
Total protein, which is considered as a positive bias when mixing normal patients’ serum with albumin in the previous study, is of no significant association with difference between Jaffe and IDLCMS, also enzymatic and IDLCMS 21. However, if look deeper, we can find albumin is a strong negative bias for Jaffe method, and globulin (total protein minus albumin) is a strong positive bias. But the effect is less significant for enzymatic method (0.05 < P < 0.1). The reason why total protein is of no significant association is likely to be that it is the summation of albumin and globulin. The reason that globulin is a strong positive bias may be that globulin can form a pricrate‐protein compound as a positive chromatogen 21. The reason that albumin is a strong negative bias could be that albumin is a good carrier that can combine with many substances, such as dopamine, total bilirubin, etc. and may be some of these substances are accumulated in uremic patients’ serum, which act as strong negative biases for Jaffe method 20, 21. Although total bilirubin has been identified as a negative bias previously, we did not find any consisted data in this study 22. This is possibly because in our study the value of total bilirubin is too low to show the effect as a negative bias (<1.4 mg/dl).
We hypothesized that a negative bias is highly accumulated in uremic patients due to following reasons. First, the serum creatinine is constantly underestimated by either method. Second, albumin is no longer a positive bias as it is in a normal patient serum, leaving globulin to act solely as a positive bias. And it could be explained by the fact that albumin binds with this negative‐bias substance and exerts a negative‐bias effect. Third, potassium (K) and phosphorus (P) are representatives of uremic toxins and have strong association with negative bias by two methods, despite that K and P are different in molecular size and electrolyte charges.
There are some limitations for this study. First, our method only utilizes one kind of Jaffe method. So without additional confirmation with other modified Jaffe methods, the findings are applicable only to the current creatinine method used. Second, we do not know what this negative‐bias substance is and we can only speculate it is accumulated in uremic serum and combined with albumin with indirect proofs. However, it seems a logical explanation that awaits more investigation.
We proved there is a significant underestimation (6–7%) by current Jaffe or enzymatic methods in uremic serum as compared to IDLCMS standard method 23 (Table 1). Serum glucose is a strong positive bias, whereas albumin acts as a negative bias and globulin acts as a positive bias in Jaffe method. But the enzymatic method is less affected by serum sugar and protein. Although there are some indirect evidences in this study, more investigation is needed to explore this substance and to improve the clinical accuracy of serum creatinine measurement 16.
CONCLUSIONS
This is the first study to our knowledge concerning the clinical factors influencing the serum creatinine value determined by three methods (one kind of Jaffe, enzymatic, and IDLCMS methods) in HD patients. We proved there is a significant underestimation by current Jaffe and enzymatic methods in uremic serum when serum creatinine concentration is high above normal limit. Albumin acts differently in uremic serum as compared to the results of mixing them with normal serum. Serum glucose and protein had a significant effect on Jaffe method, but to a less significant extend on enzymatic method. As for uremic patients, in whom serum creatinine level is high and many of them suffered from DM as their cause of renal failure, serum creatinine can be either under‐ or overestimated by Jaffe method. Enzymatic method seems to be a better way when considering serum glucose and protein interference.
ACKNOWLEDGMENTS
This work was approved by institutional review board of Taipei Veterans General Hospital (VGHIRB No.: 201010032IC) in Taiwan.
Grant sponsor: Taipei Veterans General Hospital; Grant number: V100B‐030 and V101A‐011.
Author Wen‐Sheng Liu and Yu‐Ting Chung contributed equally to this work.
Financial disclosure and conflict of interest: none.
REFERENCES
- 1. Ocampo JH, Rosales AT, Castellanos FR. Comparison of four methods for measuring glomerular filtration rate by inulin clearance in healthy individuals and patients with renal failure. Nefrologia 2010;30:324–330. [DOI] [PubMed] [Google Scholar]
- 2. Schwartz GJ, Furth SL. Glomerular filtration rate measurement and estimation in chronic kidney disease. Pediatr Nephrol 2007;22:1839–1848. [DOI] [PubMed] [Google Scholar]
- 3. Bjornsson TD. Use of serum creatinine concentrations to determine renal function. Clin Pharmacokinet 1979;4:200–222. [DOI] [PubMed] [Google Scholar]
- 4. Weber JA, Vanzanten AP. Interferences in current methods for measurements of creatinine. Clin Chem 1991;37:695–700. [PubMed] [Google Scholar]
- 5. Blass KG, Thibert RJ, Lam LK. Study of mechanism of jaffe reaction. Zeitschrift Fur Klinische Chemie Und Klinische Biochemie 1974;12:336–343. [DOI] [PubMed] [Google Scholar]
- 6. Gottschalk EM, Holdt‐Lehmann B, Bastian M, Schmitz RA, Schuff‐Werner P. Automatisation and interference testing of a new creatinine assay using recombinant creatinine deiminase. Clin Chem 2004;50:A27–A27. [Google Scholar]
- 7. Tsikas D, Wolf A, Mitschke A, Gutzki F‐M, Will W, Bader M. GC‐MS determination of creatinine in human biological fluids as pentafluorobenzyl derivative in clinical studies and biomonitoring: Inter‐laboratory comparison in urine with Jaffe, HPLC and enzymatic assays. J Chromatogr B 2010;878:2582–2592. [DOI] [PubMed] [Google Scholar]
- 8. Deleacy EA, Brown NN, Clague AE. Nitromethane interferes in assay of creatinine by the jaffe reaction. Clin Chem 1989;35:1772–1774. [PubMed] [Google Scholar]
- 9. Booth CJ, Naidoo D, Rosenberg AR, Kainer G. Elevated creatinine after ingestion of model aviation fuel: Interference with the Jaffe reaction by nitromethane. J Paediatr Child Health 1999;35:503–504. [DOI] [PubMed] [Google Scholar]
- 10. Bruns DE. Lactulose interferes in the alkaline picrate assay for creatinine. Clin Chem 1988;34:2592–2593. [PubMed] [Google Scholar]
- 11. Feld LG, Langford DJ, Schwartz GJ. The effect of neonatal hyperbilirubinemia on the measurement of plasma creatinine. Clini Pediatr 1984;23:154–156. [DOI] [PubMed] [Google Scholar]
- 12. Linnet K, Bruunshuus I. HPLC with enzymatic detection as a candidate reference method for serum creatinine. Clin Chem 1991;37:1669–1675. [PubMed] [Google Scholar]
- 13. Guy JM, Legg EF. An improved cation exchange HPLC method for the measurement of serum creatinine. Ann Clin Biochem 1990;27(Pt 3):223–226. [DOI] [PubMed] [Google Scholar]
- 14. Seronie‐Vivien S, Galteau MM, Carlier MC, Hadj‐Aissa A, Hanser AM, Hym B, Marchal A, Michotey O, Pouteil‐Noble C, Strernberg M, Perret‐Liaudet A. Improving the interlaboratory variation for creatinine serum assay. Ann Biol Clin 2004;62:165–175. [PubMed] [Google Scholar]
- 15. Yuen PST, Dunn SR, Miyaji T, Yasuda H, Sharma K, Star RA. A simplified method for HPLC determination of creatinine in mouse serum. Am J Physiol Renal Physiol 2004;286:F1116–F1119. [DOI] [PubMed] [Google Scholar]
- 16. Myers GL, Miller WG, Coresh J, Fleming J, Greenberg N, Greene T, Hostetter T, Levey AS, Panteghini M, Welch M, Eckfeldt JH. Recommendations for improving serum creatinine measurement: A report from the laboratory working group of the National Kidney Disease Education Program. Clin Chem 2006;52:5–18. [DOI] [PubMed] [Google Scholar]
- 17. Kikuchi K, Itoh Y, Tateoka R, Ezawa A, Murakami K, Niwa T Metabolomic search for uremic toxins as indicators of the effect of an oral sorbent AST‐120 by liquid chromatography/tandem mass spectrometry. J Chromatogr B 2010;878:2997–3002. [DOI] [PubMed] [Google Scholar]
- 18. Owen LJ, Wear JE, Keevil BG. Validation of a liquid chromatography tandem mass spectrometry assay for serum creatinine and comparison with enzymatic and Jaffe methods. Ann Clin Biochem 2006;43:118–123. [DOI] [PubMed] [Google Scholar]
- 19. Delanghe JR, Louagie HK, De Buyzere ML, Leroux‐Roels GG. Glomerular filtration rate and creatinine production in adult icteric patients. Clin Chim Acta 1994;224:33–44. [DOI] [PubMed] [Google Scholar]
- 20. Apple F, Bandt C, Prosch A, Erlandson G, Holstrom V, Scholen J, Googins M. Creatinine clearance: Enzymatic vs Jaffe determinations of creatinine in plasma and urine. Clin Chem 1986;32:388–390. [PubMed] [Google Scholar]
- 21. Peake M, Whiting M. Measurement of serum creatinine—current status and future goals. Clin Biochem Rev 2006;27:173–184. [PMC free article] [PubMed] [Google Scholar]
- 22. Srisawasdi P, Chaichanajarernkul U, Teerakanjana N, Vanavanan A, Kroll MH. Exogenous interferences with Jaffe creatinine assays: Addition of sodium dodecyl sulfate to reagent eliminates bilirubin and total protein interference with Jaffe methods. J Clin Lab Anal 2010;24:123–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hetu PO, Gingras ME, Vinet B. Development and validation of a rapid liquid chromatography isotope dilution tandem mass spectrometry (LC‐IDMS/MS) method for serum creatinine. Clin Biochem 2010;43:1158–1162. [DOI] [PubMed] [Google Scholar]


