Abstract
The significance of antibodies to cardiolipin (anti‐CL) remains uncertain in patients with chronic hepatitis C (CH‐C). The main purpose of this study was to elucidate the clinical characteristics of patients with CH‐C seropositive for anti‐CL. The prevalence of anti‐CL and clinical parameters associated with anti‐CL in those patients were examined. Six of the 45 (13%) patients with CH‐C had anti‐CL. However, none of these six CH‐C patients fulfilled the criteria for antiphospholipid syndrome. Serum triglyceride and apolipoprotein B (ApoB) levels in CH‐C patients with anti‐CL were significantly higher than those in CH‐C patients without anti‐CL. Serum triglyceride levels positively correlated with serum ApoB levels. CH‐C patients with anti‐CL had significantly more progressive hepatic fibrosis than those without anti‐CL. The degree of 8‐hydroxy 2′‐deoxyguanosine (8‐OHdG) expression in the liver tissue was more severe in CH‐C patients with anti‐CL than in those without it. However, the emergence of anti‐CL in CH‐C patients was independent of insulin resistance, hepatic steatosis, and iron overload. These findings suggest that the emergence of anti‐CL is associated with oxidative stress and that CH‐C patients seropositive for anti‐CL have clinical characteristics of hypertriglyceridemia, which derives from the facilitation of ApoB synthesis, and progressive hepatic fibrosis. J. Clin. Lab. Anal. 26:342‐348, 2012. © 2012 Wiley Periodicals, Inc.
Keywords: antibodies to cardiolipin, apolipoprotein B, hepatic fibrosis, hepatitis C virus, oxidative stress, triglyceride
INTRODUCTION
It has been well established that persistent hepatitis C virus (HCV) infection often evokes autoimmune phenomena including the production of autoantibodies and/or concurrent autoimmune diseases 1, 2. The diversity of autoantibodies such as nonorgan‐specific autoantibodies and liver‐specific autoantibodies detected in the sera of patients with HCV‐related chronic liver disease has been widely discussed 3.
Antibodies to cardiolipin (anti‐CL), the serological hallmark of antiphospholipid syndrome 4, are also present in sera of patients with autoimmune liver diseases including autoimmune hepatitis 5 and primary biliary cirrhosis 6, and alcoholic liver disease 7. A recent study revealed that the emergence of anti‐CL was associated with oxidative stress in experimental mouse models 8.
Persistent HCV infection often initiates oxidative stress 9, 10 and subsequent metabolic abnormalities including insulin resistance, hepatic steatosis, and iron overload 11, 12, 13. Anti‐CL are also found in the sera of patients with HCV‐related chronic liver disease 14, 15, 16, 17, 18, 19. Patients with chronic hepatitis C (CH‐C) who are seropositive for anti‐CL are generally free from thrombocytopenia and thrombosis 15, 17, 19. Previous reports revealed the close association between the emergence of anti‐CL in CH‐C patients and concomitant extrahepatic autoimmune disease including lichen planus 20 and mixed cryoglobulinemia 21. However, other clinical significance of anti‐CL in patients with HCV‐related chronic liver disease remain unclear. The primary purposes of this study were to examine the prevalence of anti‐CL in the sera of patients with CH‐C, to analyze the clinical parameters and concurrent autoimmune disease or metabolic diseases linked to anti‐CL, and finally, to investigate the relationship between the emergence of anti‐CL and oxidative stress or subsequent metabolic abnormalities caused by persistent HCV infection.
MATERIALS AND METHODS
Study Population
Forty‐five patients with CH‐C, who had detectable serum HCV‐RNA by polymerase chain reaction and showed histological findings compatible with chronic hepatitis, were randomly selected for participation in this study. Eleven patients with chronic hepatitis B (CH‐B), eight patients with alcoholic liver diseases, ten patients with nonalcoholic steatohepatitis (NASH), and 12 cases of normal healthy control (NHC) were also enrolled as comparison groups. Patients with CH‐B had hepatitis B surface antigen (HBsAg) and hepatitis B virus DNA (HBV‐DNA) in their sera. Patients with alcoholic liver diseases included those with alcoholic hepatitis and alcoholic liver cirrhosis. Patients with NASH had NASH activity scores 22 of 5 or greater.
Clinical Assessments
Age at entry, gender and the prevalence of concurrent metabolic diseases including type 2 diabetes mellitus (DM), hypertension and hyperlipidemia, and concurrent autoimmune diseases were examined in the patients with CH‐C. Obesity was evaluated by body mass index (BMI), which was calculated in accordance with the formula of weight (kg) divided by height2 (m2).
Laboratory Assessments
Circulating anti‐CL of the immunoglobulin G (IgG) isotype were determined using commercially available enzyme‐linked immunosorbent assay (ELISA) kits (Medical and Biological Laboratories Co., Ltd., Nagoya, Japan) according to the method of Gharavi and colleagues 23. The cutoff value of the antibodies was set at 10 U/ml. Analysis of antibodies to β2‐glycoprotein I (anti‐β2‐GPI) was also performed in sera of patients with CH‐C seropositive for anti‐CL using an ELISA kit 24 (Yamasa Corporation, Tokyo, Japan, normal range: <3.5 U/ml). Peripheral platelet count and biochemical tests, including alanine aminotransferase (ALT), total cholesterol (T‐Cho), triglyceride (TG), and apolipoprotein B (ApoB) levels, were examined in the enrolled patients with CH‐C. Serum ferritin concentrations were also assessed as a serological hallmark of iron overload. Insulin resistance was determined by the Homeostasis Model for Assessment of Insulin Resistance (HOMA‐IR) method using the following equation: HOMA‐IR = Fasting insulin (μU/ml) × fasting glucose (mg/dl)/405. Quantitative detection of serum HCV‐RNA was performed by the Amplicor‐HCV monitor assay 25 (Roche Molecular Diagnostics, Tokyo, Japan). The HCV genotype was determined by the HCV‐RNA genotyping assay system 26 (Home Brew SRL Inc., Tokyo, Japan). As immunoserological assessments, antinuclear antibodies (ANA) and serum IgG levels were measured. ANA was tested at a serum dilution of 1:40 by the indirect immunofluorescence method, using HEp‐2 cells as substrates. Positive reactions were titered by double dilution to the end point.
Histological Assessments
Liver tissue specimens were obtained by liver biopsy under the guidance of ultrasound, using 16‐gauge needles, before treatment. The tissue samples were fixed in 10% formalin and embedded in paraffin. The tissue sections were stained with hematoxylin and eosin for morphological evaluation. The severity of hepatic steatosis was graded on the basis of the classification proposed by Brunt and colleagues 27. Briefly, steatosis observed in none, less than 33%, 33–66%, or more than 66% of hepatocytes was defined as grades 0, 1, 2, or 3, respectively. Fibrosis and necroinflammation in the liver were evaluated in accordance with the New Inuyama Classification system 28, which is a standard criterion for the histological assessment of chronic hepatitis in Japan, and histological activity index (HAI) scores designed by Knodell 29. The staging of hepatic fibrosis by the New Inuyama Classification was classified into F0 through F4. F0 is defined as no fibrosis in the tissue specimen, while F4 is defined as liver cirrhosis. On the other hand, the severity of grading was classified into A0 (no inflammation) through A3 (severe inflammation) 28.
The expression of 8‐hydroxy 2′‐deoxyguanosine (8‐OHdG), the hallmark for oxidatively generated DNA damage 30, in the liver tissue was examined by an immunohistochemical procedure. In brief, tissue sections were deparaffinized. Each specimen was washed by phosphate‐buffered saline (PBS) and incubated with mouse antihuman monoclonal antibody to 8‐OHdG (1 μg/ml) (Nikken Seil, Co., Ltd., Tokyo, Japan) as the primary antibody. After washing by PBS, the tissue section was reacted with biotinylated goat antimouse polyclonal antibody. Thereafter, color was developed with diaminobenzidine. Counter staining was performed with hematoxylin. The degree of 8‐OHdG expression was scored as follows: none (0 point), mild (1 point), moderate (2 points), and severe (3 points) (Fig. 1).
Figure 1.
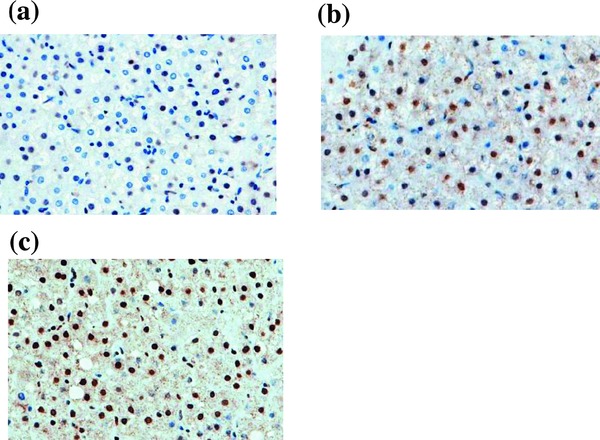
Expression of 8‐OHdG in the liver of CH‐C patients by an immunohistochemical procedure. (a) Mild, (b) moderate, (c) severe.
Statistical Analyses
Data values are represented as means ± standard deviation. The Mann–Whitney U‐test was applied for comparison of continuous variables. Linear regression analysis was used to analyze the relationships between titers of anti‐CL and serum ApoB concentrations. Fisher's exact test was used to compare the differences in frequencies. P values less than 0.05 were considered to indicate a significant difference between groups.
RESULTS
Characteristics of the Enrolled Patients with CH‐C
Among the enrolled patients with CH‐C, 29 cases were male, and 16 cases were female. HCV genotypes of the enrolled patients were 1b in 29 (62%) patients, 2a in 11 (26%) patients, and 2b in five (12%) patients. The patient ages at enrollment ranged from 23 to 76 years old. Mean BMI was 23.6 ± 3.4 kg/m2 (range, 17.1–33.5 kg/m2). No fibrosis (F0) was in two patients, while F1 in 18 patients, F2 in eight patients, and F3 in 17 patients, respectively. The severity of necroinflammation was evaluated as follows: A1 in 12 patients, A2 in 24 patients, and A3 in nine patients. On the other hand, 28 (62%) patients had no hepatic steatosis (grade 0), ten (22%) patients had grade 1 steatosis, and seven (16%) patients had grade 2 steatosis: none had grade 3 steatosis.
Prevalence of Anti‐CL in Patients with CH and Patients with Comparison Groups
Figure 2 shows the distribution and prevalence of anti‐CL in each group. Six (13%) of 45 patients with CH‐C had anti‐CL, while two of ten (20%) patients with NASH, no patients with CH‐B, no patients with alcoholic liver diseases, and no NHC did. However, there was no significant difference in the prevalence of anti‐CL between patients with CH‐C and NHC.
Figure 2.
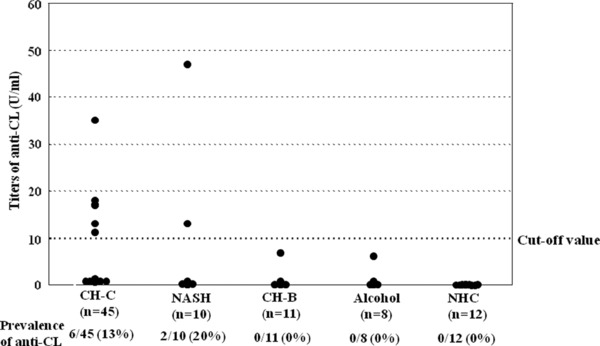
Distribution of anti‐CL titers in CH‐C patients and comparison patients/control groups.
Anti‐β2‐GPI was also measured in five patients with CH‐C seropositive for anti‐CL. Among these five CH‐C patients seropositive for anti‐CL, four CH‐C patients, whose titers of the antibodies did not exceed 20 U/ml, were seronegative for anti‐β2‐GPI. Only the rest CH‐C patient seropositive for anti‐CL had a high titer of anti‐β2‐GPI (anti‐CL: 35 U/ml, anti‐β2‐GPI :18.1 U/ml).
Association of Anti‐CL with Demographic Factors
Table 1 summarizes demographic factors in the enrolled patients with CH‐C. As shown in the table, CH‐C patients with anti‐CL were male‐predominant, although the difference was not significant (P = 0.3846). The incidence of concomitant hyperlipidemia in CH‐C patients seropositive for anti‐CL was significantly higher than that in those patients seronegative for anti‐CL (50% vs. 10%, P = 0.0394). However, there was no significant difference in the prevalence of concomitant type 2 DM or hypertension between the groups.
Table 1.
Comparisons of Demographic Factors Between CH‐C Groups Seropositive and Seronegative for Anti‐CL
| Anti‐CL (+) (n = 6) | Anti‐CL (−) (n = 39) | P‐ values | |
|---|---|---|---|
| Age (year) | 58.8 ± 7.9 | 59.4 ± 11.7 | N.S. |
| Gender (male/female) | 5/1 | 24/15 | N.S. |
| BMI (kg/m2) | 23.5 ± 1.7 | 23.7 ± 3.7 | N.S. |
| Concomitant metabolic | |||
| disease | |||
| Hyperlipidemia | 3 (50%) | 4 (10%) | 0.0394 |
| Hypertension | 1 (17%) | 7 (18%) | N.S. |
| Type 2 DM | 2 (33%) | 8 (21%) | N.S. |
| Concomitant autoimmune | |||
| disease | |||
| Autoimune thyroiditis | 0 (0%) | 1 (3%) | N.S. |
| Membranous nephropathy | 0 (0%) | 1 (3%) | N.S. |
| Lichen planus | 1 (17%) | 0 (0%) | N.S. |
N.S., not significant.
In terms of concomitant autoimmune diseases, one CH‐C patient seropositive for anti‐CL had oral lichen planus, while one CH‐C patient without anti‐CL had autoimmune thyroiditis, and another CH‐C patient without it had membranous nephropathy, respectively. No significant difference in the prevalence of concomitant autoimmune diseases was apparent between the groups.
Association of Anti‐CL with Laboratory Data
Laboratory data analyses were performed to detect the clinical parameters associated with the emergence of anti‐CL. As shown in Table 2, peripheral platelet counts in CH‐C patients with anti‐CL were almost equivalent to those in CH‐C patients without anti‐CL. The result indicates that the emergence of anti‐CL in patients with CH‐C was independent of thrombocytopenia. There were no significant differences in the levels of serum ALT and T‐Cho between the groups seropositive and seronegative for anti‐CL. However, CH‐C patients with anti‐CL had significantly higher serum TG and ApoB concentrations than CH‐C patients without anti‐CL (TG: 139 ± 35 vs. 79 ± 18 mg/dl, P = 0.0048, ApoB: 79 ± 19 vs. 53 ± 17 mg/dl, P = 0.0145).
Table 2.
Comparisons of Laboratory and Histological Data Between CH‐C Groups Seropositive and Seronegative for Anti‐CL
| Anti‐CL (+) (n = 6) | Anti‐CL (−) (n = 39) | P‐ values | |
|---|---|---|---|
| Plt (×104/μl) | 15.1 ± 3.2 | 15.4 ± 4.7 | N.S. |
| ALT (IU/l) | 96 ± 74 | 82 ± 54 | N.S. |
| T‐Cho (mg/dl) | 159 ± 33 | 163 ± 33 | N.S. |
| TG (mg/dl) | 139 ± 35 | 79 ± 18 | 0.0048 |
| ApoB (mg/dl) | 79 ± 19 | 53 ± 17 | 0.0145 |
| Ferritin (ng/ml) | 273 ± 190 | 240 ± 313 | N.S. |
| HOMA‐IR value | 1.61 ± 1.14 | 2.87 ± 3.17 | N.S. |
| HCV‐genotype (1b/2a/2b) | 5/0/1 | 24/11/4 | N.S. |
| Load of HCV‐RNA (KIU/ml) | 1,113 ± 525 | 686 ± 268 | N.S. |
| IgG (mg/dl) | 1,831 ± 552 | 1,689 ± 353 | N.S. |
| Prevalence of ANA (%) | 2 (33%) | 12 (31%) | N.S. |
| Severity of 8‐OHdG (points) | 2.8 ± 0.4 | 2.0 ± 0.8 | 0.0463 |
| Staging (F0/F1/F2/F3) | 0/0/1/5 | 2/18/7/12 | 0.0228 |
| Grading (A1/A2/A3) | 0/4/2 | 12/20/7 | N.S. |
| HAI score (points) | 13.2 ± 3.0 | 9.8 ± 4.6 | N.S. |
| Steatosis (grade 0/1/2) | 5/1/0 | 23/9/7 | N.S. |
N.S., not significant.
The degree of 8‐OHdG expression in the liver of CH‐C patients was significantly more severe in those with anti‐CL than without it (2.8 ± 0.4 vs. 2.0 ± 0.8 points, P = 0.0463). The finding described above suggests that the emergence of anti‐CL was closely associated with oxidative stress in CH‐C patients. On the other hand, serum ferritin levels in CH‐C patients with anti‐CL were almost the same as those in CH‐C patients without anti‐CL. There was no significant difference in the values of HOMA‐IR between CH‐C patients seropositive and seronegative for anti‐CL. Moreover, the emergence of anti‐CL was independent of HCV genotypes and loads of HCV‐RNA.
With regard to immunological aspects, no significant differences in the levels of serum IgG and the prevalence of ANA were observed between the groups of CH‐C patients with and without anti‐CL.
Relationship between Serum TG and ApoB Levels
The relationship between serum TG and ApoB levels was examined in CH‐C patients. As shown in Figure 3, a significant positive correlation between serum TG and ApoB levels was observed in those patients (r = 0.556, P = 0.0252, n = 16).
Figure 3.
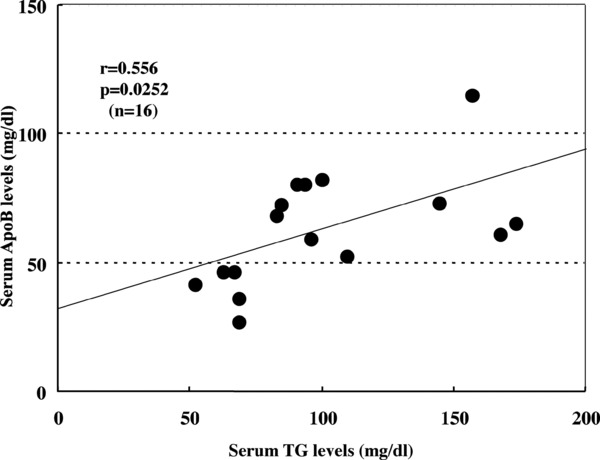
Relationship between serum TG and ApoB levels in CH‐C patients.
Histological Features in Patients with CH‐C Seropositive for Anti‐LC
Histological findings were compared between the groups seropositive and seronegative for anti‐CL (Table 2). The incidence of F3 in CH‐C patients with anti‐CL was significantly higher than that in CH‐C patients without anti‐CL (83% vs. 31%, P = 0.0228), indicating that the emergence of anti‐CL was associated with the progression of hepatic fibrosis. No significant difference was observed in the activity of liver tissue specimens between the groups. CH‐C patients with anti‐CL had a trend of higher HAI scores than CH‐C patients without anti‐CL (13.2 ± 3.0 vs. 9.8 ± 4.6 points, P = 0.1069). On the other hand, the presence of anti‐CL was independent of the severity of hepatic steatosis and histological grading.
DISCUSSION
Here, we have documented that 13% of patients with CH‐C and 20% of patients with NASH had anti‐CL, while no patients with CH‐B, or alcoholic liver diseases did, suggesting that oxidative stress may contribute to the production of anti‐CL. We confirmed that the emergence of anti‐CL was significantly associated with oxidative stress in patients with CH‐C. To the best of our knowledge, this is the first study to describe the relationship between anti‐CL and oxidative stress in patients with HCV‐related chronic liver disease. Our previous study revealed that autoantibodies to oxidized low‐density lipoprotein (anti‐oxLDL), one of oxidatively modified autoantigens, were present in sera of patients with CH‐C, and that the production of anti‐oxLDL was associated with hepatic steatosis in these patients 31. However, this study showed no association between the existence of anti‐CL in CH‐C patients and insulin resistance, severity of hepatic steatosis, or iron overload, which are characteristic metabolic abnormalities in HCV infection 11, 12, 13. Albano and colleagues documented that titers of anti‐CL were independent of hepatic steatosis in patients with nonalcoholic fatty liver disease 32. Further examinations will be required to reveal why there were no relationships between the emergence of anti‐CL and hepatic steatosis, insulin resistance, or iron overload in patients with CH‐C. On the other hand, the emergence of anti‐CL was neither associated with HCV genotypes nor loads of HCV‐RNA, implying that host factors rather than viral factors might contribute to the production of anti‐CL in CH‐C patients.
It was noteworthy that an association between the existence of anti‐CL and the elevation of serum TG levels in CH‐C patients was shown in this study. However, the increase in the levels of serum TG did not correlated with the severity of hepatic steatosis in CH‐C patients with CL, although several articles have found a relationship between TG synthesis and hepatic steatosis in patients with HCV‐related chronic liver disease 33. Vaarala and colleagues documented that the coexistence of anti‐CL and hypertriglyceridemia increased patients’ risk of myocardial infection 34. Arteriosclerosis is one of the most common extrahepatic manifestations evoked by persistent HCV infection 35. The coexistence of anti‐CL and hypertriglycemia may be a useful predictive marker for arteriosclerosis in CH‐C patients. The increase in the level of serum ApoB may account for the hypertriglyceridemia in CH‐C patients with anti‐CL 36.
The production of anti‐CL has been recognized as a result of cross‐reactivity between HCV and phospholipids. Persistent HCV infection may disrupt the cell membrane and favor the exposure of hidden phospholipids 14, 16. We speculated that the facilitation of TG synthesis by HCV infection might have a possibility to trigger the conformational change of phospholipids and thereby initiate the production of anti‐CL in patients with CH‐C. Ordi‐Ros and colleagues elucidated that anti‐CL detected in patients with infection did not require β2‐GPI for binding to CL 37, while these autoantibodies in autoimmune disease including systemic lupus erythematosus were cofactor (β2‐GPI) dependent 38. We confirmed that CH‐C patients had low‐to‐moderate titers of anti‐CL and that the presence of anti‐CL was independent of anti‐β2‐GPI. These results indicated that the production of anti‐CL can be regarded as an HCV‐induced autoimmunity. However, a previous study revealed that the existence of anti‐CL in CH‐C patients did not affect the antiviral treatment, although the prevalence of anti‐CL was increased by the treatment 15.
No association of the existence of anti‐CL and the presence of ANA or serum IgG levels was found in this study, although Leroy and colleagues documented a correlation between the emergence of anti‐CL and ANA in patients with CH‐C 16. Another interesting article represented a correlation between the emergence of anti‐CL and smooth muscle antibodies in CH‐C patients 14. We failed to detect an association between concurrent autoimmune diseases and anti‐CL in CH‐C patients in this study, although Nagao and colleagues previously reported the strong correlation between the emergence of anti‐CL and oral lichen planus in patients with CH‐C 20.
The present study revealed that CH‐C patients seropositive for anti‐CL had more progressive hepatic fibrosis than those seronegative for anti‐CL. It has been widely recognized that the hepatic fibrosis in CH‐C patients with ANA is more severe than those without ANA as a result of HCV‐induced autoimmunity 39, 40. Moreover, a high prevalence of anti‐CL in patients with liver cirrhosis has been demonstrated though the etiology was not established 14, 41. The present study did not show that the emergence of anti‐CL was associated with histological grading (activity) or serum ALT levels in patients with CH‐C.
In conclusion, the emergence of anti‐CL was likely to result from HCV‐induced autoimmunity and to be linked to oxidative stress in CH‐C patients. Hypertriglyceridemia caused by the facilitation of ApoB synthesis and progressive hepatic fibrosis were the clinical characteristics of CH‐C patients seropositive for anti‐CL.
REFERENCES
- 1. Pawlotsky JM, Yahia MB, Andre C, et al. Immunological disorders in C virus chronic active hepatitis: A prospective case‐control study. Hepatology 1994;19:841–848. [PubMed] [Google Scholar]
- 2. Hadziyannis SJ. Nonhepatic manifestations and combined disease in HCV infection. Dig Dis Sci 1996;41:63s–74s. [DOI] [PubMed] [Google Scholar]
- 3. Himoto T, Nishioka M. Autoantibodies in hepatitis C virus‐related chronic liver diseases. Hepat Mon 2008;8:295–303. [Google Scholar]
- 4. Roubey RAS. Immunology of the antiphospholipid syndrome. Arthritis Rheum 1996;39:1444–1454. [DOI] [PubMed] [Google Scholar]
- 5. Liaskos C, Rigopoulou E, Zachou K, et al. Prevalence and clinical significance of anticardiolipin antibodies in patients with type 1 autoimmune hepatitis. J Autoimmun 2005;24:251–260. [DOI] [PubMed] [Google Scholar]
- 6. Zachou K, Liaskos C, Rigopoulou E, et al. Prevalence of high avidity anticardiolipin antibodies in patients with autoimmune cholestatic liver diseases. Clin Immunol 2006;119:203–212. [DOI] [PubMed] [Google Scholar]
- 7. Rolla R, Vay D, Mottaran E, et al. Antiphospholipid antibodies associated with alcoholic liver disease specifically recognize oxidized phospholipids. Gut 2001;49:852–859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Alves JD, Mason LJ, Ames PRJ, et al. Antiphospholipid antibodies are associated with enhanced oxidative stress, decreased plasma nitric oxide and paraoxonase activity in an experimental mouse model. Rheumatology 2005;44:1238–1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Koike K, Miyoshi H. Oxidative stress and hepatitis C viral infection. Hepatol Res 2006;34:65–73. [DOI] [PubMed] [Google Scholar]
- 10. Okuda M, Li K, Beard MR, et al. Mitochondrial injury, oxidative stress, and antioxidant gene expression are induced by hepatitis C virus core protein. Gastroenterology 2002;122:366–375. [DOI] [PubMed] [Google Scholar]
- 11. Koike K. Hepatitis C as a metabolic disease: Implication for the pathogenesis of NASH . Hepatol Res 2005;33:145–150. [DOI] [PubMed] [Google Scholar]
- 12. Lonardo A, Adinolfi LE, Loria P, et al. Steatosis and hepatitis C virus: Mechanisms and significance for hepatic and extrahepatic disease. Gastroenterology 2004;126:588–597. [DOI] [PubMed] [Google Scholar]
- 13. Farinati F, Cardin R, DeMaria N, et al. Iron storage, lipid peroxidation and glutathione turn over in chronic anti‐HCV positive hepatitis. J Hepatol 1995;22:449–456. [DOI] [PubMed] [Google Scholar]
- 14. Prieto J, Yuste JR, Beloqui O, et al. Anticardiolipin antibodies in chronic hepatitis C: implication of hepatitis C virus as the cause of the antiphospholipid syndrome. Hepatology 1996;23:199–204. [DOI] [PubMed] [Google Scholar]
- 15. Leroy V, Arvieux J, Jacob MC, et al. Prevalence and significance of anticardiolipin, anti‐β2 glycoprotein I and anti‐prothrombinantibodies in chronic hepatitis C . Br J Haematol 1998;101:468–474. [DOI] [PubMed] [Google Scholar]
- 16. Munoz‐Rodriguez FJ, Tassies D, Font J, et al. Prevalence of hepatitis C virus infection in patients with antiphospholipid syndrome. J Hepatol 1999;30:770–773. [DOI] [PubMed] [Google Scholar]
- 17. Ordi‐Ros J, Villarreal J, Monegal F, et al. Anticardiolipin antibodies in patients with chronic hepatitis C virus infection: characterization in relation to antiphospholipid syndrome. Clin Diagn Lab Immunol 2000;7:241–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Dalekos GN, Kistis KG, Boumba DS, et al. Increased incidence of anti‐cardiolipin antibodies in patients with hepatitis C is not associated with aetiopathogenetic link to anti‐phospholipid syndrome. Eur J Gastroenterol Hepatol 2000;12:67–74. [DOI] [PubMed] [Google Scholar]
- 19. Harada M, Fujisawa Y, Sakisaka S, et al. High prevalence of anticardiolipin antibodies in hepatitis C virus infection: Lack of effects on thrombocytopenia and thrombotic complications. J Gastroenterol 2000;35:272–277. [DOI] [PubMed] [Google Scholar]
- 20. Nagao Y, Tsubone K, Kimura R, et al. High prevalence of anticardiolipin antibodies in patients with HCV associated oral lichen planus. Int J Mol Med 2002;9:293–237. [DOI] [PubMed] [Google Scholar]
- 21. Cacoub P, Musset L, Amour Z, et al. Anticardiolipin, anti‐β2‐glycoprotein I, and antinucleosome antibodies in hepatitis C virus infection and mixed cryoglobulinemia. J Rheumatol 1997;24:2139–2144. [PubMed] [Google Scholar]
- 22. Kleiner DE, Brunt EM, Natta MV, et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology 2005;41:1313–1321. [DOI] [PubMed] [Google Scholar]
- 23. Gharavi AE, Harris EN, Asherson RA, et al. Anticardiolipin antibodies: isotype distribution and phospholipid specifity. Ann Rheum Dis 1987;46:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kaburai J, Kuwana M, Yamamoto M, et al. Phospholipid‐dependent anti‐β2‐glycoprotein I (β2‐GPI) antibodies and phospholipid syndrome. Intern Med 1996;35:105–110. [DOI] [PubMed] [Google Scholar]
- 25. Lau JY, Davis GL, Kinffen J, et al. Significance of serum hepatitis C virus RNA levels in chronic hepatitis C . Lancet 1993;341:1501–1504. [DOI] [PubMed] [Google Scholar]
- 26. Simmonds P, Alberti A, Alter HJ, et al. A proposed system for nomenclature of hepatitis C viral genotypes. Hepatology 1994;19:1321–1324. [PubMed] [Google Scholar]
- 27. Brunt EM, Janney CG, Bisceglie D. Nonalcoholic steatohepatitis: A proposal for grading and staging the histological lesions. Am J Gastroenterol 1994;94:2467–2474. [DOI] [PubMed] [Google Scholar]
- 28. Ichida F, Tsuji T, Omata M, et al. New Inuyama classification: New criteria for histological assessment of chronic hepatitis. Int Hepatol Commun 1996;6:112–119. [Google Scholar]
- 29. Knodel RG, Ishak KG, Black WC, et al. Formulation and application of a numerical scoring system for assessing histological activity in asymptomatic chronic active hepatitis. Hepatology 1981;1:431–435. [DOI] [PubMed] [Google Scholar]
- 30. Fujita N, Sugimoto R, Ma N, et al. Comparison of hepatic oxidative DNA damage in patients with chronic hepatitis B and C . J Viral Hepat 2008;15:498–507. [DOI] [PubMed] [Google Scholar]
- 31. Himoto T, Yoneyama H, Deguchi A, et al. Relationship between the production of autoantibodies to oxidized low‐density lipoprotein and hepatic steatosis in patients with chronic hepatitis C . Exp Ther Med 2010;1:663–338. [Google Scholar]
- 32. Albano E, Mottaran E, Vidali M, et al. Immune response towards lipid peroxidation products as a predictor of progression of non‐alcoholic fatty liver diseas to advance fibrosis. Gut 2005;54:987–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Poynard T, Ratziu V, McHutchison J, et al. Effect of treatment with peginterferon or interferon alfa‐2b and ribavirin on steatosis in patients infected with hepatitis C . Hepatology 2003;38:75–85. [DOI] [PubMed] [Google Scholar]
- 34. Vaarala O, Mänttäri M, Manninen V, et al. Anti‐cardiolipin antibodies and risk of myocardial infarction in a prospective cohort of middle‐aged men. Circulation 1995;91:23–27. [DOI] [PubMed] [Google Scholar]
- 35. Ishizaka N, Ishizaka Y, Takahashi E, et al. Association between hepatitis C virus seropositivity, carotid‐artery plaque, and intima‐media thickening. Lancet 2002;359:133–135. [DOI] [PubMed] [Google Scholar]
- 36. Negro F. Mechanisms and significance of liver steatosis in hepatitis C virus infection. World J Gastroenterol 2006;12:6756–6765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ordi‐Ros J, Selva‐O'Callaghan A, Monegal‐Ferran F, et al. Prevalence, significance and specificity of anti‐phospholipid antibodies in Q fever. Clin Infect Dis 1994;18:213–217. [DOI] [PubMed] [Google Scholar]
- 38. Hunt JE, McNeil P, Morgan GJ, Crameri RM, Krilis SA. A phospholipid‐β2‐glycoprotein I complex is an antigen for anticardiolipin antibodies occurring in autoimmune disease but not with infection. Lupus 1992;1:75–81. [DOI] [PubMed] [Google Scholar]
- 39. Czaja A, Carpenter HA. Histological findings in chronic hepatitis C with autoimmune features. Hepatology 1995;26:459–466. [DOI] [PubMed] [Google Scholar]
- 40. Himoto T, Nakai S, Kinekawa F, et al. Clinical characteristics of patients with hepatitis C virus‐related chronic liver disease seropositive for anticentromere antibody. Dig Dis Sci 2009;54:360–368. [DOI] [PubMed] [Google Scholar]
- 41. Quintarelli C, Ferro D, Valensi G, Basili S, Tassone G, Violi F. Prevalence of lupus anticoagulant in patients with cirrhosis: Relationship with β2‐glycoprotein I plasma levels. J Hepatol 1994;21:1086–1091. [DOI] [PubMed] [Google Scholar]


