Abstract
Background
Mycobacterium abscessus group belongs to a group of rapidly growing mycobacteria (RGM) and, following Mycobacterium avium complex, is the second most common pathogen responsible for lung disease caused by nontuberculous mycobacteria (NTM). Clarithromycin is known to be the key drug in the treatment of M. abscessus group disease, but a high failure rate of treatment response is reported due to clarithromycin inducible resistance.
Methods
Using the results from a clarithromycin susceptibility test we examined the proportion of clarithromycin inducible resistant M. abscessus (sensu stricto; hereafter referred to as M. abscessus) clinical strains. Also, we attempted to detect the clarithromycin resistant strains, using the amplification refractory mutation system‐PCR (ARMS‐PCR) and real‐time PCR methods for rapid detection of single‐nucleotide polymorphisms (SNPs) at position 28 (T or C) of the erm(41) gene of M. abscessus leading to resistance to clarithromycin.
Results
Of the 157 M. abscessus clinical strains, clarithromycin susceptible, resistant, and inducible resistant strains accounted for 10.83% (n = 17), 22.29% (n = 35), and 66.88% (n = 105), respectively. Clarithromycin resistant strains were able to separate from clarithromycin susceptible strains by ARMS‐PCR and real‐time PCR identical to DNA sequence analysis.
Conclusion
Most M. abscessus clinical strains in Korea are resistant to clarithromycin, and ARMS‐PCR and real‐time PCR are useful tools for the rapid detection of single‐nucleotide polymorphisms (SNPs) at position 28 of the erm(41) gene.
Keywords: amplification refractory mutation system‐PCR, real‐time PCR, erm(41), clarithromycin
INTRODUCTION
Mycobacterium abscessus group accounts for approximately 65–85% of lung disease caused by rapidly growing mycobacteria (RGM; 1, 2, 3). It is the second most common pathogen, following Mycobacterium avium complex, responsible for lung disease caused by nontuberculous mycobacteria (NTM) in South Korea 4, 5. Infections due to M. abscessus are difficult to treat because this mycobacterium is intrinsically resistant, not only to the classical antituberculous drugs, but also to most of the antibiotics that are currently available 6, 7, 8, 9. Combination therapy of intravenous amikacin with cefoxitin or imipenem and oral macrolide (clarithromycin or azithromycin) have been recommended by the American Thoracic Society/Infectious Disease Society of America (ATS/IDSA; 10).
Clarithromycin is known to be the key drug in the treatment of M. abscessus group disease, but its application is undermined by clarithromycin inducible resistance of M. abscessus, associated with single‐nucleotide polymorphisms (SNPs) in the erm(41) gene at position 28 (T or C) recently 11, 12. For these reasons, the Clinical and Laboratory Standards Institute (CLSI) recommendations were based on the presence of the erm(41) gene and inducible resistance whatever the polymorphism at nucleotide 28 is 13.
In the present study, we examined the proportion of clarithromycin inducible resistant M. abscessus clinical strains, by using the results from clarithromycin susceptibility test and the correlation between the mutation of the erm(41) at position 28 and the minimum inhibitory concentrations (MICs) of clarithromycin for M. abscessus strains. In addition, we applied to the rapid detection methods for clarithromycin resistance using amplification refractory mutation system and polymerase chain reaction (ARMS‐PCR) and real‐time PCR methods for detecting erm(41) gene mutation.
MATERIALS AND METHODS
Strains
A total of 157 M. abscessus clinical strains, submitted to the drug susceptibility test from July 2009 to December 2010, were obtained for this study. All clinical strains were identified using a polymerase chain reaction and restriction length polymorphism (PCR‐RFLP) method based on the rpoB gene 4. Also, they were differentiated from Mycobacterium massiliense and Mycobacterium bolletii based on the PCR product size and ‐35 promoter sequence for erm(41) gene in the M. abscessus group 11.
Clarithromycin Susceptibility Test
A broth microdilution method was used to determine the MIC of clarithromycin for M. abscessus strains at pH 7.4, and were interpreted according to the CLSI document M24‐A2 in 2011 13. Strains were considered resistant if the MIC of clarithromycin was ≥8 mg/l for 3 days, and susceptible if the MIC of clarithromycin was ≤ 2 mg/l for 14 days. Also, strains were considered inducible resistant if the MIC of clarithromycin was ≤ 2 mg/l for 3 days and ≥8 mg/l for 14 days.
Sequence Analysis of the erm(41) Gene at Position 28
The mycobacterial DNA preparations for 157 M. abscessus strains were obtained by heating method (100°C, 15 min). PCR was performed to amplify the erm(41) gene according to the method described by Nash et al. 14. The PCR products were sequenced using the BigDye Terminator v3.1 Cycle Sequencing kit and sequencing was performed on a 3730XL DNA analyzer (Applied Biosystems, Foster city, CA). Using BLAST, the erm(41) gene sequences from clinical strains were analyzed for homology with the erm(41) gene sequence from M. abscessus strain MAB30 (GenBank accession number EU590129), which is susceptible to clarithromycin (MIC ≤ 0.5 mg/l).
ARMS‐PCR
The mycobacterial DNA preparations for 157 M. abscessus strains were obtained by heating method (100°C, 15 min). Primers were designed using Primer3 software based on the erm(41) sequence in M. abscessus MAB30 (GenBank accession number EU590129; Table 1). For the detection of clarithromycin‐resistant strains, ermf1 and ermR were used for amplification of 522 bp product. Also, ermf3 and ermR were used for amplification of 300 bp for the erm(41) gene control. The reaction mixtures were prepared by adding the following to 1 μl of DNA solution: 0.5 (ermf1), 0.25 (ermf3), 0.0625 (ermR) pmol of each primer; 1 U of EX Taq polymerase (5 U/μl [Takara Bio, Inc., Otsu, Japan]; 2 μl of 2.5 mM dNTP mixture; 2 μl of 10× PCR buffer; and using sterile purified water were made to a total volume of 20 μl. The reactions were performed using a GeneAmp 9700 PCR system (Applied Biosystems). PCR conditions were as follows: 1 cycle of 95°C for 2 min; 35 cycles of 95°C for 30 sec, 69°C for 30 sec, 72°C for 30 sec; and 1 cycle of 72°C for 5 min. The PCR products were electrophoresed in a 2% agarose gel. When 522 bp and 300 bp were present, the strain was judged to be a clarithromycin‐resistant (T28 type). But the strain was judged to be a clarithromycin susceptible when only 300 bp was present (C28 type).
Table 1.
Primer Sequences and Probe Sequences Used ARMS‐PCR and Real‐Time PCR
| Sequence (5′ → 3′) | |
|---|---|
| Primers for ARMS‐PCR | |
| ermf1 | GGCCAATGGTCGTGCCGCCacTa |
| ermf3 | GCGAGCCCGCCCTACCAAGTCA |
| ermR | GACTTCCCCGCACCGATTCCAC |
| Primers for real‐time PCR | |
| erm‐real‐F | GGAGCATGGGCATATTCA |
| erm‐real‐R | TGCGCCCAGATCCACAA |
| Probes | |
| erm28T‐sencor | LC Red 640‐TACCAGCCCCACTGGCG‐Phosphate |
| erm28T‐anchor | CCGCCCAGTCATCAGTGAGCG‐fluorescein |
Small letter indicate mismatched nucleotides.
Nucleotide at position 28.
Real‐Time PCR of erm(41) and Melting Curve Analysis
The mycobacterial DNA preparations for 67 M. abscessus strains were obtained by heating method (100°C, 15 min). Real‐time PCR and melting curve analysis were performed using a LighCycler® 480 instrument and Gene Scanning software version 1.5 (Roche Diagnostics, Mannheim, Germany). Primers and probes sequences were shown in Table 1. For real‐time PCR, reaction volumes of 20 μl were applied containing 0.5 μM of each primer, 0.2 μM of each HybProbe probe, 4 μl Genotyping Master Mix (LightCycler® Genotyping Master version 7.0, Roche Diagnostics), 8 μl of PCR grade water, and 5 μl of target DNA template. Real‐time PCR conditions were 95°C for 10 min and 45 cycles of 95°C for 10 sec, 54°C for 10 sec and 72°C for 10 sec; after amplification a melting curve was obtained by one cycle of 95°C for 1 min, 40 for 2 min, and 95°C for 0 sec (acquisition mode continuous, 0.1°C/s), followed by a cooling step (40°C for 30 sec).
All PCR products were confirmed by gel electrophoresis and sequenced using the BigDye Terminator v3.1 Cycle Sequencing kit and sequencing was performed on 3730XL DNA analyzer (Applied Biosystems).
RESULTS
Clarithromycin Susceptibility Test
Clarithromycin susceptibility was determined for 157 strains by the broth microdilution method. Of the 157 M. abscessus clinical strains, clarithromycin‐susceptible, clarithromycin‐resistant, and clarithromycin inducible resistant strains accounted for 10.83% (n = 17), 22.29% (n = 35), and 66.88% (n = 105), respectively (Table 2).
Table 2.
Clarithromycin Susceptibility Test Results by Microdilution Method of 157 M. abscessus Clinical Strains
| MIC (μg/ml) | |||
|---|---|---|---|
| Result Number (%) | 3 Days | 14 Days | Number |
| Susceptible | ≤0.5 | ≤0.5 | 13 |
| 17 (10.83) | 1 | 1 | 1 |
| 2 | 2 | 3 | |
| Resistant | 8 | 11 | |
| 35 (22.29) | 16 | 3 | |
| 32 | 2 | ||
| 64 | 7 | ||
| >64 | 12 | ||
| Inducible resistant | ≤0.5 | 8 | 5 |
| 105 (66.88) | 16 | 12 | |
| 32 | 9 | ||
| 64 | 13 | ||
| >64 | 16 | ||
| 1 | 8 | 1 | |
| 16 | 4 | ||
| 32 | 4 | ||
| 64 | 7 | ||
| >64 | 8 | ||
| 2 | 64 | 15 | |
| >64 | 11 | ||
Sequence Analysis of the erm(41) Gene at Position 28
We carried out DNA sequence analysis to confirm the association of clarithromycin resistance with single‐nucleotide polymorphism (SNP) in the erm(41) gene at position 28 (T or C). We confirmed that 17 susceptible strains had nucleotide C and 140 resistant strains had nucleotide T at position 28, and these results were identical to those of the susceptibility test.
ARMS‐PCR
The 300 bp amplification products, which all strains yielded, were used as an erm(41) positive control, and clarithromycin‐resistant strains (T28 type) yield 522 bp amplification products with 300 bp as a result. We observed 522 and 300 bp amplification products in 140 of the 140 clarithromycin‐resistant strains, while we observed only 300 bp amplification products in 17 clarithromycin‐susceptible strains (C28 type; Fig. 1, Table 3).
Figure 1.
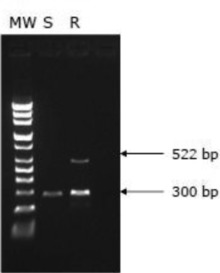
Products of ARMS‐PCR. S, clarithromycin‐susceptible strain; R, clarithromycin‐resistant strain; MW, molecular weight marker.
Table 3.
Comparison of the Results of Sequence Analysis of erm(41) Gene, ARMS‐PCR and Real‐Time PCR With the Clarithromycin Susceptibility Test 157 M. abscessus Clinical Strains
| Sequence analysis | ARMS‐PCR | Real‐time PCR | |||||
|---|---|---|---|---|---|---|---|
| Clarithromycin susceptibility | Total number | C28 | T28 | C28 | T28 | C28 | T28 |
| Susceptible | 17 | 17 | 0 | 17 | 0 | 17 | 0 |
| Resistant | 140 | 0 | 140 | 0 | 140 | 0 | 50 |
Real‐Time PCR of erm(41) and Melting Curve Analysis
In order to investigate the usefulness of real‐time PCR for detecting SNPs at position 28 in erm(41) gene, 17 clarithromycin‐susceptible and 50 clarithromycin‐resistant strains, determined by the clarithromycin susceptibility test, sequence analysis, and ARMS‐PCR, were applied to melting curve analysis using a LighCycler® 480 instrument and Gene Scanning software version 1.5.
Susceptible and resistant strains displayed different melting curves (Fig. 2, Table 3). Melting peaks of 47°C were displayed by all clarithromycin‐susceptible strains and 69°C melting peaks were displayed by all clarithromycin‐resistant strains. This result illustrated that 47°C is the melting peak of susceptible (C28 type) and 69°C is the melting peak of resistant (T28 type) strains.
Figure 2.
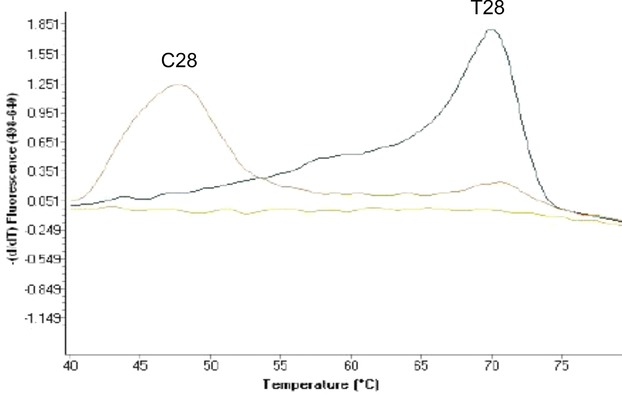
Melting peaks for genotyping of SNP in erm(41) gene. Melting peak for clarithromycin‐susceptible strain (C28 type) at 47°C, melting peak for clarithromycin‐resistant strain (T28 type) at 69°C.
DISCUSSION
ATS/IDSA has recommended the administration of multidrug therapy, including a macrolide and one or more parenteral agents (amikacin, cefoxitin, or imipenem) over several months, in order to help to control symptoms and progression of M. abscessus lung disease 10. Since Nash et al. published the mechanism of resistance to macrolides in the M. abscessus group as clarithromycin inducible resistance conferred by the erm(41) gene, several reports showed the association with SNPs in the erm(41) gene at position 28 (T or C; 11, 14, 15). Some report revealed that the lack of efficacy of antibiotic therapy against M. abscessus is due to the clarithromycin inducible resistance, that is, although a strain may appear susceptible after 3 days of in vitro incubation, the strain becomes clarithromycin‐resistant on increasing the incubation period to 14 days 12. For these reasons, it is recommended in the newest CLSI document that in order to ensure detection of inducible macrolide resistance in RGM, including M. abscessus, the final reading for clarithromycin is to be taken after 14 days, unless resistance (MIC ≥8 mg/l) is recognized earlier 13.
In this study, we aimed to examine the proportion of clarithromycin inducible resistant M. abscessus clinical strains in Korea using a comparison of the results from clarithromycin susceptibility tests, and confirm the correlation between the results of drug susceptibility tests and mutations in the erm(41) gene in M. abscessus strains. In addition, we evaluated the usefulness of two systems for the rapid detection of clarithromycin resistance, using the ARMS‐PCR and real‐time PCR methods, reducing the reading time from that recommended by the newest CLSI document.
M. abscessus clinical strains were identified using a PCR‐RFLP method based on the rpoB gene, and were differentiated from M. massiliense and M. bolletii based on their PCR product size and ‐35 promoter sequence for erm(41) gene in the M. abscessus group 11. M. abscessus strains were present in almost equal proportions with M. massiliense in the M. abscessus group in Korea (data not shown). We excluded M. massiliense clinical strains because they have nonfunctional methyltransferase based on the deleted erm(41) gene, and are able to interpret the results for drug susceptibility test for 3∼5 days. In the M. bolletii strain, we identified one clinical strain (T28 type) and applied it to ARMS and real‐time PCR methods. We found same results in M. bolletii as those of M. abscessus.
This study was the largest of its kind, performed with 157 clinical strains of M. abscessus, and also isolated the most number of clarithromycin‐susceptible strains (17 strains), conferring and confirming those strains identified by Bastian et al. (10/43 strains), Nash et al. (2/11 strains), and Kim et al. (5/39 strains; 11, 14, 15). This study identified that clarithromycin inducible resistant strains accounted for 66.88% of Korean M. abscessus clinical strains. It was also identified that clarithromycin‐resistant strains occupied almost 90% of Korean M. abscessus clinical strains, adding supportive data to the previous report that it is very difficult to treat M. abscessus lung disease 12.
By performing the drug susceptibility test and erm(41) gene sequencing analysis, it was confirmed that C28 type erm(41) gene is clarithromycin‐susceptible and T28 type is clarithromycin‐resistant. However, Bastian et al. reported that two M. abscessus strains acquired resistance to clarithromycin with mutation in rrl (23S rRNA gene) were C28 type and that no strains in the M. abscessus with T28 type had mutations in rrl. They mentioned that there might be less selection of resistant rrl mutants for those strains expressing inducible resistance, than for strains not expressing inducible resistance 11. In this study, we found all clarithromycin‐resistant strains had the T28 type erm(41) gene, and two of them also had mutations in the rrl gene. These results are inconsistent with Bastian et al. 11.
For the rapid detection of M. abscessus clarithromycin resistance, we applied ARMS‐PCR and real‐time PCR methods for a point mutation at nucleotide position 28 within erm(41) gene. ARMS‐PCR method was first described by Newton et al. as a general technique for the analysis of any point mutation 16. A typical ARMS test, which can detect a known SNP polymorphism, consists of two complementary reactions: one containing an ARMS primer specific for the normal DNA sequence that cannot amplify mutant DNA at a given locus, and the other one containing a mutant‐specific primer that also does not amplify normal DNA. We applied the latter only within this study.
Melting curve analysis is an assessment of the dissociation characteristics of double‐stranded DNA during heating. The information gathered can be used to infer the presence and identity of SNPs. The probe‐based technique is sensitive enough to detect SNP and can distinguish between homozygous wild type, heterozygous, and homozygous mutant alleles by virtue of the dissociation patterns produced. With higher resolution instruments and advanced dyes, amplicon melting analysis of base variants is now possible with several instruments commercially available.
In this study, both methods were shown to be able to differentiate clarithromycin‐resistant strains (T28 type) from clarithromycin‐susceptible strains (C28 type). The differences between the two methods lay in the required cost and time. While ARMS‐PCR is a simple PCR application by changing of only primer sequence, real‐time PCR needs an expensive real‐time PCR instrument and probes. In terms of the required time, real‐time PCR has advantage in detecting the result simultaneously without any additional method. The time required for real‐time PCR takes 2 hr and for ARMS‐PCR takes 3∼4 hr. Our results showed both methods are simple, rapid, and effective methods for the detection of clarithromycin resistance, especially when compared to conventional drug susceptibility test.
In conclusion, both methods can likely be used for the detection of clarithromycin resistance and can contribute to the appropriate and rapid management of patients with M. abscessus infections. In light of the proportion of clarithromycin‐resistant clinical strains, treatment response rates for clarithromycin‐based antibiotic therapy are low in patients with M. abscessus lung disease. Clearly, further study is needed to investigate more effective antibiotics against M. abscessus infections.
ACKNOWLEDGMENTS
This study was supported by the funds from the Korean Institute of Tuberculosis and a grant of the Korean Health Technology R&D Project, Ministry of Health & Welfare, Republic of Korea (HI13C0891).
Grant sponsor: Korean Institute of Tuberculosis, Korean Health Technology R&D Project, Ministry of Health & Welfare.
REFERENCES
- 1. Griffith DE, Girard WM, Wallace RJ Jr. Clinical features of pulmonary disease caused by rapidly growing mycobacteria. An analysis of 154 patients. Am Rev Respir Dis 1993;147:1271–1278. [DOI] [PubMed] [Google Scholar]
- 2. Han XY, De I, Jacobson KL. Rapidly growing mycobacteria: Clinical and microbiologic studies of 115 cases. Am J Clin Pathol 2007;128:612–621. [DOI] [PubMed] [Google Scholar]
- 3. Wallace RJ Jr, Swenson JM, Silcox VA, Good RC, Tschen JA, Stone MS. Spectrum of disease due to rapidly growing mycobacteria. Rev Infect Dis 1983;5:657–659. [DOI] [PubMed] [Google Scholar]
- 4. Koh WJ, Kwon OJ, Jeon K, et al. Clinical significance of nontuberculous mycobacteria isolated from respiratory specimens in Korea. Chest 2006;129:341–348. [DOI] [PubMed] [Google Scholar]
- 5. Ryoo SW, Shin S, Shim MS, et al. Spread of nontuberculous mycobacteria from 1993 to 2006 in Korean. J Clin Lab Anal 2008;22:415–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Brown‐Elliott BA, Wallace RJ Jr. Clinical and taxonomic status of pathogenic nonpigmented or late‐pigmenting rapidly growing mycobacteria. Clin Microbiol Rev 2002;15:716–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Jarand J, Levin A, Zhang L, Huitt G, Mitchell JD, Daley CL. Clinical and microbiologic outcomes in patients receiving treatment for Mycobacterium abscessus pulmonary disease. Clin Infect Dis 2011;52:565–571. [DOI] [PubMed] [Google Scholar]
- 8. Jeon K, Kwon OJ, Lee NY, et al. Antibiotic treatment of Mycobacterium abscessus lung disease: A retrospective analysis of 65 patients. Am J Respir Crit Care Med 2009;180:896–902. [DOI] [PubMed] [Google Scholar]
- 9. Lyu J, Jang HJ, Song JW, et al. Outcomes in patients with Mycobacterium abscessus pulmonary disease treated with long‐term injectable drugs. Respir Med 2011;105:781–787. [DOI] [PubMed] [Google Scholar]
- 10. Griffith DE, Aksamit T, Brown‐Elliott BA, et al. An official ATS/IDSA statement: Diagnosis, treatment, and prevention of nontuberculous mycobacterial disease. Am J Respir Cirt Care Med 2007;175:367–416. [DOI] [PubMed] [Google Scholar]
- 11. Bastian S, Veziris N, Roux AL, et al. Assessment of clarithromycin susceptibility in strains belonging to the Mycobacterium abscessus group by erm(41) and rrl sequencing. Antimicrob Agents Chemother 2011;55:775–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Koh WJ, Jeon K, Lee NY, et al. Clinical significance of differentiation of Mycobacterium massiliense from Mycobacterium abscessus . Am J Respir Crit Care Med 2011;183:405–410. [DOI] [PubMed] [Google Scholar]
- 13. Clinical and Laboratory Standards Institute : Susceptibility Testing of Mycobacteria, Nocardia, and Other Aerobic Actinomycetes, Approved Standard M24‐A2. Wayne, PA: CLSI; 2011. [PubMed] [Google Scholar]
- 14. Nash KA, Brown‐Elliott BA, Wallace RJ Jr. A novel gene, erm(41), confers inducible macrolide resistance to clinical isolates of Mycobacterium abscessus but is absent from Mycobacterium chelonae . Antimicrob Agents Chemother 2009;53:1367–1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kim HY, Kim BJ, Kook Y, et al. Mycobacterium massiliense is differentiated from Mycobacterium abscessus and Mycobacterium bolletii by erythromycin ribosome methyltransferase gene (erm) and clarithromycin susceptibility patterns. Microbiol Immunol 2010;54:347–353. [DOI] [PubMed] [Google Scholar]
- 16. Newton CR, Graham A, Heptinstall LE, et al. Analysis of any point mutation in DNA. The amplification refractory mutation system (ARMS). Nucleic Acids Res 1989;17:2503–2516. [DOI] [PMC free article] [PubMed] [Google Scholar]


