Abstract
Background
The performance of Bactec Fx Plus Aerobic/F (PA), Mycosis IC/F (MF), Myco/F Lytic (ML) media and BacT/Alert 3D FA (FA) media in detecting 15 Candida isolates in blood cultures to which fluconazole had been added was investigated.
Methods
PA with resin, MF, ML media (n = 360), and FAmedia (n = 120) were tested against Candida albicans, C. tropicalis, C. parapsilosis, and C. krusei. As the peak plasma concentration after single oral doses of fluconazole 100, 200, and 400 mg was equivalent to peak level of 1.9, 4.7, and 6.7 mg/l, respectively, corresponding fluconazole was added. Time to detection (TTD) was measured.
Results
Overall TTD (mean hour ± standard deviation) for PA, FA, MF, and ML was as follows: 24.5 ± 7.3, 27.0 ± 7.5, 31.9 ± 21.3, and 37.7 ± 30.1, respectively. TTD of PA was shorter compared to other media. The effect of fluconazole was limited in PA and FA, but MF and ML showed delayed TTD. Larger inoculum size showed shorter TTDin PA and FA.
Conclusion
TTD of Bactec Fx Plus Aerobic/F was more than 2.5 hr faster among the tested media. As thus system and media are unaffected by added fluconazole, it could be used for the diagnosis of candidemia in the clinical settings including the patients who have been treated empirically with fluconazole at the time when blood cultures were drawn.
Keywords: Bactec, BacT/Alert, Candida species, fluconazole, automated blood culture system
INTRODUCTION
Candida species is one of common causes of blood stream infection 1, 2 and the incidence of infection with these species has increased in Korea 3, 4. Candida species accounted for 13.6–19.6% of all cases of blood stream infection in intensive care units (ICU) 5. This incidence is slightly higher than that of other reports 6, 7. The main pathogens of candidemia are Candida albicans, C. parapsilosis, C. tropicalis, and C. glabrata 8. Candida albicans is known to be the most common pathogen of blood stream infections among the Candida species 9 but non‐albicans Candida species have been increasing in frequency 3, 8. Candidemia is associated with ICU and hospital stays and has variable mortality from 5% to 71% 10. Therefore, it is important to identify and treat Candida species as early as possible to reduce mortality 8, 11. Prophylactic or empirical treatment is also suggested in patients at high risk for candidemia 8, 12, and so antifungal treatment is often administered before the collection of blood cultures. However, according to Riedel et al., this may have an impact on microbiological recovery of yeast from patients’ blood culture 13. The most common diagnostic method of detecting candidemia is blood culture in spite of its low sensitivity and the delay in diagnosis 8. Blood cultures are usually performed by using commercially available automatic blood culture systems (ABCSs) 14, which include Bactec Fx (BD Diagnostics, Sparks, MD) and BacT/Alert 3D (bioMerieux, Durham, NC). Because these systems use different detection methods (fluorometry vs. colorimetry) with different media (resin vs. charcoal containing), they have different detection rates and times for detection 15, 16. A few studies that evaluated the performance of these two ABCSs in detecting Candida species have been reported 15.
In this study, we evaluated the performance of the Bactec Fx Plus Aerobic/F, Mycosis IC/F, Myco/F Lytic media and BacT/Alert 3D FA media for detecting the growth of Candida species in seeded blood cultures. We examined the performance in relation to inoculum size and various levels of a fluconazole.
MATERIALS AND METHODS
The study was approved by the Institutional Review Board of Yeouido St. Mary's Hospital, Seoul, Korea (No. KC09FZZZ0222). A total of 512 seeded blood culture bottles were tested for comparing the Bactec Fx to BacT/Alert 3D. The tested media were 120 bottles of Bactec Fx Plus Aerobic/F (PA, aerobic), 120 bottles of Bactec Mycosis IC/F (MF, mycology), and 120 bottles of Bactec Myco/F Lytic (ML, for the recovery of yeast, fungi, and mycobacteria) for the Bactec Fx system, and 120 bottles of FA (aerobic) for the BacT/Alert 3D system. For the positive control, 32 bottles were tested. We did not test the anaerobic media of both ABCSs because some studies have reported their poor performance in recovering Candida species 15, 17.
Tested Isolates
The Candida species used in this study were recovered from clinical specimens using BacT/Alert 3D, which were as follows: blood, C. albicans (n = 3), C. parapsilosis (n = 3), C. krusei (n = 1); urine, C. tropicalis (n = 2), C. krusei (n = 1), and sputum, C. tropicalis (n = 1). In addition, each one of ATCC isolate was included: C. albicans, ATCC 10231; C. parapsilosis, ATCC 22019; C. tropicalis, ATCC 96745; C. krusei, ATCC 6258, respectively. The isolates were stored frozen (−70℃) until tested.
Antifungal Susceptibility Testing (AST)
AST for the clinically isolated Candida species was performed using fluconazole and the minimal inhibitory concentration (MIC) was measured. MICs of all Candida species was determined by the reference broth microdilution method described by the Clinical and Laboratory Standards Institute (CLSI) M27‐A2 after 24‐hr and 48‐hr incubation at 35°C 18.
Preparation of Blood Donation
Each 400 ml of fresh whole blood was drawn from 12 healthy volunteers after obtaining their written informed consent. The donors were selected from volunteers who met the criteria outlined in our national blood management act.
Fluconazole Solution Preparation
The Fluconazole has been widely used as the prophylactic and empirical antifungal agent. The stock solutions with three different levels were prepared. To make the stock solution for the final peak concentration of 1.9 mg/l, 3.952 mg of fluconazole powder was dissolved in 40 ml of 0.85% sterile saline. For the final peak concentrations of 4.7 mg/l and 6.7 mg/l of the stock solutions, 9.776 mg and 13.936 mg of fluconazole powder was dissolved in each 40 ml of 0.85% sterile saline. A total of 0.1 ml aliquot of each stock solution was used for making one inoculation solution.
Yeast Inoculum Preparation
The final suspensions containing the test strains were diluted and resulted in 1 yeast cell/ml and 5 yeast cells/ml, respectively. For preparation of the test strains that had been maintained at –70°C, strains were subcultured onto blood agar plates and were incubated overnight at 37°C. A suspension of each strain was made by addition of 0.85% sterile saline and adjusted to a 0.5 McFarland standard, and the resulting suspension contained approximately 106 yeast cells/ml 15. Two serial 1:100 dilution and 1:2 dilution of each yeast suspension with 0.85% sterile saline were then performed to produce a density of 50 yeast cells/ml in the final suspension. Serial 1:100 dilution, 1:10 dilution, and 1:4 dilution of each yeast suspension were performed to produce a density of 250 yeast cells/ml in the final suspension. A 0.1 ml aliquot of each final suspension was used for making one inoculation solution. Inoculum concentration was verified by plating a 0.1 ml aliquot from the final dilutions onto sheep blood agar to establish a CFU count, which would be 5 and 25 yeast cells/ml, respectively.
Blood Culture Bottles Inoculation/Incubation
To make a simulated model of candidemia, the inoculation solutions were prepared by mixing whole blood, 0.85% sterile saline, fluconazole solution, and solution of tested strains in sequence. As the actual blood volume loaded in a blood culture bottle is around 5 ml in our institution, 5 ml of whole blood was mixed for making one inoculation solution with 0.1 ml of each stock solution of fluconazole and tested strains. The total volume for one inoculation solution was 5.2 ml and 0.85% sterile saline was mixed to adjust the total volume.
Blood culture bottles were mixed and installed at respective ABCSs. Bottles were incubated at 35°C with continuous agitation in their respective ABCSs. The PLUS, MF, MY, and FA blood culture bottles were incubated for up to 5 days, 14 days, and 42 days according to the manufacturers’ instructions.
When BC bottles were flagged as positive by the ABCS, time to detection (TTD, in hours) was documented. Yeast growth within positive BC bottles was verified by Gram stain. Terminal subcultures were performed on blood bottles with negative flags after the end of incubation period to confirm negative growth of yeast species.
Statistics
The independent variables, which were fluconazole concentration, Candida species, inoculum size, and culture media, were compared with the dependent variable, which was TTD. The TTD for positive blood cultures was analyzed using the Mann–Whitney U‐test. Statistical analysis of data was performed with Medcalc software 9.0 (Medcalc, Mariakerke, Belgium).
RESULTS
All clinically isolated strains were susceptible or showed dose‐dependent susceptibility to fluconazole after 24 hr of incubation, except the C. krusei species, which is intrinsically resistant to fluconazole (Table S1, available in Supplementary Information online) 19. Two strains of this species switched to being susceptible (in a dose‐dependent manner) after 48‐hr incubation, but the remaining strains had the same ASTs (Supporting Information Table S1). All the seeded blood culture bottles recovered Candida species. The 32 negative control bottles all had negative results and the 120 positive control bottles all had positive results. The TTD of PA, FA, MF, and ML by fluconazole level, Candida species, and inoculum size are listed in Table 1. TTD of all the tested PA, FA, MF, and ML media showed that average TTD of PA was 24.5 hr, which was the shortest time among the tested media, and this result had statistical significance.
Table 1.
Average Time to Detection (TTD) of Culture Media by Fluconazole Concentration, Candida Species, and Inoculum Size and Comparison of Average TTD Within Each Media
| Time to detection (hour) | ||||
|---|---|---|---|---|
| PA | FA | MF | ML | |
| Fluconazole concentration | ||||
| zero | 24.3 ± 7.2 | 25.8 ± 6.9 | 21.6 ± 6.5 | 25.3 ± 7.0 |
| 1.9 mg/l | 24.3 ± 7.1 | 26.2 ± 7.3 | 26.6 ± 9.1 | 30.4 ± 8.0 |
| 4.7 mg/l | 24.7 ± 7.3 | 27.1 ± 7.1 | 36.4 ± 23.2 | 42.1 ± 25.1 |
| 6.7 mg/l | 25.3 ± 7.8 | 28.7 ± 6.8 | 42.1 ± 29.4 | 53.0 ± 50.4 |
| Candida species | ||||
| C. albicans | 26.8 ± 2.6 | 26.0 ± 2.5 | 27.3 ± 5.8 | 29.0 ± 7.9 |
| C. tropicalis | 17.2 ± 1.3 | 21.1 ± 3.2 | 25.9 ± 9.4 | 33.5 ± 10.2 |
| C. parapsilosis | 33.9 ± 4.3 | 36.9 ± 4.6 | 52.3 ± 30.2 | 62.3 ± 49.0 |
| C. krusei | 19.3 ± 1.7 | 22.0 ± 7.1 | 17.9 ± 1.5 | 22.0 ± 1.5 |
| Inoculum | ||||
| 1 CFU/ml | 26.2 ± 7.7 | 28.4 ± 7.5 | 33.7 ± 22.5 | 40.7 ± 36.5 |
| 5 CFU/ml | 22.9 ± 6.3 | 25.4 ± 6.2 | 29.8 ± 19.2 | 34.7 ± 21.9 |
| Total | 24.5 ± 7.3 | 27.0 ± 7.5 | 31.9 ± 21.3 | 37.7 ± 30.1 |
| P‐values of each media | ||||
| Fluconazole concentration | ||||
| zero vs. 1.9 mg/l | ns | ns | 0.019 | 0.002 |
| zero vs. 4.7 mg/l | ns | ns | <0.001 | <0.001 |
| zero vs. 6.7 mg/l | ns | ns | <0.001 | <0.001 |
| 1.9 mg/l vs. 4.7 mg/l | ns | ns | ns | 0.036 |
| 1.9 mg/l vs. 6.7 mg/l | ns | ns | 0.007 | 0.013 |
| 4.7 mg/l vs. 6.7 mg/l | ns | ns | ns | ns |
| Candida species | ||||
| C. albicans vs. C. tropicalis | <0.001 | <0.001 | ns | ns |
| C. albicans vs. C. parapsilosis | <0.001 | <0.001 | <0.001 | <0.001 |
| C. albicans vs. C. krusei | <0.001 | <0.001 | <0.001 | <0.001 |
| C. tropicalis vs. C. parapsilosis | <0.001 | <0.001 | <0.001 | 0.006 |
| C. tropicalis vs. C. krusei | <0.001 | 0.004 | <0.001 | <0.001 |
| C. parapsilosis vs. C. krusi | <0.001 | <0.001 | <0.001 | <0.001 |
| Inoculum | ||||
| 1 CFU/ml vs. 5 CFU/ml | 0.006 | 0.012 | ns | ns |
PA, Bactec Fx Plus Aerobic/F media; FA, BacT/Alert 3D FA media; MF, Bactec Mycosis IC/F media; ML, Bactec Myco/F Lytic media; ns, nonspecific.
Comparison by Fluconazole Level
The comparison of the zero, 1.9 mg/l, 4.7 mg/l, and 6.7 mg/l fluconazole levels within each media was analyzed with the Mann–Whitney U‐test and the results are shown in Table 1. Only the results with a statistically significant P value are listed. TTD (mean hour) with 95% confidence interval of each media is plotted in Figure 1. Comparison of TTD within each media was performed. TTD of PA and FA media showed no statistical significance in simulated fluconazole concentrations, indicating that influence of fluconazole for recovery of Candida species was limited in PA and FA media. Comparison of TTD of MF for zero fluconazole and other concentrations showed that TTD of MF was the shortest with statistical significance and TTD of 1.9 mg/l was lower than that of 6.7 mg/l. ML showed a similar result to MF and TTD of 1.9 mg/l and 4.7 mg/l also showed significant results.
Figure 1.
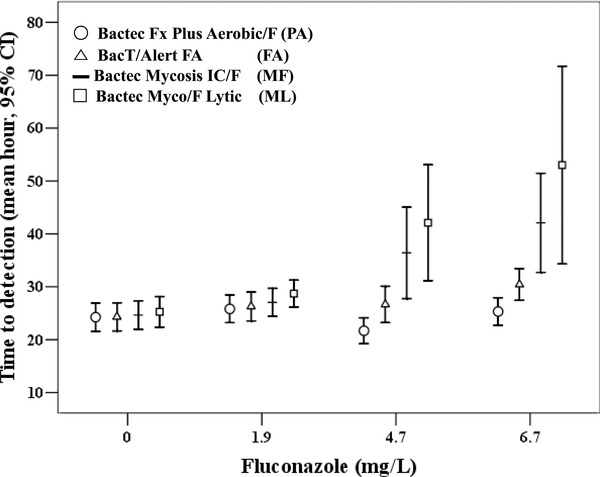
Time to detection of each blood culture media classified by concentration of fluconazole level.
Comparison between each media is listed in Table 2. TTD of PA and FA media showed no statistical significance, indicating that PA and FA showed similar performances, regardless of fluconazole concentrations. Comparison of TTD of 4.7 mg/l PA and 6.7 mg/l MF was statistically significant. Comparison of TTD of PA and ML showed statistical significance in all concentrations except at zero concentration. TTD of MF was lower than FA at zero concentration. Comparison of TTD of FA and ML also showed statistical significance except at zero concentration. TTD of MF was shorter than ML at zero and at 1.9 mg/l fluconazole concentration.
Table 2.
Comparison of Time To Detection (TTD) Between Culture Media
| PA vs. FA | PA vs. MF | PA vs. ML | FA vs. MF | FA vs. ML | MF vs. ML | |
|---|---|---|---|---|---|---|
| Fluconazole concentration | ||||||
| zero | ns | ns | ns | 0.004 | ns | 0.003 |
| 1.9 mg/l | ns | ns | 0.007 | ns | 0.023 | 0.041 |
| 4.7 mg/l | ns | 0.014 | <0.001 | ns | 0.001 | ns |
| 6.7 mg/l | ns | 0.002 | <0.001 | ns | 0.002 | ns |
| Candida species | ||||||
| C. albicans | ns | ns | ns | ns | ns | ns |
| C. tropicalis | <0.001 | <0.001 | <0.001 | ns | <0.001 | 0.002 |
| C. parapsilosis | 0.003 | 0.011 | <0.001 | ns | 0.011 | ns |
| C. krusei | <0.001 | 0.005 | <0.001 | <0.001 | ns | <0.001 |
| Inoculum | ||||||
| 1 CFU/ml | ns | ns | <0.001 | ns | 0.01 | 0.029 |
| 5 CFU/ml | 0.016 | ns | <0.001 | ns | 0.003 | 0.01 |
| Total | 0.004 | 0.01 | <0.001 | ns | <0.001 | 0.001 |
PA, Bactec Fx Plus Aerobic/F media; FA, BacT/Alert 3D FA media; MF, Bactec Mycosis IC/F media; ML, Bactec Myco/F Lytic media; ns, nonspecific.
Comparison by Candida Species
The comparison of TTD of Candida species within each media performed by the Mann–Whitney U‐test are shown in Table 1. Only those results that had a P‐value with statistical significance were listed. TTD (mean hour with 95% confidence interval) of each bottle is plotted in Figure 2. The comparison of TTD within each media was performed. In the case of PA, TTD of C. tropicalis was the fastest and C. parapsilosis was the slowest compared to C. albicans and C. krusei, and these results had significance. As in PA, FA showed a similar result, with C. tropicalis being the fastest and C. parapsilosis being slowest, with statistical significance. With respect to MF, TTD of C. tropicalis was higher compared to C. krusei, with statistical significance (P = 0.046). As for ML, the results showed no significance with regard to the Candida species.
Figure 2.
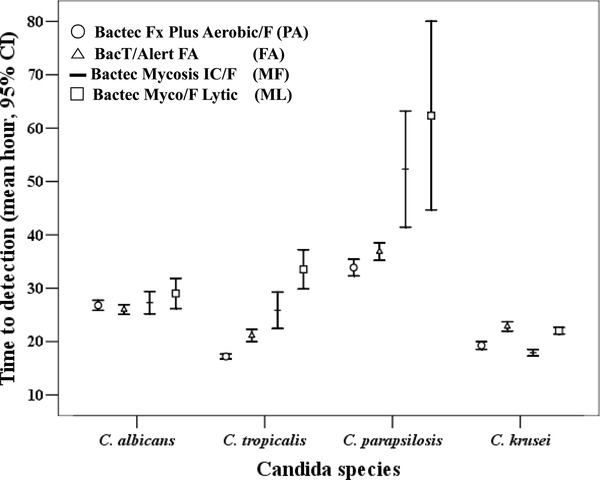
Time to detection of each blood culture media classified by Candida species.
The comparisons of TTD of the blood culture bottles are listed in Table 2 and Figure 2. TTD of PA and FA showed similar outcomes with regard to C. albicans. TTD of PA was shorter than FA for C. tropicalis, C. parapsilosis, and C. krusei, and this result had statistical significance. TTD of PA was significantly shorter compared to MF for C. albicans and C. parapsilosis. TTD of PA was shorter compared to ML for all tested Candida species. TTD of FA was shorter compared to MF and ML for C. albicans. TTD of FA was shorter compared to ML for C. parapsilosis. TTD of MF was shorter compared to ML media for C. albicans and C. parapsilosis.
Comparison by Inoculum Size
The results of the comparison of TTD by inoculum size within each media performed by the Mann–Whitney U‐test are shown in Table 1. TTD (mean hour with 95% confidence interval) of each bottle is plotted in Figure 3. The comparison of TTD within each media was performed and there was a trend toward faster Candida species growth detection with increasing inoculum size. PA and FA showed the inoculum effect, and Candida species loaded with 5 CFU/ml showed faster TTD compared to 1 CFU/ml.
Figure 3.
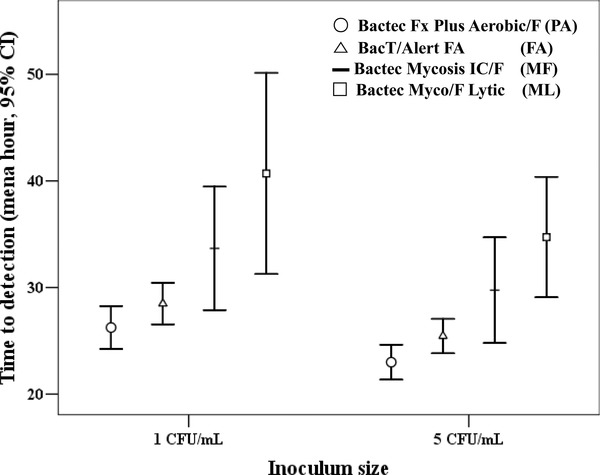
Time to detection of each blood culture media classified by concentration of inoculums size.
The comparisons between PA, FA, MF, and ML are listed in Table 2. TTD of PA was shorter compared to FA in 5 CFU/ml. Comparison of TTD of PA and MF, ML was statistically significant in 1 CFU/ml and 5 CFU/ml, respectively. Comparison of TTD of FA and MF showed no statistical significance, but FA showed shorter TTD compared to ML in both inoculums. TTD of MF was lower than ML at both inoculum sizes.
DISCUSSION
Candida species ranks fourth among common pathogens isolated from the blood of hospitalized patients with risk factors such as ICU stay, transplantation, malignancy, and immunosuppression 6, 13. Empirical therapy has been suggested for patients at high risk of Candida infection 13, 20 and fluconazole is currently one of the most commonly used antifungal agents. Therefore, this study not only simulated clinical practice conditions, but also fluconazole concentration, blood volume, and loading of Candida were controlled to minimize a bias.
Bactec Fx Plus Aerobic/F (PA) and BacT/Alert 3D FA (FA) media utilize cationic‐exchange, adsorbent nonionic resin and charcoal, respectively, to remove certain antibiotics, which drives the difference in time for growth 15, 21. In this study, comparison of PA and FA showed no statistical difference in regard to fluconazole concentration. However, TTD of PA was faster than that of FA for detecting C. tropicalis, C. parapsilosis, and C. krusei. In the experiment with the change of inoculum size, the TTD of PA was shorter compared to FA in 5 CFU/ml.
In the previous literature, Bactec Mycosis IC/F media (MF) that is formulated for the isolation of fungi in blood showed a higher recovery rate of Candida species, and TTD for C. albicans was significantly shorter compared to PA media and the difference of TTD for C. tropicalis, C. parapsilosis, and C. krusei was not significant 22. This result is discrepant with our study. This could be explained by the differences in TTD due to varying amounts of fluconazole, which resulted in delayed detection in MF. Comparison of TTD of MF for zero fluconazole and other concentrations showed that TTD of MF for zero fluconazole was the shortest with statistical significance and TTD of 1.9 mg/l was lower than that of 6.7 mg/l, implying that fluconazole was associated with delayed recovery of Candida species.
Bactec Myco/F Lytic media (ML) supports the growth of yeast, fungi, and mycobacteria. In addition, it was reported that the difference of TTD was insignificant between ML and standard Bactec culture media except for C. glabrata 23. In this study, TTD of PA was faster than that of ML, which also could be explained by the addition of fluconazole.
TTD of all the tested PA, FA, MF, and ML media showed that TTD of PA was the fastest among the media tested. The TTD varied greatly depending on the Candida species as in the previous literature 14. Among the Candida species, TTD of C. parapsilosis was longer compared with other species, which was in line with previous studies 14, 24, 25. TTD of FA for C. parapsilosis was 36.9 hr and the previous data showed that TTD of FA for C. parapsilosis ranged from 37.3 hr to 39.3 hr) 14, 25. Candida glabrata was reported to have the longest TTD among the Candida species, but C. glabrata was not included in this study. Comparison of PA and FA showed that TTD of PA was faster than FA by 3.3 hr for C. tropicalis, C. paraspilosis, and C. krusei.
The number of inoculated cells was rather less than that of previous studies, in which concentrations ranging from 10 CFU/ml to 1,000 CFU/ml were used 13, 14, 15. The TTD results in this study might be longer due to smaller inoculated cells and comparison with other studies might be inaccurate. However, it has been suggested that patients with candidemia can have less than 10 CFU/ml with some having even less than 1 CFU/ml of blood 22. The limitation of this study is that the C. glabrata, which was reported to have longer TTD, was not included in this study, as this species was not available during the study period.
CONCLUSION
Overall TTD of Bactec Fx Plus Aerobic/F was more than 2.5 hr faster (P = 0.004) for C. albicans, C. tropicalis, C. parapsilosis, and C. krusei compared to other tested media. As Bactec Fx Plus Aerobic/F media was unaffected by added fluconazole, it could support diagnosis of candidemia in the patients receiving fluconazole prophylactic or empirical therapy.
Supporting information
Disclaimer: Supplementary materials have been peer‐reviewed but not copyedited.
Table S1. Fluconazole susceptibility test results for selected Candida species.
ACKNOWLEDGMENTS
We acknowledge the following microbiologist and staff for their contribution to the study: Mi Ran Lee, Yeon Sil Seo, Gil Bong Jung, Min Gyu Choi, Jung Hwa Shim, and Su Kyung Park for laboratory support.
REFERENCES
- 1. Edmond MD, Wallance SE, McClish DK, Pfaller MA, Jones RN, Wenzel RP. Nosocomial bloodstream infections in United States hospitals; a three‐year analysis. Clin Infec Dis 1999;29:239–244. [DOI] [PubMed] [Google Scholar]
- 2. Tiraboschi IN, Bennett JE, Kauffman CA, Rex JH, Girmenia C, Sobel JD, Menichetti F. Deep Candida infections in the neutropenic and non‐neutropenic host: An ISHAMsymposium. Med Mycol 2000;38:199–204. [PubMed] [Google Scholar]
- 3. Oh BJ, Choi HW, Lee JS, et al. Clinical and laboratory features of candidemia caused by different Candida species. Korean J Lab Med 2005;25:317–323. [Google Scholar]
- 4. Han SS, Yim JJ, Yoo CG, et al. Clinical characteristics and risk factors for nosocomial candidemia in medical intensive care units: Experience in a single hospital in Korea for 6.6 years. J Korean Med Sci 2010;25:671–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Korean Nosocomial Infections Surveillance System (KONIS) . KONIS official report. Available at: http://www.kosnic.org/bbs/zboard.php?id=konisop/, October 16, 2007.
- 6. Wisplinghoff H, Bischoff T, Tallent SM, Seifert H, Wenzel RP, Edmond MB. Nosocomial bloodstream infections in US hospitals: Analysis of 24,179 cases from a prospective nationwide surveillance study. Clin Infect Dis 2004;39:309–317. [DOI] [PubMed] [Google Scholar]
- 7. Pfaller MA, Diekema DJ. Epidemiology of invasive candidiasis: A persistent public health problem. Clin Microbiol Rev 2007;20:133–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Echeverria PM, Kett DH, Azoulay E. Candida prophylaxis and therapy in the ICU . Semin Respir Crit Care Med 2011;32:159–173. [DOI] [PubMed] [Google Scholar]
- 9. Diekema DJ, Messer SA, Brueggemann AB, et al. Epidemiology of candidemia: 3‐year results from the emerging infections and the epidemiology of Iowa organisms study. J Clin Microbiol 2002;40:1298–1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Falagas ME, Apostolou KE, Pappas VD. Attributable mortality of candidemia: A systematic review of matched cohort and case‐control studies. Eur J Clin Microbiol Infect Dis 2006;25:419–425. [DOI] [PubMed] [Google Scholar]
- 11. Morrell M, Fraser VJ, Kollef MH. Delaying the empiric treatment of Candida bloodstream infection until positive blood culture results are obtained: A potential risk factor for hospital mortality. Antimicrob Agents Chemother 2005;49:3640–3645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Playford EG, Lipman J, Sorrell TC. Prophylaxis, empirical and preemptive treatment of invasive candidiasis. Curr Opin Crit Care 2010;16:470–474. [DOI] [PubMed] [Google Scholar]
- 13. Riedel S, Eisinger SW, Dam L, Stamper PD, Carroll KC. Comparison of BD Bactec Plus Aerobic/F medium to VersaTREK Redox 1 blood culture medium for detection of Candida spp. in seeded blood culture specimens containing therapeutic levels of antifungal agents. J Clin Microbiol 2011;49:1524–1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Horvath LL, George BJ, Hospenthal DR. Detection of fifteen species of Candida in an automated blood culture system. J Clin Microbiol 2007;45:3062–3064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Horvath LL, George BJ, Murray CK, Harrison LS, Hospenthal DR. Direct comparison of the Bactec 9240 and BacT/Alert 3D automated blood culture systems for Candida growth detection. J Clin Microbiol 2004;42:115–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Endimiani A, Tamborini A, Luzzaro F, Lombardi G, Toniolo A. Epidemiology of bloodstream infections and time to detection of positive blood cultures: An evaluation of the automated BacT/Alert and Bactec 9240 systems. New Microbiol 2002;25:9–16. [PubMed] [Google Scholar]
- 17. George BJ, Horvath LL, Hospenthal DR. Effect of inoculum size on detection of Candida growth by the Bactec 9240 automated blood culture system using aerobic and anaerobic media. J Clin Microbiol 2005;43:433–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Clinical and Laboratory Standards Institute (CLSI). Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts: Approved Standard M27‐A2. Wayne, PA: Clinical and Laboratory Standards Institute (CLSI) 2002. [Google Scholar]
- 19. Clinical and Laboratory Standards Institute (CLSI) . Zone Diameter Interpretive Standards, Corresponding Minimal Inhibitory Concentration (MIC) Interpretive Breakpoints, and Quality Control Limits for Antifungal Disk Diffusion Susceptibility Testing of Yeasts; Third Informational Supplement. M44‐S3. Wayne, PA: CLSI, 2002. [Google Scholar]
- 20. Pappas P. Clinical practice guidelines for the management of candidiasis: 2009 update by the Infectious Disease Society of America. Clin Infect Dis 2009;48:503–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Endimiani A, Tamborini A, Luzzaro F, Lombardi G, Toniolo A. Epidemiology of bloodstream infections and time to detection of positive blood cultures: An valuation of the automated BacT/Alert and Bactec 9240 systems. New Microbiol 2002;25:9–16. [PubMed] [Google Scholar]
- 22. Meyer MH, Letscher‐Bru V, Jaulhac B, Waller J, Candolfi E. Comparison of Mycosis IC/F and plus Aerobic/F media for diagnosis of fungemia by the Bactec 9240 system. J Clin Microbiol 2004;42:773–777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kirby J, Delaney M, Qian Q, Gold H. Optimal use of Myco/F lytic and standard Bactec blood culture bottles for detection of yeast and mycobacteria. Arch Pathol Lab Med 2009;133:93–96. [DOI] [PubMed] [Google Scholar]
- 24. Horvath LL, Hospenthal DL, Murray CK, Dooley DP. Detection of simulated candidemia by the Bactec 9240 system with PLUS Aerobic/F and Anaerobic/F blood culture bottles. J Clin Microbiol 2003;41:4714–4717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Fernandez J, Erstad BL, Petty W, Nix DE. 2009. Time to positive culture and identification for Candida blood stream infections. Diagn Microbiol Infect Dis 2009;64:402–407. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Disclaimer: Supplementary materials have been peer‐reviewed but not copyedited.
Table S1. Fluconazole susceptibility test results for selected Candida species.


