Abstract
Background
The measurements of platelet count and mean platelet volume (MPV) are routinely available nowadays. The aim of this study was to evaluate the platelet count and MPV trend in infectious and inflammatory processes. We also investigated whether these parameters were associated with the known markers of disease activity, erythrocyte sedimentation rate (ESR) and C‐ reactive protein (CRP).
Methods
This cross‐sectional study was conducted on 100 children with diagnosis of infectious and inflammatory diseases. Platelet count, MPV, ESR, and CRP were measured at the time of hospitalization and thereafter in the recovery phase.
Results
Mean platelet count increased in the patients at the time of admission in the hospital compared to the recovery and discharge time (mean 430,820 ± 134,643/μl vs. 350,970 ± 99,374/μl, P < 0.001). However, MPV decreased significantly during the same period (8.2 ± 1.1 fl vs. 8.7 ± 0.9, P < 0.001). Platelet count was directly correlated with CRP (mean 6.4 ± 0.3 mg/l), (r = 0.49, P < 0.001) and ESR (mean 10.9 ± 1.1 mm/hr), (r = 0.32, P = 0.003). On the other hand, MPV was inversely correlated with CRP (r = 0.39, P < 0.001) and ESR (r = −0.24, P = 0.034).
Conclusions
This study demonstrated a higher level of platelet count and lower MPV in the patients with active disease compared to the recovered patients. These parameters were well correlated with the known disease activity markers. We propose that platelet parameters can be considered as reliable markers for assessment of disease activity and response to treatment.
Keywords: platelet count, mean platelet volume, disease activity, erythrocyte sedimentation rate, C‐reactive protein
Infectious and inflammatory diseases are the most common diseases that delay the diagnosis and inadequate treatment in these cases can increase the mortality and morbidity and also cause a lot of costs for national health services. Because of the importance of these cases, some kinds of diagnostic examinations are always undertaken. In case of inflammatory stimulations, the first reaction step of this system is creating a local inflammation, in which the intrinsic immunity cells are called to be in the inflammation area. This response can be associated with the symptoms, including fever, fatigue, and anorexia 1, 2.
C‐reactive protein (CRP) is one of the acute phase reaction proteins, which is synthesized from liver and increases during inflammatory reactions. Elevated erythrocyte sedimentation rate (ESR) is another marker of inflammatory reactions 3. ESR and CRP are widely requested by the physicians in order to estimate the presence and severity of infectious and inflammatory diseases.
Platelets, as another part of the natural immune system, can be elevated in response to “acute phase reaction” during the inflammation process. This increase in platelets can reflect the bone marrow cells activity in response to the inflammation phase interleukins, such as IL‐1 and IL‐6 4, 5.
Nowadays, platelet count as well as platelets size can be easily measured by automatic counter devices; however, only a few studies have been done with regard to the clinical importance of using platelet markers fluctuations as an indicator of “acute phase response” for assessment of disease activity and response to the treatment.
In 1983, Robbins and Barnard performed a cohort study and found out that there were at least two different patterns in changes of platelet parameters in response to infection: increase in platelet count and decrease in mean platelet volume (MPV). Nevertheless, a primary increase in MPV along with thrombocytopenia could occur in acute sepsis 6. Since then, some other studies have been conducted on platelet parameters changes during infectious and inflammatory processes. From all the previous studies, it can be concluded that in case of an active infection or inflammation (apart from the acute sepsis), there is an increase in platelet count, but a decrease in MPV 7.
The study by Kapsoritakis et al. on the cohort of patients with intestine inflammation showed a significant correlation between MPV reduction and some other acute response phase markers, such as CRP and ESR and, as a result, MPV can be used as an independent marker in assessment of disease activity 8.
The present study aims to assess the changes in platelet parameters, such as platelet count and MPV, and the relation between each of those markers with ESR and CRP in infectious and inflammatory diseases. Considering the role the platelets play in the pathophysiology of infection and inflammation and the fact that platelet parameters are very easy and simple lab procedures to perform, by collecting more information of how these markers change, it has been tried to show the clinical importance of these changes that can be considered as the criteria to assess the disease activity and response to treatment.
This analytic, cross‐sectional study was conducted on 100 children (51 male and 49 female) with infectious and inflammatory diseases admitted in pediatric immunology and infectious ward of Nemazee Hospital, Shiraz University of Medical Science, Shiraz, Iran.
All the patients who had been diagnosed with any infectious or inflammatory disease were enrolled into the study.
All the eligible patients were included after taking consents from their parents or their legal guardians. The study was approved by the Ethics Committee of Shiraz University of Medical Sciences, Shiraz, Iran.
Platelet parameters, including platelet count and MPV, were measured automatically using Sysmex k 1000 (Sysmex Inc., Japan). Serum level of CRP was calculated by using semiquantitative agglutination on latex kite (Human Company, Germany). ESR was also measured using ESR auto analyzer (Electa, Italy).
Proper data collection form was designed to record the patients’ demographic information, contact number, residential place, disease name, and the paraclinical findings. Statistical analyses were performed using the SPSS statistical software (v. 15, Chicago, IL, USA).
The platelet counts and MPV of admission and recovery times were compared using paired t‐test. In addition, Pearson correlation test was used in order to determine the relationship between the platelet count as well as MPV and ESR and CRP. P values less than 0.05 were considered as statistically significant.
The mean age of the participants was 5.5 ± 4.1 years with the age range 1–15 y/o. There were 13 patients with central nervous system infection, seven patients with gastrointestinal infection, 48 patients with infection and inflammation of respiratory system, nine patients with dermal infections, five patients with joint infections, six patients with bone infections, eight patients with rheumatoid arthritis, and four patients suffering from Kawasaki disease.
According to the laboratory data, 39% of the children had a platelet count of more than 450,000/μl at the time of admission, while this number was within the normal range for other children. In 55% of the cohort, the MPV was less than 8.5 fl, while the rest of our cohort had a normal MPV of 8.5–12.5 fl.
In total patients, mean platelet count was 430,820 ± 134,643/μl at the admission time that reached to mean 350,970 ± 99,374/μl after the treatment period and clinical symptoms relief (P < 0.001).
The mean MPV of the cohort at the time of admission was 8.2 ± 1.1 fl that reached to 8.7 ± 0.9 fl after the treatment period and symptom relief (P < 0.001).
The study results revealed a negative correlation between the platelet count and MPV in the admission time (P < 0.001, r = −0.67). Similar results were also obtained during the treatment time in the hospital as well as during the time of discharge from the hospital (P < 0.001, r = −0.6).
The serum CRP level was found to be ≥6 mg/l in 76% of the patients during the hospital stay (mean 6.4 ± 0.3 mg/l), while it was reported to be negative after the treatment at the recovery time. Moreover, a positive correlation was found between the platelet count and the serum CRP level during the treatment period in the hospital (P < 0.001, r = 0.49), but a negative correlation between MVP and the serum CRP level during the same period (P < 0.001, r = – 0. 39) (Fig. 1).
Figure 1.
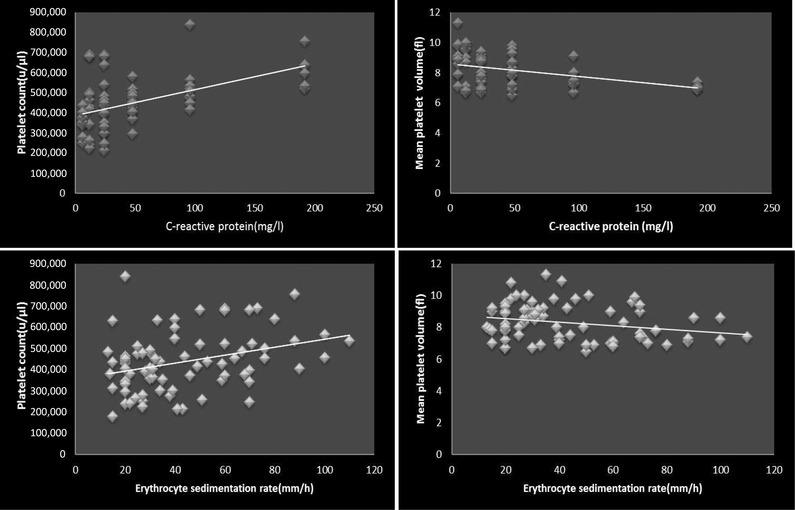
The relationship between platelet count and MPV with the serum level of CRP (top) and ESR (bottom).
At the admission time, 78% of the patients had an ESR more than the normal range of 10 mm/hr (mean 10.9 ± 1.1 mm/hr). In this group, a significant positive correlation was observed between the platelet count and ESR during the hospital stay (P = 0.003, r = 0.32). On the other hand, an inverse relationship was found between MPV and ESR during the same period (P = 0.0 34, r = –0. 24) (Fig. 1).
At the end of the treatment period, statistically significant relationships were found neither between the platelet count and ESR (P = 0.12, r = 0.17) nor between MPV and ESR (P = 0.6, r = –0.06).
Serum CRP level and ESR are very well recognized and the most common laboratory tests that along with clinical symptoms can be applied to assess the disease activity and treatment response 9, 10.
Platelets are also one of the cellular parts of the immunity system whose changes during the infectious and inflammatory processes would reflect the bone marrow activity. These changes, as some criteria of acute phase response, can be considered for patient clinical assessments. Infection and inflammatory reactions usually lead to low MPV with a high platelet count that suggests a reactive thrombocytosis 11, 12, 13, 14.
In 2001, Kapsoritakis et al. reported a significant increase in platelet count and a decrease in MPV during the disease activity phase. They evaluated the relationship between MPV and CRP, ESR, and WBC count, and reported a significant inverse relationship between MPV and other disease activity markers. Finally, the study results showed that MPV could even be used as an independent marker in disease activity assessments 8.
Nilsson and Milovanovic conducted a study on the patients with rheumatoid arthritis in 2003 and concluded that platelet count significantly increased during the disease activity 15 and Balbaloglu et al. found a significant difference in the MPV level between the patients with synovitis associated with osteoarthritis of knee and patients with knee osteoarthritis, and the patients with synovitis associated with osteoarthritis of knee and the control group 16. Also it was shown that platelet counts are significantly higher and MPV values were significantly lower in patients with acute rheumatic fever during the acute stage when compared to controls 17.
Considering platelet count and other inflammatory markers, it has been shown that there is a direct relationship between platelet count and CRP as well as IL‐6. However, no relationship has been reported between MPV and other inflammatory markers in those studies, which is against what Kapsoritakis reported in his study 8.
Douda et al. also conducted a study in 2006 and came to the point that MPV reduction was an independent marker for disease activity assessment, but its predictive value was not as high as platelet count and serum CRP 7.
In our study, mean platelet count and MPV were evaluated before treatment and after treatment. Comparing the platelet count and MPV at the time of disease activity with relief time after the treatment indicated similar results with the previous studies showing a significant increase in the patients’ platelet count, but a decrease in MPV at the time of disease activity.
The current study also assessed the relationship between the platelet count and MPV at the time of disease activity as well as the recovery time and the statistical analyses demonstrated a strong inverse relationship between platelet count and MPV in both circumstances. Hence, it can be concluded that platelet count and MPV changes have a negative correlation with each other. In other words, platelet count reduction and MPV increase are the signs of the patients moving from disease acute phase to the recovery phase.
In our study, 76% of the children had a CRP ≥ mg/l during the hospital stay, while it was measured zero at the recovery time after the treatment period. A direct relationship was also reported between platelet count and serum CRP level during the disease activity period. These results are in line with what Milovanovic and Nilsson have found in their studies 15. Also, we reported a negative relationship between MPV and serum CRP level, which is again consistent with what Kapsoritakis et al. had previously stated 8.
The results of the present study revealed no significant relationship between platelet count as well as MPV and ESR after the treatment at the recovery time.
The results also showed a significant increase in platelet count and a significant decrease in MPV at the time of disease activity. In addition, these changes have a fair correlation with the other disease activity markers (ESR and CRP), which can be used to assess the disease activity and response to treatment.
REFERENCES
- 1. Sachin J, Vidhi G, Sania N. Acute –phase proteins: As diagnostic tool. J Pharm Bioallied Sci 2011;3(1):118–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Simon L, Gauvin F, Amre DK, Saintlouis P, lacroix J. Serum procalcitonin and C – reactive protein levels as markers of bacterial infection: A systematic review and meta‐analysis. Clin Infect Dis 2004;39:206–217. [DOI] [PubMed] [Google Scholar]
- 3. Saadeh C. The erythrocyte sedimentation rate: Old and new applications. South Med J 1998;91(3):220–225. [PubMed] [Google Scholar]
- 4. Unsal E, Aksaray S, Koksal D, Sipit T. Potential role of interleukin‐6 in reactive thrombocytosis and acute phase response in pulmonary tuberculosis. Postgrad Med J 2005;81:604–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Dan K, Gomi S, Inokuchi K, Ogata K, Yamada T, Ohki I. Effects of interleukin‐1 and tumor necrosis factor on megakaryocytopoiesis: Mechanism of reactive thrombocytosis. Acta Hematol 1995;93:67–72. [DOI] [PubMed] [Google Scholar]
- 6. Robbins G, Barnard DL. Mean platelet volume changes in infection. J Clin Pathol 1983;36(11):1320–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Douda T, Bures J, Rejchrt S, Kopacova M, Pecka M, Maly J. Mean platelet volume (MPV) in crohn's disease patients. Cas Lek Cesk 2006;145(11):870–873. [PubMed] [Google Scholar]
- 8. Kapsoritakis AN, Koukourakis MI, Sfiridaki A, et al. Mean platelet volume: A useful marker of inflammatory bowel disease activity. Am J Gastroentrol 2001;96(3):776–781. [DOI] [PubMed] [Google Scholar]
- 9. Hameed MA, Waqas S. Physiologic basis and clinical utility of erythrocyte sedimentation rate. Pak J Med Sci 2006;22:2214–2218. [Google Scholar]
- 10. Brigden M. Clinical utility of the erythrocyte sedimentation rate. Am Fam Physician 1999;60:1443–1450. [PubMed] [Google Scholar]
- 11. Gabay C, Kushner I. Acute phase proteins and other systemic responses to inflammation. N Engl J Med 1999;340:448–454. [DOI] [PubMed] [Google Scholar]
- 12. Grys E, Toussaint MJ, Niewold TA, Koopmans SJ. Acute phase reaction and acute phase proteins .J Zhejiang Univ Sci B 2005;6(11):1045–1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Black S, Kushner I, Samols D. C‐reactive protein. J Biol Chem 2004;279:48487–48490. [DOI] [PubMed] [Google Scholar]
- 14. Reeves G. C‐reactive protein. Aust Prescr 2007;30(3):74–76. [Google Scholar]
- 15. Milovanovic M, Nilsson E, Jaremo P. Relationships between platelets and inflammatory markers in rheumatoid arthritis. Clin Chim Acta 2004;343:237–240. [DOI] [PubMed] [Google Scholar]
- 16. Balbaloglu O, Korkmaz M, Yolcu S, Karaaslan F, Beceren NG. Evaluation of mean platelet volume (MPV) levels in patients with synovitis associated with knee osteoarthritis. Platelets 2013. Mar 7. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 17. Sert A, Aypar E, Odabas D. Mean platelet volume in acute rheumatic fever. Platelets 2013;24(5):378–382. [DOI] [PubMed] [Google Scholar]


