Abstract
Objectives
The objectives of this article are to investigate the serum lipid hydroperoxide (LOOH) levels and paraoxonase‐1 (PON1) and arylesterase (ARE) activity in patients with lung, breast, and colorectal cancer.
Design and methods
Serum PON1 and ARE activities and LOOH levels were measured in 110 patients with cancer and same number of age‐ and sex‐matched controls.
Results
Serum LOOH levels were found to be increased while serum PON1 and ARE activities were found to be decreased in patients compared to controls. PON1 activity was found to be lower in patients with breast cancer than in patients with lung and colorectal cancer. There were positive correlations between the serum PON1 and ARE activities in patients with colorectal cancer.
Conclusion
We concluded that decreased PON1 and ARE activities and increased LOOH levels might have a connection to carcinogenesis. PON1 activity is decreased in all patients but it does not seem to be related to metastase status except for colorectal cancer. J. Clin. Lab. Anal. 26:155‐160, 2012. © 2012 Wiley Periodicals, Inc.
Keywords: paraoxonase‐1, arylesterase, lipid hydroperoxide, cancer
INTRODUCTION
Lipid peroxidation is a well‐known example of oxidative damage in cell membranes, lipoproteins, and other lipid‐containing structures. Peroxidative modification of unsaturated phospholipids, glycolipids, and cholesterol can occur in reactions triggered by (i) free radical species such as oxyl radicals, peroxyl radicals, and hydroxyl radicals derived from iron‐mediated reduction of hydrogen peroxide or (ii) nonradical species such as singlet oxygen, ozone, and peroxynitrite generated by the reaction of superoxide with nitric oxide 1. Overproduction of lipid peroxidation byproducts and disturbances in antioxidant defense system have been implicated in the pathogenesis of cancer.
High‐density lipoproteins (HDLs) are major carriers of plasma lipid hydroperoxides (LOOHs) in animal models of atherosclerosis and in humans. HDLs are also carriers of enzymes that destroy the lipid hydroperoxides that oxidize low‐density lipoprotein (LDL) phospholipids. These enzymes include paraoxonase‐1 (PON1) and PON3, and possibly glutathione phospholipid peroxidase 2. The enzyme may remove lipid peroxidation products. Such oxidatively damaged macromolecules have been implicated in aging and in diseases ranging from ischemia‐reperfusion injury to arthritis to cancer 3. Oxidative stress is a known mediator of cancer.
Human PON1 is a 355 amino acid (43–45 kDa) calcium‐dependent enzyme with at least two N‐linked carbohydrate chains. Glycosylation heterogeneity may explain the existence of isoforms. In blood, PON1 is bound to a category of HDL. Historically termed paraoxonase, this enzyme has no known physiological substrate and no clear biological function 4. Besides its capability to hydrolyze organophosphorus (OP) [(e.g. chlorpyrifos oxon, diazoxon) as well as nerve agents such as soman, sarin, or VX], PON1 was shown to hydrolyze aromatic carboxyl esters such as phenyl acetate, and thus to be involved in the metabolism of drugs and xenobiotics. However, PON1 does not hydrolyze directly the parent compounds of such insecticides (i.e., parathion, chlorpyrifos, diazinon), or several other OPs (e.g., malaoxon, dichlorvos) 5. PON1 hydrolyzes also various lactones, including naturally occurring lactone metabolites. The catalytic activity of PON1 spans five orders of magnitude between phenylacetate (the best substrate known) and L‐homocysteine thiolactone (a metabolite known as a risk factor for atherosclerotic vascular disease) 4.
PON1 was named after its ability to hydrolyze the organophosphate substrate paraoxon (PON activity, EC 3.1.8.1), which is the toxic metabolite of the insecticide parathion. Because PON1 could also hydrolyze aromatic esters, such as phenylacetate [arylesterase activity (ARE), EC 3.1.1.2], the term “A‐esterase” was introduced for the enzyme hydrolysing both compounds 6, 7.
Concentration and activity of PON1 are highly variable in human populations. The quantity and quality of the enzyme in serum is likely to be important in an individual's response to OP poisoning or risk of developing vascular disease. As such, it is vital to understand the factors that influence serum levels of PON1 in vivo, particularly if it is considered a target for therapeutic intervention 8.
LOOHs are a large family of the first by‐products of oxidized lipids, and their quantification could become a useful biomarker 9. Lipid hydroperoxides are formed nonenzymatically by the action of reactive oxygen species (ROS) on polyunsaturated fatty acids (PUFAs) 10 or enzymatically from lipoxygenases (LOXs) 11 and cyclooxygenases (COXs) 12.
Previous studies have shown reduced rates of lipid peroxidation in the tumor tissue of various types of cancer 13, 14, 15, 16. The aim of this study was to investigate the serum PON1 and ARE activities and LOOH levels in patients with patients lung, breast, and colorectal cancer, compared to that of healthy controls, and to investigate possible alterations in relation to the type of cancer.
MATERIALS AND METHODS
The Study Population
This study was conducted at the Department of Internal Medicine, Cerrahpasa Medical Faculty, University of Istanbul, Turkey. A total of 110 patients were admitted during the period. Twenty‐five age‐ and sex‐matched healthy women were enrolled into this study. Cases either study group or control, who had pathologies that could cause secondary lipid disorders, cardiovascular diseases, diabetes mellitus, renal failure, chronic infection and inflammation, alcohol abuse, and those who used antilipidemic and antioxidant drugs, were excluded from the study. PON1 activity is influenced by diet as well as endocrine and other environmental factors 17. Therefore, we have had a questionnaire that included questions on demographics, diet, and other lifestyle factors performed. After doing this questionnaire, we have used the patients who have similar diet and lifestyle. All patients with previously performed chemotherapy, radiotherapy, and surgery were also excluded from the study.
The remaining 45 patients with previously untreated, histopathologically verified newly diagnosed as of lung cancer (LC). Subjects’ history and physical examinations were documented. The diagnosis of LC was based on histopathologic findings. Seven of 45 patients were small cell LC, 38 patients were nonsmall cell LC (NSCLC).
This study included 25 consecutive patients with primary breast cancer who attended the Faculty of them had distant metastases at the time of diagnosis. We evaluated clinicopathological features (histology, menopausal status, estrogen receptor (ER), and progesterone receptor (PR) status, number of axillary lymph nodes involved, grade, tumor size, and stage according to the American Joint Committee on Cancer staging system).
Forty patients with newly diagnosed and histologically confirmed primary colorectal cancer were included in this prospective study. Tumor staging was performed according to the Dukes’ and TNM Classification of Malignant Tumors (TNM).
The protocol for sample collection was approved by the İstanbul University Cerrahpasa Medicine Faculty Ethics Committee. The study was performed in accordance with the Helsinki Declaration and informed consent was obtained from all patients and controls prior to their inclusion in the study.
Serum Preparation
Blood was drawn after 12–14 hr of fasting in the morning. Serum were obtained after at least 30 min of clotting by centrifugation at 2,500 × g for 15 min. Serum were removed and used directly for measurements of biochemical parameters and tumor markers. Other serum were stored at −70°C until assayed for determination of all parameters. All icteric or haemolytic blood samples were discarded. All parameters were analyzed in all samples together in a single batch, after we had finished our protocol (control and patient samples were analysed in the same batch).
Determination of PON1 Arylesterase Activity
Arylesterase activity was also measured spectrophotometrically using phenylacetate (Sigma Co, London, UK) as the substrate. The assay mixture contained 100 μl of 10 mmol/l substrate solution, 5 μl serum, and 1 mmol/l CaCl2 (Sigma, USA) in 50 mmol/l Tris buffer (Fluka Chemie, Switzerland), pH = 8. Production of phenol was determined spectrophotometrically after 2 min at 270 nm. The assay mixture was prepared daily before use. PON1 arylesterase activity was monitored in triplicate and the results are presented as μmol/min per ml 18. Mean intra‐assay and inter‐assay coefficients of variation were up to 5% and 8%, respectively.
Determination of PON1 Paraoxonase Activity
PON1 activity was assayed using synthetic paraoxon (diethyl‐p‐nitrophenyl phosphate) as substrate. PONl activity was determined by measuring the initial rate of substrate hydrolysis to p‐nitrophenol, whose absorbance was monitored at 412 nm in the assay mixture containing 2.0 mM paraoxon, 2.0 mM CaCl2, and 20 μl of plasma in 100 mM Tris‐HCI buffer (pH = 8.0). The blank sample containing incubation mixture without plasma was run simultaneously to correct for spontaneous substrate breakdown. The enzyme activity was calculated from E412 of p‐nitrophenol (18.290 per M/cm) and was expressed as U/ml; 1 U of enzyme hydrolyzes 1 nmol of paraoxon/min 19. Mean intra‐assay and inter‐assay coefficients of variation for this analysis were 9.7% and 10.9%, respectively.
Determination of Serum LOOHLevels
Serum LOOH levels were determined spectrophotometrically according to the method of ferrous oxidation with xylenol orange version 2 (FOX 2) 20. Hydroperoxides oxidize ferrous to ferric ions selectively in dilute acid and the resultant ferric ions can be determined by using ferric‐sensitive dyes as an indirect measure of hydroperoxide concentration. Xylenol orange binds ferric ions with high selectivity to produce a coloured (blue‐purple) complex with an extinction coefficient of 1.5 × 104M−1 × cm−1. Ninety microliter aliquots of plasma were transferred into microcentrifuge reaction vials. Triphenylphosphine (TPP) (10 mM in methanol; 10 ml) was added to vials to remove hydroperoxides. Methanol alone (10 ml) was added to the remaining vials. The LOOH content in the plasma samples is determined as a function of the absorbance difference of samples with and without elimination of LOOHs by TPP. The samples were vortexed and subsequently incubated at room temperature for 30 min. FOX2 reagent (900 ml) was added and the samples were vortexed. After incubation at room temperature for 30 min, the samples were centrifuged at 15,000 g at 20°C for 10 min, the supernatant was carefully decanted into a cuvette, and absorbance was read at 560 nm. The coefficients of intra‐ and inter‐assay variations were 2.6% (n = 10), and 2.5% (n = 12), respectively.
Serum total protein, albumin, total cholesterol, HDL, and LDL cholesterol were measured using an Olympus AU 800 autoanalyzer (Olympus, Japan). The methods were Biuret for total protein, BCG (Bromocresol green) for albumin. Total cholesterol, HDL cholesterol, and triglycerides were measured by enzymatic methods in all samples.
Statistical Analysis
Statistical analyses were performed using the SPSS software package, version 10.0 for Windows. Clinical laboratory data were expressed as mean ± standard deviation. The analysis of variance (ANOVA) test was used to compare biochemical parameters among lung, breast, and colorectal cancer. Mann–Whitney U test was used to assess the mean differences between metastatic and nonmetastatic cancer patients. The Pearson's correlation analysis was used to assess the relationship between PON1 and ARE activity. A value of P < 0.05 was considered as statistically significant.
RESULTS
The demographic features and biochemical parameters of all groups are presented in the Table 1. Age and triglycerides levels were not different among the groups. The serum total cholesterol levels in controls and patients with breast cancer were significantly lower compared to patients with lung and colorectal cancer (P < 0.05) (Table 1). Serum HDL cholesterol was significantly lower (P < 0.05) and LDL cholesterol levels were significantly higher (P < 0.001) in the cancer patients as compared with controls. However, there was no significant correlation between HDL and PON1 activity in all groups.
Table 1.
Demographic features, paraoxonase, and arylesterase activity and lipid hydroperoxide parameters in controls and patient group
| Control (n:25) | Lung (n: 45) | Colorectal (n: 40) | Breast (n: 25) | P | |
|---|---|---|---|---|---|
| Age (year) | 52.40 ± 11.32 | 59.37 ± 10.83 | 54.28 ± 12.46 | 53.74 ± 10.71 | .062 |
| Total cholesterol (mmol/l) | 4.68 ± 0.74 | 5.28 ± 0.81 | 5.47 ± 1.37 | 4.84 ± 0.64 | .003** |
| Triglyceride (mmol/l) | 1.10 ± 0.63 | 1.24 ± 0.56 | 1.42 ± 1.17 | 1.08 ± 0.53 | .364 |
| HDL cholesterol (mmol/l) | 1.43 ± 0.26 | 1.26 ± 0.22 | 1.27 ± 0.19 | 1.24 ± 0.13 | .017* |
| LDL cholesterol (mmol/l) | 2.75 ± 0.65 | 3.45 ± 0.61 | 3.55 ± 1.19 | 3.10 ± 0.57 | .000*** |
| PON1 (U/ml) | 124.89 ± 21.70 | 67.64 ± 22.01 | 65.94 ± 22.57 | 42.99 ± 7.98 | .000*** |
| ARE (U/ml) | 98.55 ± 18.82 | 65.62 ± 27.14 | 57.55 ± 13.34 | 54.51 ± 11.57 | .000*** |
| LOOH (μmol/l) | 1.08 ± 0.24 | 2.96 ± 0.43 | 2.38 ± 0.63 | 1.97 ± 0.50 | .000*** |
Data are expressed as mean ± SD.
*P < 0.05; **P < 0.01; *** P < 0.001
PON: paraoxonase; ARE: arylesterase; LOOH: lipid hydroperoxide.
PON1 and ARE acivities was found to be decreased (P < 0.001) in serum of patients with cancer compared to controls. When the patient group was categorized, PON1 activity was found to be lower in patients with breast cancer than in patients with lung and colorectal cancer. There was established positive correlation between serum PON1 activity and serum ARE activity in patients with colorectal cancer (r = 0356; P < 0.05) (Fig. 1).
Figure 1.
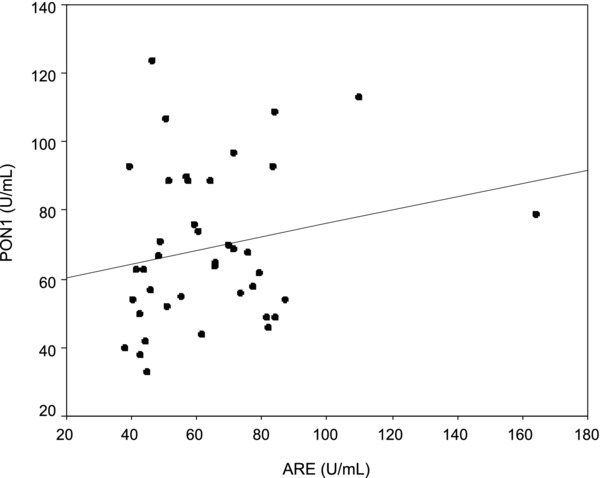
Positive correlation between serum PON1 and AREactivity in the colorectal cancer group (r = 0.356 P = 0.033).
LOOH levels were significantly different in all of the groups (P < 0.001). LOOH levels were found to be higher in lung cancer cases, and lower in breast cancer. No significant difference was found in PON1 activities among the patients groups with metastase except for patients with colorectal cancer (P < 0.05) (Table 2).
Table 2.
Paraoxonase and arylesterase activity and lipid hydroperoxide in metastatic and nonmetastatic cancer patients
| Nonmetastatic | Metastatic | P | |
|---|---|---|---|
| Lung cancer | (n: 27) | (n:18) | |
| PON 1(U/ml) | 66.19 ± 22.36 | 69.83 ± 21.93 | .586 |
| ARE (U/ml) | 59.06 ± 17.39 | 75.45 ± 35.68 | .086 |
| LOOH (μmol/l) | 2.90 ± 0.44 | 3.05 ± 0.41 | .366 |
| Colorectal cancer | (n:15) | (n:25) | |
| PON 1(U/ml) | 75.99 ± 24.65 | 59.91 ± 19.30 | .040* |
| ARE (U/ml) | 61.39 ± 13.77 | 55.21 ± 12.80 | .192 |
| LOOH (μmol/l) | 2.65 ± 0.72 | 2.22 ± 0.51 | .052 |
| Breast cancer | (n:17) | (n:8) | |
| PON 1(U/ml) | 43.98 ± 7.71 | 40.88 ± 8.64 | .440 |
| ARE (U/ml) | 53.56 ± 11.10 | 56.51 ± 13.08 | .588 |
| LOOH (μmol/l) | 1.98 ± 0.57 | 1.97 ± 0.36 | .588 |
*P < 0.05
DISCUSSION
Research on lipid peroxidation has intensified in recent years largely because of increasing awareness that this process may play an important role in UV‐induced skin cancer, atherosclerosis, neurodegeneration, and various other disorders. LOOHs are well‐known intermediates of peroxidative reactions that are generally more long‐lived than any free radical precursors, making intermembrane translocation within a cell, between cells, or between lipoproteins and cells possible 21. In general, ROS appear to be key regulatory factors in molecular pathways linked to tumor development and tumor dissemination, which offer potential therapeutic invention points 22.
Serum PON1 binds to high‐density lipoprotein and contributes to the detoxification of OP compounds, such as paraoxon, and carcinogenic lipid soluble radicals from lipid peroxidation 23, 24, 25. A recent study also reported a decreased level of PON1 and ARE activities in radiology workers exposed for more than 5 years to ionizing radiation 26. In a preliminary study of Elkiran et al. 27, it was reported that serum PON1 and ARE activities were significantly lower in LC patients compared to healthy subjects in a Turkish population. These observations suggested the hypothesis that defects in the antioxidant system capacity and altered PON1 activity may be involved in the pathogenesis of LC. Results of our study are enough to confirm it in LC patients in the same population.
Both serum PON1 activity, part of the lipid peroxidation scavenging system, and LOOH levels have not been studied in lung cancer. In our study, serum LOOH levels were found to be increased while serum PON1 and ARE activities were found to be decreased in patients compared to controls. Kaynar et al. 28 studied the activities of antioxidant enzymes and demonstrated that erythrocyte malondialdehyde, nitric oxide, total glutathione levels, and erythrocyte superoxide dismutase, catalase, and xanthine oxidase activities were significantly higher in patients with LC than in controls. This study indicates significant changes in antioxidant defence system in NSCLC and SCLC patients, which may lead to enhanced action of oxygen radical, resulting in lipid peroxidation.
The gastrointestinal tract, especially the colon, is constantly exposed to ROS originating from endogenous and exogenous sources 29. LOOH levels were also significantly increased in colorectal cancer in this study. There were positive correlations between the serum PON1 and ARE activities in patients with colorectal cancer. Another important finding of our study significant difference was not found in PON1 activities among the patients groups with metastase except for patients with colorectal cancer. No significant difference was found in ARE activities and LOOH levels among the patients groups with metastase with colorectal cancer. Di Giacomo et al. 30 demonstrated that a significant decrease in nonproteic antioxidant status and in total thiol groups with respect to healthy controls, whereas oxidized proteins and lipid hydroperoxide levels were significantly increased in colon cancer patients. This study supports our observations in colorectal cancer.
Gönenc et al 31 have demonstrated lipid peroxidation in serum and tissue of benign breast disease is greater than in breast cancer. Total antioxidant status is lower in benign tissue than in cancerous tissue, probably to compensate for this elevated free radical production. Serum LOOH levels was found to be increased while serum PON1 and ARE activities were found to be decreased in patients compared to controls. Our results and another study 32, 33 support these observations in breast cancer. Diminished PON and ARE activity, and increased LOOH levels are also associated with particular stage, grade, and CA‐125 level of ovarian cancer 34.
Protection against organophosphorus insecticide and nerve agent poisoning and reduction of the risk of cardiovascular disease in individuals who have low PON1 activity represent examples of future utilizations of this enzyme 35. Human serum PON binds to high‐density lipoprotein and contributes to the elimination of carcinogenic lipid‐soluble radical from lipid peroxidation. Decreased PON1 activity is a consequence of cancer and not an underlying cause in this study. Therefore, future utilizations of this enzyme may contribute to the elimination of carcinogenic radicals.
Chronic inflammation is closely associated with angiogenesis 36 and PON1, also decreases during inflammation 37. The decreased PON1 activity may be in response to inflammation in patients with lung, breast, and colorectal cancer. In future studies, it will be important to determine whether PON1 and ARE activities play a causal role in cancer.
Low plasma paraoxonase/arylesterase activities and the concentration of the PON1 enzyme, high lipid peroxide levels were observed in all cancer patients 38. LOOH levels appear to play a role in the development of lung, breast, and colorectal cancer in this study. Significant increases in the levels of lipid hydroperoxide and decreases in PON1 and ARE activities suggest the possible involvement of oxidative stress in lung, breast, and colorectal cancer. This shift of equilibrium is probably influencing the cancer pathogenesis. Our findings also indicate that in future clinical trials testing the anticarcinogenic effect of antioxidative supplements, the screening of metastase status of patients with colorectal cancer according to the PON1 activity may be assured.
LIMITATION OF STUDY
The activity of PON1 is under genetic and environmental regulation. Due to economical problems, we couldn't detect genotypes of PON1 of cancer patients.
CONFLICT OF INTEREST
The authors declare that they have no conflict of interest related to the publication of this article.
AUTHOR CONTRIBUTIONS
All authors contributed equally to the research and writing of this article.
REFERENCES
- 1. Girotti AW. Lipid hydroperoxide generation, turnover, and effector action in biological systems. J Lipid Res 1998;39:1529–1542. [PubMed] [Google Scholar]
- 2. Barter PJ, Nicholls S, Rye KA, Anantharamaiah GM, Navab M, Fogelman AM. Antiinflammatory properties of HDL . Circ Res 2004;95:764–772. [DOI] [PubMed] [Google Scholar]
- 3. Ames BN, Shigenaga MK, Hagen TM. Oxidants, antioxidants, and the degenerative disease of aging. Proc Natl Acad Sci 1993;90:7915–7922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Rochu D, Chabrière E, Masson P. Human paraoxonase: A promising approach for pre‐treatment and therapy of organophosphorus poisoning. Toxicology 2007;233:47–59. [DOI] [PubMed] [Google Scholar]
- 5. Costa LG, Cole TB, Vitalone A, Furlong CE. Measurement of paraoxonase (PON1) status as a potential biomarker of susceptibility to organophosphate toxicity. Clinica Chimica Acta 2005;352:37–47. [DOI] [PubMed] [Google Scholar]
- 6. Aldridge WN. Serum esterases. II. An enzyme hydrolysing diethyl pnitrophenyl phosphate (E600) and its identity with the A‐esterase of mammalian sera. Biochem J 95;53:117–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Aldridge WN. Serum esterases. I. Two types of esterase (A and B) hydrolysing p‐nitrophenyl acetate, propionate and butyrate, and a method for their determination. Biochem J 1953;53:110–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Deakin SP, James RW. Genetic and environmental factors modulating serum concentrations and activities of the antioxidant enzyme paraoxonase‐1. Clin Sci 2004;107:435–447. [DOI] [PubMed] [Google Scholar]
- 9. Arab K, Steghens JP. Plasma lipid hydroperoxides measurement by an automated xylenol orange method. Anal Biochem 2004;325:158–163. [DOI] [PubMed] [Google Scholar]
- 10. Marnett LJ. Oxyradicals and DNAdamage. Carcinogenesis 2000;21:361–370. [DOI] [PubMed] [Google Scholar]
- 11. Brash AR. Lipoxygenases: Occurrence, functions, catalysis, and acquisition of substrate. J Biol Chem 1999;274:23679–23682. [DOI] [PubMed] [Google Scholar]
- 12. Lee SH, Williams MV, Dubois RN, Blair IA. Cyclooxygenase‐2‐mediated DNA damage. J Biol Chem 2005;280:28337–28346. [DOI] [PubMed] [Google Scholar]
- 13. Tanaka T. Effect of diet on human carcinogenesis. Crit Rev Oncol Hematol 1997;25:73–95. [DOI] [PubMed] [Google Scholar]
- 14. Tanaka, K , Kawabata, K , Kakumoto, M . Citrus auraptene exerts dose dependent chemopreventive activity in rat large bowel tumorigenesis: The inhibition correlates with suppression of cell proliferation and lipid peroxidation and with induction of Phase IIdrug metabolizing enzymes. Cancer Res 1998;58:2550–2556. [PubMed] [Google Scholar]
- 15. Manoj GR, Thampi BSH, Menon VP, Leelamma, S . Influence of dietary fibre from coconut kernel (Cocus nucifera) on the 1,2‐dimethylhydrazine‐induced lipid peroxidation in rats. J Nutr Biochem 1999;10:555–560. [DOI] [PubMed] [Google Scholar]
- 16. Cheeseman KH, Collins M, Proudfoot K, et al. Studies on lipid peroxidation in normal and tumor tissues. The Novikoff rat liver tumor. Biochem J 1986;235:507–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Costa LG, Giordano G, Furlong CE. Pharmacological and dietary modulators of paraoxonase 1 (PON1) activity and expression: the hunt goes on. Biochem Pharmacol 2011;81:337–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Haagen L, Brock A. A new automated method for phenotyping arylesterase (EC3.1.1.2) based upon inhibition of enzymatic hydrolysis of 4‐nitrophenyl acetate by phenyl acetate. Eur J Clin Chem Clin Biochem 1992;30:391–395. [DOI] [PubMed] [Google Scholar]
- 19. Cakatay U, Kayali R, Uzun H. Relation of plasma protein oxidation parameters and paraoxonase activity in the ageing population. Clin Exp Med 2008;8:51–57. [DOI] [PubMed] [Google Scholar]
- 20. Nourooz‐Zadeh J. Ferrous ion oxidation in presence of xylenol orange for detection of lipid hydroperoxides in plasma. Methods Enzymol 1999;300:58–62. [DOI] [PubMed] [Google Scholar]
- 21. Girotti AW. Lipid hydroperoxide generation, turnover, and effector action in biological systems. J Lipid Res 1998;39:1529–1542. [PubMed] [Google Scholar]
- 22. Behrend L, Henderson G, Zwacka RM. Reactive oxygen species in oncogenic transformation. Biochem Soc Trans 2003;31:1441–1444. [DOI] [PubMed] [Google Scholar]
- 23. Humbert R, Adler DA, Disteche CM, Hassett C, Omiecinski CJ, Furlong CE. The molecular basis of the human serum paraoxonase activity polymorphism. Nat Genet 1993;3:73–76. [DOI] [PubMed] [Google Scholar]
- 24. Davies HG, Richter RJ, Keifer M, Broomfield CA, Sowalla J, Furlong CE. The effect of the human serum paraoxonase polymorphism is reversed with diazoxon, soman and sarin. Nat Genet 1996;14:334–336. [DOI] [PubMed] [Google Scholar]
- 25. Shih DM, Gu L, Xia YR, et al. Mice lacking serum paraoxonase are susceptible to organophosphate toxicity and atherosclerosis. Nature 1998;394:284–287. [DOI] [PubMed] [Google Scholar]
- 26. Serhatlioglu S, Gursu MF, Gulcu F, Canatan H, Godekmerdan A. Levels of paraoxonase and arylesterase activities and malondialdehyde in workers exposed to ionizing radiation. Cell Biochem Funct 2003;21:371–375. [DOI] [PubMed] [Google Scholar]
- 27. Elkiran ET, Mar N, Aygen B, Gursu F, Karaoglu A, Koca S. Serum paraoxonase and arylesterase activities in patients with lung cancer in a Turkish population. BMC Cancer 2007;7:48--55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kaynar H, Meral M, Turhan H, Keles M, Celik G, Akcay F. Glutathione peroxidase, glutathione‐S‐transferase, catalase, xanthine oxidase, Cu‐Zn superoxide dismutase activities, total glutathione, nitric oxide, and malondialdehyde levels in erythrocytes of patients with small cell and non‐small cell lung cancer. Cancer Lett 2005;227:133–139. [DOI] [PubMed] [Google Scholar]
- 29. Blau S, Rubinstein A, Bass P, Singaram C, Kohen R. Differences in the reducing power along the rat GI tract: Lower antioxidant capacity of the colon. Molec Cell Biochem 1999;194:185–191. [DOI] [PubMed] [Google Scholar]
- 30. Di Giacomo C, Acquaviva R, Lanteri R, Licata F, Licata A, Vanella A. Nonproteic antioxidant status in plasma of subjects with colon cancer. Exp Biol Med (Maywood) 2003;228:525–528. [DOI] [PubMed] [Google Scholar]
- 31. Gönenç A, Erten D, Aslan S, Akinci M, Simşek B, Torun M. Lipid peroxidation and antioxidant status in blood and tissue of malignant breast tumor and benign breast disease. Cell Biol Int 2006;30:376–380. [DOI] [PubMed] [Google Scholar]
- 32. Yeh CC, Hou MF, Tsai SM, et al. Superoxide anion radical, lipid peroxides and antioxidant status in the blood of patients with breast cancer. Clin Chim Acta 2005;361:104–111. [DOI] [PubMed] [Google Scholar]
- 33. Sener DE, Gönenç A, Akinci M, Torun M. Lipid peroxidation and total antioxidant status in patients with breast cancer. Cell Biochem Funct 2007;25:377–382. [DOI] [PubMed] [Google Scholar]
- 34. Camuzcuoglu H, Arioz DT, Toy H, Kurt S, Celik H, Erel O. Serum paraoxonase and arylesterase activities in patients with epithelial ovarian cancer. Gynecol Oncol 2009;112:481–485. [DOI] [PubMed] [Google Scholar]
- 35. Costa LG, Vitalone A, Cole TB, Furlong CE. Modulation of paraoxonase (PON1) activity. Biochem Pharmacol 2005;69:541–550. [DOI] [PubMed] [Google Scholar]
- 36. Jackson JR, Seed MP, Kircher CH, Willoughby DA, Winkler JD. The codependence of angiogenesis and chronic inflammation. FASEB J 1997;11:457–465. [PubMed] [Google Scholar]
- 37. Chait A, Han CY, Oram JF, Heinecke JW. Thematic review series: The immune system and atherogenesis. Lipoprotein‐associated inflammatory proteins: Markers or mediators of cardiovascular disease? J Lipid Res 2005;46:389–403. [DOI] [PubMed] [Google Scholar]
- 38. Samra ZQ, Pervaiz S, Shaheen S, Dar N, Athar MA. Determination of oxygen derived free radicals producer (xanthine oxidase) and scavenger (paraoxonase1) enzymes and lipid parameters in different cancer patients. Clin Lab 2011;57:741–747. [PubMed] [Google Scholar]


