Abstract
Objective
Chagas disease constitutes a major public health problem in Latin America. Correctly designed pharmacokinetic, safety, and bioequivalence studies are desirable in order to fill the knowledge gaps that presently exist on available drugs. It is necessary to develop accurate, simple, reproducible, and sensitive high‐performance liquid chromatography (HPLC)/UV methods for the quantization of benznidazole (BNZ) in human plasma and urine for clinical applications, specially in pediatric patients.
Methods
Quantization of BNZ in human plasma involved freeze‐drying and re‐suspension in organic solvent followed by reverse phase HPLC with UV detection. Analysis of BNZ in urine involved liquid/liquid extraction followed by reverse phase HPLC with UV detection.
Results
Limits of quantization (LOQ) were 0.32 μg/ml for plasma and 5.2 μg/ml for urine. No metabolite interferences were showed in both methods.
Conclusion
The LOQ of methods seems appropriate in pediatric clinical contexts. Both procedures were applied with good results, to the quantization of BNZ in plasma and urine of patients treated for Chagas disease.
Keywords: HPLC, Chagas Disease, Benznidazole, Urine, Plasma
INTRODUCTION
Chagas disease, a protozoan infection caused by the kitenoplastid Trypanosoma cruzi, constitutes a major public health problem in Latin America. According to current data, there are over seven million infected people in the Americas 1 with an incidence of more than 40,000 new cases per year 2. Also, Chagas disease has expanded to nonendemic regions such as North America and Europe countries via immigration of infected individuals 3 and has been rising in the ranking of international health priorities due to the growing migration flows from endemic to nonendemic areas 4. Most infections take place in children, mostly by vector or congenital transmission 5.
Correctly designed pharmacokinetic, safety, and bioequivalence studies are desirable in order to fill the know‐ledge gaps that presently exist on available drugs. In this sense, it would be very helpful to establish the therapeutic range of benznidazole (BNZ) in order to optimize dosage, and to validate new pharmaceutical formulations for patients with Chagas disease in the acute phase, parti‐cularly children.
The aim of this work was to develop and validate fast, accurate, precise, reproducible, and specific methods to quantify BNZ in human plasma and urine, for further application in clinical research, safety and regulatory purposes, and for routine clinical use.
EXPERIMENTAL
Materials and Reagents
Trichloroacetic acid (TCA) and dichloro methane (DCM) were purchased from Biopack (Buenos Aires, Argentina). Dimethyl sulfoxide (DMSO), anhidrous sodium sulfate, and sodium hydroxide were obtained from Anedra (Buenos Aires, Argentina). Ethyl acetate was obtained from Dorwil (Buenos Aires, Argentina). Glycine was obtained from Parafarm (Buenos Aires, Argentina). Hydrochloric acid was obtained from J.T. Baker (USA). All these reagents were proanalysis grade. Chromatographic grade demineralized water (<0.2 μsiemens) was obtained in our laboratory with ionic interchange resins. Sodium octanesulfonate (Tedia, USA) and acetonitrile (J.T. Baker, USA) high‐performance liquid chromatography (HPLC)‐grade drug were used. BNZ was obtained from Hoffmann‐La Roche Ltd. (Buenos Aires, Argentina). Radanil® (BNZ 100 mg) was obtained from Roche (São Paulo, Brazil).
A Rolco 2036 centrifuge and a rotary evaporator Heidolph Laborota 4010 equipped with a ROTAVAP valve control equipment were used for the pretreatment procedures. A certified analytical balance 0.1 mg (Ohaus‐Pionner, USA) was used in weighing operations. All micropipettes were calibrated before use. All HPLC solvents were degassed with a vacuum pump (Pascal, Buenos Aires, Argentine). An ultrasonic homogenizer (FAETA, Argentina) and a Genesis G12 (VIRTIS®, New York, NY) freeze‐drier were also used.
Stock and Working Solutions
Stock control solution was prepared with 112.8 mg of BNZ in 5 ml of DMSO to complete dissolution and then accurately diluted to 25.00 ml in a calibrated volumetric flask, with acetonitrile to obtain a 4.512 mg/ml stock control solution. This stock control solution was stored at 4°C. Variable volumes of the stock control solution were diluted in the mobile phase to obtain the standard working solutions (SWS). Standard control curve was made in a duplicated mode at 0.10, 0.50, 1.00, 5.00, 20.00, and 45.00 μg/ml with these SWS.
Three SWS at 0.50, 1.00, and 5.00 μg/ml were stored for three months at 4°C. After this time were measured in plasma chromatographic conditions.
HPLC Instrumentation and Calculation
The instrumental analytic methods development was performed with an LC system consisting of an HPLC Merck‐Hitachi LC‐6200A and Merck‐Hitachi UV/Vis L‐4250 detector (Japan). Separations were carried out at room temperature using a C18 column 5 μm, 100 mm × 4.6 mm I.D. Lichrospher‐100 RP18 (Merck, USA). Samples were injected with a manual injector system with a 20 μl sample loop. Peak areas were integrated automatically by Merck‐Hitachi D‐2500 Chromato‐Integrator.
All the calculations concerning the quantitative analysis were performed with an external standardization by the measurement of peak areas of a sample specimen series. Limits of detection (LOD) were established at 3.3 times of intercept coefficient standard error/slope coefficient ratio. Limits of quantization (LOQ) were established at nine times of intercept coefficient standard error/slope coefficient ratio. Accuracy and precision of the assays were calculated based on the analysis of three replicates for each level of the standard curve. Total uncertainty was calculated as the sum of accuracy and precision. Sensitivity calculation was carried out as the product of total uncertainty with LOQ.
Plasma Samples
Preparation of control plasma samples
Drug‐free plasma (2.000 ml), as a control specimen, was spiked with the appropriate volume of stock control solution to attain standards with different BNZ concentrations. Plasma samples specimens were spiked at 0.10, 0.50, 1.00, 5.00, 10.00, 15.00, and 20.00 μg/ml (two minors concentrations duplicated), which were used to make calibration curve. These standards were freeze‐dried and stored at 4°C until analysis. The stability of BNZ in lyophilized plasma (1.00 μg/ml) was studied at six months after storage at −21°C, through an HPLC measure.
Patients’ plasma samples
Plasma samples were obtained from pediatric patients treated for Chagas infection with Radanil® (5–8 mg/kg/day) aged between 2 and 12. These samples came from patients in one of three groups: group I (patients at beginning of treatment), group II (patients in advanced in the treatment—i.e., at steady state), and group III (patients at the end of treatment). All plasma samples were freeze‐dried, transported, and stored at 4°C until analysis. Some random duplicated samples were stored for six months for stability studies.
Pretreatment of plasma control specimens and patients’ samples
Two sample volumes (4.000 ml) of ethyl acetate were added to the lyophilized plasma for drug extraction. The mixture was manually shaken and deproteinization was carried out with 200 μl (1/10 sample volume) of TCA (30% p/v), vortexed for one minute, and sonicated for five minutes. The mixture was then centrifuged at 8000 g for 10 min, the supernatant was placed into a round bottom glass flask and rotoevaporated to dryness at 40°C and 90–120 bar until the organic solvent was eliminated. The residue was resuspended in 600 μl of the chromatographic mobile phase and injected into the HPLC system.
Chromatographic conditions for plasma analysis
HPLC analysis was performed by isocratic elution with a flow rate of 1.0 ml/min. The mobile phase composition was glycine buffer/acetonitrile (75:25 v/v). The glycine buffer was an aqueous solution of glycine 0.20 M and sodium octanesulfonate 5 mM at pH 2.5. Hydrochloric acid 50% v/v for pH adjustment was used. All solvents were filtered through a 0.45 μm nylon membrane and degassed before use.
The maximum UV absorption of BNZ is at 313 nm in this chromatographic mobile phase, so this wavelength was chosen for the method. A value of 0.030 absorbance units (a.u.) threshold was used. Triplicate injections were made for each plasma control specimen to test reproducibility of the detector response at each concentration level. The peak area was plotted against the concentration to obtain the calibration graph. Regression analysis was used to calculate the calibration equation and correlation coefficients.
Urine Samples
Preparation of urine control specimen
Drug‐free human duplicated urine specimens of 2.500 ml were spiked with the appropriate volume of stock control solution to attain urine control specimens at 5.0, 10.0, 20.0, 30.0, and 45 μg/ml final BNZ concentrations in a duplicate series. These controls were mixed and stored at 4°C until pretreatment. Three random controls were stored for six months at ‒21°C for stability studies.
Patients’ urine samples
Twenty‐four hour urine samples were obtained from Chagas disease pediatric patients and from healthy adult volunteers who received Radanil® at 5–8 mg/kg/day dose. All urine samples were stored at ‒21°C until analysis.
Pretreatment of urine control specimens and samples
Human urine samples (2.500 ml) were deproteinizated with 250 μl of an aqueous solution of TCA (30% v/v). After shaking for one minute, 225 μl of an alkaline solution of NaOH (10% w/v) was added to adjust to pH 7.00 ± 0.02. A 1.250 ml volume of DCM (1/2 sample volume) and 200.0 mg of anhydrous sodium sulfate (near saturation) were added, shaking the mixture for two minutes. The organic phase of two consecutive liquid/liquid extraction procedures was recovered. Both fractions were rotoevaporated to dryness at 50°C and 40–80 bar in round bottom glass flasks. The residue was resuspended in 1.500 ml of the mobile phase and then injected into the chromatographic system.
Chromatographic conditions for urine analysis
HPLC analysis was performed by isocratic elution with a flow rate of 1.0 ml/min. The mobile phase composition was water/acetonitrile (80:20 v/v). All solvents were filtered through a 0.45 μm nylon membrane and degassed before use.
The maximum UV absorption of BNZ is at 313 nm in this chromatographic solvent, so this wavelength was chosen for the method. A value of 0.100 absorbance units threshold was used. Triplicate injections were made for each urine control specimen to test reproducibility of the detector response at each concentration level. The peak area was plotted against the concentration to obtain the calibration graph. This graph was subjected to regression analysis to calculate the calibration equation and correlation coefficients.
RESULTS
No significant loss was observed in the BNZ concentration in DMSO/acetonitrile solutions (0.50, 1.00, and 5.00 μg/ml) and in lyophilized plasma (1.00 μg/ml) after three and six months, respectively. In addition, analyte showed no decomposition products in the chromatogram.
After several trials glycine buffer/acetonitrile (75:25 v/v) as the mobile phase was found to achieve maximum separation and sensitivity for plasma analysis. A wavelength of 313 nm was selected for UV detection. Flow rates between 0.8 and 1.5 ml/min were tested. A flow rate of 1.0 ml/min gave an optimal signal‐to‐noise ratio with a reasonable separation time. The retention time for BNZ was 6.30 min with a 1.5% intraday CV and a 3.2% interday CV. Total run time was eight minutes per sample.
Table 1 presents correlation coefficient, F factor of ANOVA analysis, and values of the slope and intercept with relative standard deviation (RSD) for BNZ in plasma samples. Good linearity was obtained with r 2 = 0.99484. The LOD and LOQ were calculated to be 0.14 μg/ml and 0.32 μg/ml, respectively.
Table 1.
Correlation Coefficients of Human Plasma Curve
| R value | 0.9975 | |
| Adjusted‐R squared | 0.9948 | |
| Residual sum of squares | 7.04826 × 107 | |
| Observations | N = 27 | |
| ANOVA | FN ‒2 = 7903.91451 | Prob > FN ‒2 = 0 |
| Coefficients | Standard error | |
| Intercept (b) | 547.33247 | 221.49793 |
| Slope (m) | 6564.92771 | 50.24426 |
The linear range was verified with linearity of extended calibration curves and was based on the expected concentrations in probe samples. The linear ranges were determinate between LOD and 20.0 μg/ml. Recovery at 1.00 μg/ml level was 89%.
Table 3 summarizes accuracy and precision. Total uncertainty was below 10%. Sensitivity was 0.04 μg/ml, below to the LOD value. The residual‐lag‐plot, constructed by plotting residual (i) against residual (i‐1), shows a nonrandom pattern in a lag‐plot (Fig. 1A). This suggests that the variance is not random and the total CV is the same in all the concentration range.
Table 3.
Validation Parameters
| Human plasma | Human urine | |
|---|---|---|
| Accuracy | 6.3% | 6.1% |
| Precision | 3.4% | 2.8% |
| Sensitivity | 0.04 μg/ml | 0.5 μg/ml |
| LOD | 0.14 μg/ml | 1.7 μg/ml |
| LOQ | 0.32 μg/ml | 5.2 μg/ml |
| Linear range | 0.30–20.00 μg/ml | 5.0–45.0 μg/ml |
| Selectivity | without metabolite interferences | |
| Preanalysis handled time | 20 min | 15 min |
| Analytical instrumental time | 9 min | 7 min |
| Eco‐compatibility | without chlorated or cancerigen solvents; minimum organic solvents use | |
Figure 1.
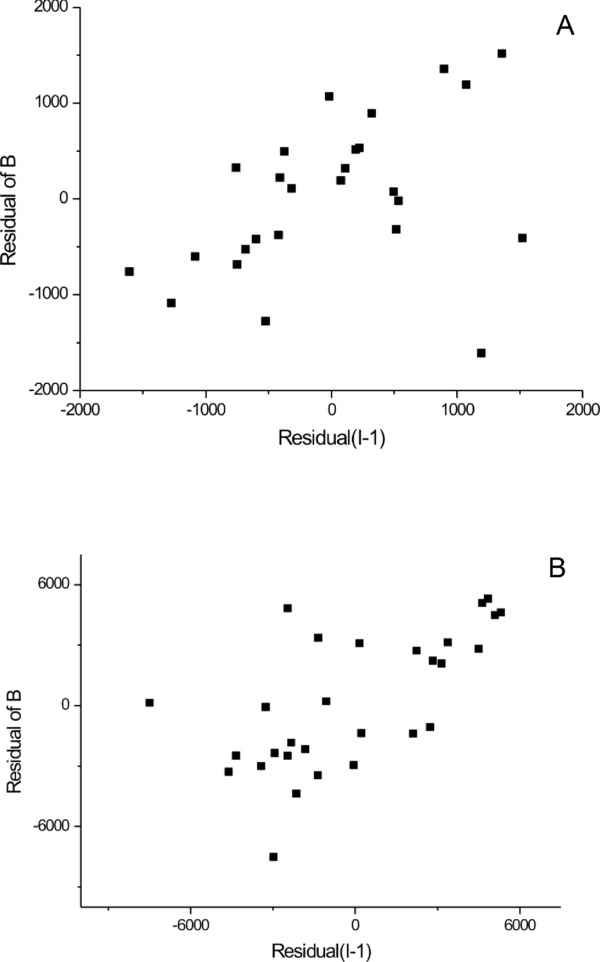
Residual‐lag‐plot on human plasma curve (A) and on human urine curve (B). Both showing that the error term is sufficiently independent.
Chromatographic parameters such as resolution, selectivity, and peak asymmetry were satisfactory for BNZ determination with this technique. Interestingly, a second peak was observed in some plasma samples belonging to patients who were in the advanced treatment (i.e., after taking BNZ for several days). This peak eluted one minute before BNZ (about 5.50 minutes; Fig. 2), and was not present in samples belonging to patients at the beginning or at the end of the medical treatment.
Figure 2.
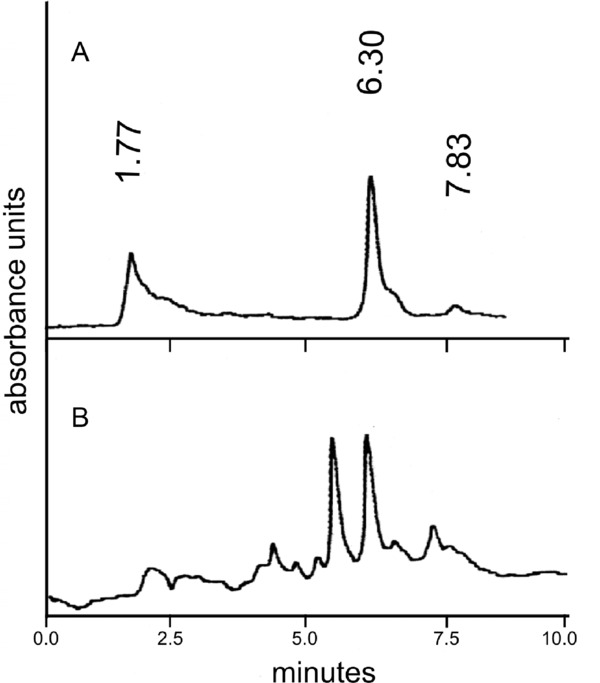
Representative chromatogram of plasma group I patients (A) and plasma group II patients (B).
In urine analysis, a mobile phase of water/acetonitrile (80:20 v/v) was chosen to achieve maximum separation and sensitivity. Same conditions to those used in plasma analysis were used (wavelength, flow rates, and technical specification of column). Retention times for BNZ were 5.30 min, with an 8.9% intraday CV. Total run time was seven minutes per sample. Chromatographic parameters such as resolution, selectivity, and peak asymmetry were satisfactory for determination of BNZ with this technique.
Table 2 presents correlation coefficient, F factor of ANOVA analysis, and values of the slope and intercept with RSD for BNZ in urine samples. Good linearity was obtained with r 2 = 0.98832. LOD and LOQ were 1.70 μg/ml and 5.20 μg/ml, respectively. Detection range was linear between LOD and 45.00 μg/ml. Recovery at 15.00 μg/ml level was 70%.
Table 2.
Correlation Coefficients of Human Urine Curve
| R value | 0.9965 | |
| Adjusted‐R squared | 0.9883 | |
| Residual sum of squares | 3.41682 × 108 | |
| Observations | N = 30 | |
| ANOVA | FN ‒2 = 2454.27904 | Prob > FN ‒2 = 0 |
| Coefficients | Standard error | |
| Intercept (b) | 2096.22642 | 1083.85506 |
| Slope (m) | 2100.7084 | 42.4037 |
Table 3 shows the accuracy and precision of the urine assay. Total uncertainty was below 10% and sensitivity was 0.5 μg/ml, below the LOD value. Residual‐lag‐plot analysis shows a nonrandom pattern (Fig. 1B) that suggests nonrandom variance in all the concentration range.
Plasma samples obtained from 21 patients and urine of 2 patients were tested to evaluate the performance of the chromatographic methods. Results are shown in Table 4.
Table 4.
Sample Results
| N | Range | Mean | SD | |
|---|---|---|---|---|
| Plasma samples | ||||
| Group I patients | 7 | 0.60–6.21 | 2.81 | 2.35 |
| Group II patients | 7 | <LOQ–7.47 | 4.49 | 2.81 |
| Group III patients | 7 | 0.82–4.94 | 3.17 | 1.68 |
| Urine samples | 2 | 9.7–25.2 | – | – |
DISCUSSION
Only two drugs are currently available for the treatment of Chagas disease, nifurtimox and BNZ, both with similar effectiveness 5, 6. Currently BNZ is the most commonly used drug in South America for the treatment of Chagas disease mainly due to availability issues 6. Unfortunately, little information is available on the pharmacokinetics of BNZ and the knowledge on its metabolism and the identity of the main metabolites is very scarce, and was not obtained in human beings. This situation is significantly worse in the case of children and it is also complicated by the fact that appropriate pediatric formulations are not available. Actually, administration of BNZ to children often requires tablet fractionation 7 and pediatric dosing is based on incomplete pharmacologic data corresponding to adults patients 5.
BNZ has been determined in biological matrices by differential pulse polarography 8, UV spectrometry 9, and HPLC 10, 11, 12, 13, 14, 15, 16 methods. A DNA‐electrochemical biosensor method was proposed for the application in biological matrices but was not validated 17. Only three of all these methods were applied to human samples in a clinical context 8, 10, 14.
HPLC is a widely used methodology for drug analysis and is the preferred method for BNZ quantization. However, there are no standardized HPLC methods proposed for routine use in human clinical studies, with only one method published so far for analysis of adult human plasma 14 and none for urine. In human samples, matrix specificity is very important due to potential metabolite interferences in the detection and quantization of the drug.
Although it can be considered as a tedious procedure in clinical chemistry, freeze‐drying of plasma samples provides advantages in storage, transportation, deproteinization, and drug extraction of samples. Moreover, it neither interfered with the detection of the drug, nor did it lead to a decrease in sensitivity. It must be considered that the chagasic patients are often far away from the analytical laboratories, even in rural areas. The freeze‐dried samples better preserved during the transport for long distances or storage for long times. Freeze‐dryer equipment can be found in some sanitary facilities. A simple liquid–liquid extraction of plasma samples could be applicable, but it would not present any advantage for transportation, storage, and drug extraction in comparison with the described method.
On the preanalytical procedure, the evaporation of the solvent was carried out under a stream of nitrogen, without significant analytical differences with the use of a rotoevaporator. Thus, due to the high performance it had, this technical procedure was carried out in our laboratory using a rotary evaporator.
In our measurements, all real human plasma samples values were within the linear range. The values ranged from 0.20 μg/ml to 15.80 μg/ml, similar to previously reported data 18. The values of validation parameters justify the use of calibration curve with external calibration.
There is an interest on the determination of clinically significant plasma BNZ concentrations. To our knowledge, there are two different scenarios: adult plasma concentrations and pediatric plasma concentrations. Based on in vitro data and on pharmacokinetic adult human studies, a therapeutic range between 3 and 6 μg/ml in adult plasma samples was originally proposed. However, lower values were observed in pediatric patients 19. The LOQ of the method here described, 0.32 μg/ml, seems to be appropriate in both contexts. In this work, most of the pediatric samples normally obtained were of 2 ml of plasma. It is important to note that minor sample volumes would result in higher LOQ values. Nevertheless, with this methodology, we could also have been working with lower amount of sample, being still suitable in pediatric context. Finally, it has been reported that higher BNZ plasma concentrations are associated with higher risk of toxicity 20, 21 but toxicity risks are low in pediatric populations 7. The higher linear limit of our plasma sample method is adequate for patient evaluation in both clinical contexts.
The use of wavelengths between 313 nm and 324 nm is described in the literature for detection of BNZ by HPLC‐UV in biological samples. In our experience, detection at 313 nm wavelength offered the best sensitivity without any significant noise increase. LOD and LOQ of our method are among the lowest reported in the literature and we postulate that this is possibly the technical limit for any HPLC analysis with UV detection.
The second chromatographic peak observed in many specimens of a subgroup of plasma samples, had lower retention times and similar UV absorption characteristics as BNZ peak. The analysis of BNZ in these samples using a direct spectrophotometric UV method without chromatographic resolution 9 could be notoriously interfered with this second substance. This peak also shows similar chromatographic features to the findings of a previous work using mice as model 11, in which this signal is assigned to the aminoderivative metabolite of BNZ. In this study, the metabolite was detected in plasma samples taken three hours after the administration of BNZ. Glioxal, another proposed BNZ metabolite 22, is not compatible with this feature. In this sense, our method bears similarities to the method described by Walton and coworkers, being plausible that this signal corresponds to a nitroreduced BNZ or similar metabolite with benzyl chromophore. If this would be the case, it suggests that our methodology could be used to detect and measure it. The results here obtained also indicate that it is unlikely that the peak assigned to BNZ has any interference from BNZ metabolites. At this time, there are no reports for plasmatic metabolites identity of BNZ in human beings.
In order to determine the clinical relevant concentrations range in urine samples, previous studies of urinary excretion of BNZ were analyzed. They showed that a minor portion of the parent drug is excreted in urine within four days after an oral dose 8, but these data have never been confirmed by other authors. Our 24‐hour urine samples showed 9.7 μg/ml and 25.2 μg/ml values, reflecting ∼3% and ∼7% of BNZ of the oral doses. For this matrix, the values of validation parameters justify the use of calibration curve with external calibration.
CONCLUSIONS
We developed HPLC methods for the quantization of BNZ in human plasma and urine. The methods described are specific, accurate, precise, and sufficiently sensitive for the quantization of BNZ in these matrices. Samples preparation involves simple steps and the methods are sufficiently fast. These methodologies showed to be suitable for the analysis of human urine and plasma samples collected during pediatric clinical studies.
Supporting this main aspect, the described plasma dosage technique is currently being used for the measurement of BNZ in clinical samples of pediatric patients treated for Chagas disease in a clinical trial (registry: clinicaltrials.gov # NCT00699387).
ACKNOWLEDGMENT
The authors acknowledge all the grant sponsors of this work. M.E.M. specially acknowledges “Salud Investiga” Program of National Health Authority for the provided scholarship.
Grant sponsor: Comisión de Investigaciones Científicas de la Provincia de Buenos Aires (CIC); Grant sponsor: Facultad de Ciencias Exactas (UNLP); Grant sponsor: Bunge & Born Foundation. Grant sponsor: DAAD (Deutscher Akademischer Austauschdienst, Germany) for the purchase of the rotary evaporator equipment.
REFERENCES
- 1. Barrett MP, Burchmore RJ, Stich A, et al. The trypanosomiases. Lancet 2003;362:1469–1480. [DOI] [PubMed] [Google Scholar]
- 2. Moncayo A, Silveira AC. Current epidemiological trends for Chagas disease in Latin America and future challenges in epidemiology, surveillance and health policy. Mem Inst Oswaldo Cruz 2009;104(suppl 1):17–30. [DOI] [PubMed] [Google Scholar]
- 3. Schmunis GA. Epidemiology of Chagas disease in non‐endemic countries: The role of international migration. Mem Inst Oswaldo Cruz 2007;102:75–85. [DOI] [PubMed] [Google Scholar]
- 4. Di Girolamo C, Bodini C, Marta BL, Ciannameo A, Cacciatore F. Chagas disease at the crossroad of international migration and public health policies: Why a national screening might not be enough. Euro Surveill 2011;16:1–5. [PubMed] [Google Scholar]
- 5. Garcia‐Bournissen F, Altcheh J, Della Védova CO, Giglio N, Mastrantonio G, Koren G. Pediatric clinical pharmacology studies in Chagas disease: Focus on Argentina. Paediatr Drugs 2009;11:33–37. [DOI] [PubMed] [Google Scholar]
- 6. Jannin J, Villa L. An overview of Chagas disease treatment. Mem Inst Oswaldo Cruz 2007;102(Suppl 1):95–97. [DOI] [PubMed] [Google Scholar]
- 7. Altcheh J, Moscatelli G, Moroni S, Garcia‐Bournissen F, Freilij H. Adverse events after the use of benznidazole in infants and children with Chagas disease. Pediatrics 2011;127:212–218. [DOI] [PubMed] [Google Scholar]
- 8. Raauflaub J, Ziegler WH. Single‐dose pharmacokinetics of the trypanomicide benznidazole in man. Arzneim Forsch Drug Res 1979;29:1611–1614. [PubMed] [Google Scholar]
- 9. Bulffer RF, Castro JA, Fanelli SL. UV methodology for determination of antichagasic drugs Nifurtimox and Benznidazole in blood. Acta Bioquím Clín Latinoam 2011;45:463–470. [Google Scholar]
- 10. Workman P, White RAS, Walton MI, Owen LN, Twentyman PR. Preclinical pharmacokinetics of benznidazole. Br J Cancer 1984;50:291–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Walton MI, Workman P. Reversed‐phase high performance liquid chromatographic method for the simultaneous determination of the 2‐nitroimidazole benznidazole and its amine metabolite in biological fluids. J Chrom 1986;375:190–196. [Google Scholar]
- 12. Morilla MJ, Benavidez P, Lopez MO, Bakas L, Romero EL. Development and in vitro characterization of a benznidazole liposomal formulation. Int J Pharm 2002;249:89–99. [DOI] [PubMed] [Google Scholar]
- 13. de Castro CR, Montalto de Mecca M, Fanelli SL, de Ferreyra EC, Díaz EG, Castro JA. Benznidazole‐induced ultrastructural and biochemical alterations in rat esophagus. Toxicology 2003;191:189–198. [DOI] [PubMed] [Google Scholar]
- 14. Guerrero L, Pinazo MJ, Posada E, Gascón J, Ribas J, Soy D. A high‐performance liquid chromatographic method for benznidazole quantitation in plasma of patients with Chagas disease. Clin Chem Lab Med 2011;49:77–82. [DOI] [PubMed] [Google Scholar]
- 15. Bulffer RF, Castro JA, Fanelli SL. Benznidazole levels in blood vary with age in rats. Mem Inst Oswaldo Cruz 2011;106:374–377. [DOI] [PubMed] [Google Scholar]
- 16. Moreira da Silva R, Teixeira Oliveira L, Silva Barcellos NM, de Souza J, de Lana M. Preclinical monitoring of drug association in experimental chemotherapy of Chagas’ disease by a new HPLC‐UV method. Antimicrob Agents Chemother 2012;56(6):3344–3348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. La‐Scalea MA, Serrano EI, Ferreira AM, Brett AM. Voltammetric behavior of benznidazole at a DNA‐electrochemical biosensor. J Pharm Biom Anal 2002;29:561–568. [DOI] [PubMed] [Google Scholar]
- 18. Raaflaub J. Multiple‐dose kinetics of the trypanosomicide benznidazole in man. Arzneim Forsch Drug Res 1980;30:2192–2194. [PubMed] [Google Scholar]
- 19. Altcheh J, Moscatelli G, Mastrantonio G, et al. Pediatric clinical pharmacology population pharmacokinetics study of benznidazole in children with Chagas disease. Basic Clin Pharmacol Tox 2010;107(Suppl. 1):162–692. [Google Scholar]
- 20. Castro JA, Montalto de Mecca M, Bartel LC. Toxic side effects of drugs used to treat Chagas´ disease (American trypanosomiasis). Hum Exp Tox 2006;25:471–479. [DOI] [PubMed] [Google Scholar]
- 21. Viotti R, Viggliano C, Lococo B. Side effects of benznidazole treatment in chronic Chagas disease: Fears and realities. Expert Rev Anti Infect Ther 2009;7:157–163. [DOI] [PubMed] [Google Scholar]
- 22. Hall BS, Wilkinson SR. Activation of benznidazole by Trypanosomal Type I Nitroreductases results in glyoxal formation. Antim Agents Chemother 2012;56:115–123. [DOI] [PMC free article] [PubMed] [Google Scholar]


