Abstract
Background
Biological variation (BV) data of analytes have been used to evaluate the significant changes in serial results (reference change value, RCV) of healthy individuals in clinical laboratories. However, BV data of healthy subjects may not be identical to the analytes of patients with ongoing clinical condition. The aim of this study was to calculate intra‐(CVw) (coefficient of variation for intra‐individual BV) and inter‐individual (CVg) BV, index of individuality, and RCV of nine serum analytes of renal posttransplant patients.
Methods
Six serum specimens were obtained in an interval of two months in a one‐year period from 70 transplant patients who had been stable for three years. Each time creatinine, uric acid, urea, sodium, potassium, calcium, inorganic phosphate, total protein, and albumin of these patients were analyzed with an integrated clinical chemistry/immunoassay auto‐analyzer. ANOVA tests were used to calculate the variations. Results were compared with the data of healthy subjects obtained from BV database.
Results
CVw of all nine analytes of the renal transplant patients were higher than the healthy subjects. RCVs of these analytes were calculated as 14.5% for creatinine, 16.5% for urea, 13.7% for urate, 12.57% for albumin, 8.26% for total protein, 3.25% for sodium, 12.81% for potassium, 5.88% for calcium, and 21.57% for inorganic phosphate.
Conclusion
RCV concept for predicting the clinical status in posttransplant population represents an optimization of laboratory reporting and could be a valuable tool for clinical decision.
Keywords: analytical variation, index of individuality, intra‐individual BV, reference change value, renal transplantation
INTRODUCTION
Physiological state of renal posttransplant patients are unstable that they should be monitored with well‐defined protocols, which are strictly followed by clinicians 1. For this purpose, clinicians use several approaches in the interpretation of routine laboratory tests of these patients. These include comparison with predetermined cut‐off values or reference intervals, or a comparison between two sequential results for a specific analyte 2. Comparison between two sequential results is not as straightforward as it seems. It should be remembered that each result has its own inherent random variation associated with laboratory activity (analytical imprecision, CVa) and biological variation (BV) 3. BV is composed of intra‐ and interindividual variations. In mathematical terms, these are usually expressed as coefficients of variation (CV) and termed as CVw for intra‐individual BV and CVg for interindividual BV 4. The method for estimating the components of BV, based on nested analysis of variance (ANOVA), has been fully described 4, 5.
BV data have several important clinical and laboratory applications that include: setting analytical quality specifications, assessing the utility of population‐based reference intervals, and determining the standards of performance required to facilitate optimal patient care 6, 7. In addition, using the BV data is the best way to detect changes of a patient's health status through a comparison between serial analytical results rather than comparison with population‐based reference intervals. This is because of the marked individuality of the majority of analytes. Hence, values obtained in consecutive analyses of samples from a patient may fall within the reference interval, but show a significant change. When the significant change exceeds a certain value, known as the reference change value (RCV), a change in the patient's condition is indicated 8, 9.
The usefulness of reference intervals has been addressed by the concept of biological individuality, also referred to as the “index of individuality” (II), which is a ratio of CVw to CVg. When the index is lower than 1, which is usual for the majority of analytes compiled up‐to‐date, two consecutive results from a subject may be outside the RCV but well stay within the population‐based reference interval. As a new concept, comparison of the result of a single test with the population‐based reference interval is a satisfactory practice only for analytes with the II higher than 1 10, 11.
The individual RCV for many analytes could be calculated using BV data from the healthy population 2. A comprehensive database constituted from BV data of nearly 320 analytes, which is updated every two years serves as a useful reference for many clinical laboratories 12, 13. However, this database, which is created from the healthy population data, may not be identical to that of patients with nonacute, ongoing clinical conditions such as renal posttransplant patients. Although BV data of ongoing pathological conditions have been the subject of various studies in recent years, the available information is still limited 14, 15, 16, 17, 18, 19, 20.
The aim of this study was to evaluate the objective analytical indicators in order to detect potential subclinical changes in renal posttransplant patients based on the BV data and RCV concept. For this purpose, CVw, CVg, II, and RCV of some of the routine analytes (creatinine, uric acid, urea, sodium, potassium, calcium, inorganic phosphate, total protein, albumin) expected to reflect instability/rejection were calculated by using serial results obtained from patients during the stable period of renal posttransplant patients. The underlying goal of this effort was to evaluate the usefulness of RCV model in daily practice in order to extract the best information from routine laboratory data and offer the clinician an improved tool for patient care.
MATERIALS AND METHODS
Patients
The study group consisted of 70 renal posttransplant patients (49 men and 21 women, 22–69 years old) who had been stable for a period of at least three years following transplantation. Stability in posttransplant patients was routinely verified through a combination of clinical, analytical, and imaging parameters: clinical normality as indicated by symptomatology, physiological constants, diuresis, weight, physical examination, etc.; analytical profile with particular attention directed to the stability of creatinine results (expected to differ <25% between two consecutive samplings); and renal arterial doppler ultrasonography.
Sample Collection
Serum specimens were collected according to the standard hospital follow‐up protocol designed by nephrologists for renal posttransplant patients. Peripheral blood samples were collected after informed consent. Results of six samples per patient after the third year on transplantation were used for the calculations, which were obtained during a period of one year in two months interval. Blood collections were performed in standardized conditions in order to minimize sources of preanalytical variation. Venipuncture was performed after an overnight fast, between 8 and 10 a.m. in the antecubital vein of the subjects. Specimens were collected into evacuated blood‐collection tubes without anticoagulant. The specimens were allowed to clot at room temperature and centrifuged at 3000 g for 10 min.
Measurement of Analytes
Specimens were analyzed at the time of collection, not stored for batched analysis. Nine serum analytes (creatinine, uric acid, urea, sodium, potassium, calcium, inorganic phosphate, total protein, albumin) were analyzed with Beckman Coulter kits (CA), which were manufactured to use on Beckman UniCel DXC 800 Synchron (Beckman Coulter Inc., CA) auto‐analyzer.
The analytical method used for each analyte included:
Creatinine: Jaffe method (rate‐blanked kinetic alkaline picrate) measured at 520 nm, at 37°C (ref: 472525).
Uric acid: Enzimatic reaction with uricase and peroxidase measured at 520 nm, at 37°C (ref: 442785).
Urea: Enzymatic method with urease and glutamate dehydrogenase measured at 340 nm, at 37°C (ref: 442820).
Sodium, potassium, calcium: Ion selective electrodes method (ref: A28937).
Inorganic phosphate: Measurement of phosphorus concentration by a timed rate method at 365 nm, at 37°C (ref: A09426).
Total protein: Biuret method measured at 545 nm, at 37°C (ref no: 465986).
Albumin: Bichromatic endpoint method using bromcresol purple reagent measured at 600 nm, at 37°C (ref no: 467858).
One analyst performed all the analyses, employing the same batches of reagent, quality control material, and calibrators. Control material was included in each analytical series to guarantee that analytical imprecision was within the acceptable limits according to our internal quality control protocol. In addition, external quality assurance program (Bio‐Rad Program, CA) was performed to all analytes during the period of the study.
Statistical Analyses
Microsoft Office Excel 2007 and ANOVA tests were used for the calculations. Analytical coefficient of variation (CVa) was calculated retrospectively through control materials, averaging the routine data for 12 months, and using the control concentration closest to the mean of the concentration values found in the 70 patients studied. Mean and standard deviation (SD) of the results of control materials for each analyte were calculated and the following formula, CVa = SD/mean, was used to calculate CVa. In clinical practice, it is widely accepted that CVa should be less than one‐half the average of the CVw [CVa < 0.50CVw], 4. However, this concept can be expanded, as there are still some tests, such as sodium, calcium, total protein, and albumin measurements for which the desirable performance standards are not attainable with the current technology and methodology. For this second group of tests, the CVa could be accepted as follows: CVa < 0.75CVw 4.
Before performing calculations with the patients’ results, the Cochran test was applied to exclude outlying values from the individual subjects, and the Reed test to eliminate mean outlying values.
For each patient mean, median, SD, and variation of the nine analytes were calculated. Figure 1 shows the median and distrubition range of the urate concentrations for each patient. The ANOVA test was used to estimate CVa plus CVw (CVa+w). CVw was calculated by a subtraction step with the two previous variables (CVa+w – CVa). Between‐subject BV (CVg) was obtained by subtracting the CVa+w from the total variation (CVt) found by using all data from all patients (Fig. 2). Additionally confident intervals (CI) of CVw and CVg of analytes were calculated as Roraas et al. suggested 21. Results were compared with the published data of healthy subjects 13.
Figure 1.
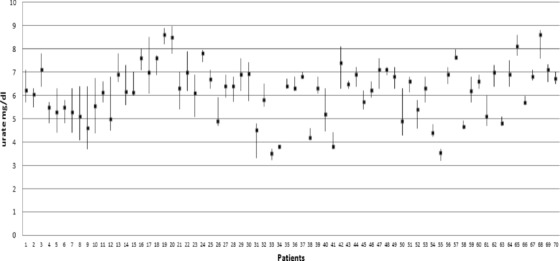
The median (square) and range of the urate concentrations of 70 posttransplant patients.
Figure 2.

Formulas used to calculate CVw, CVg, II, and RCV. CVw, within‐subject BV; CVa+w, total within‐subject BV; CVa, analytical variation; CVg, between‐subject BV; CVt, total variation; II, index of individuality; RCV, reference change value; Z, probability selected for statistical significance (Z = 1.04 at 85% confident interval).
The II was calculated by the ratio CVw/CVg 8 (Fig. 2). The RCVs for analytes at various probabilities, which were applied to a single quantity or the two quantities combined were calculated according to the formula, RCV = 21/2 × Z × (CVa2 + CVw2)1/2 (Fig. 2). Z‐score is the covering factor for a certain probability and could be chosen as 1.04 for 85% CI, 1.96 for 95% CI, and 2.58 for 99% CI. Smellie mentioned that 95% level of significance may not always be practical or desirable in different disease states. He suggested that clinicians required to use clinical knowledge to find a suitable balance between the false positives and false negatives 22. Additionally, Biosca et al. suggested that 85% CI showed the best combination of sensitivity, specificity, and positive and negative predictive values for the purpose of detecting potential rejections in renal posttransplantation. Therefore, in this study we selected 85% CI (Z = 1.04) for desired level of significance 23.
RESULTS
CVw and CVg for creatinine, urea, and urate were 9.2%, 12.9%, 9.2% and 17.2%, 19%, 18.4%, respectively. The II of all three analytes were <1 (0.53 for creatinine, 0.74 for urea, and 0.5 for urate). CVw and CVg for sodium, potassium, calcium, and inorganic phosphate were 1.91%, 8.65%, 3.81%, 14.51% and 1.2%, 7.92%, 6.34%, 17.04%, respectively. Although II of sodium and potassium were >1 (II of sodium = 1.61 and II of potassium = 1.1), II of calcium and inorganic phosphate were <1 (II of calcium = 0.6 and II of inorganic phosphate = 0.85). CVw and CVg of albumin and total protein were 8.4%, 5.03% and 11.5%, 8.03%, respectively. II was calculated as 0.63 for total protein and 0.73 for albumin (Table 1).
Table 1.
Analytical (CVa), Intra‐ (CVw) and Interindividual (CVg) Biological Variations, Index of Individuality (II), and Reference Change Values (RCV) of Nine Serum Analytes of Renal Posttransplant Patients
| CVa (%) | CVw (%) | CVg (%) | II | RCV (%) | |
|---|---|---|---|---|---|
| Urea | 4.5 | 12.9 | 17.3 | 0.74 | 20.09 |
| Creatinine | 3.46 | 9.2 | 17.2 | 0.53 | 14.46 |
| Urate | 1.51 | 9.2 | 18.4 | 0.5 | 13.71 |
| Albumin | 1.56 | 8.4 | 11.5 | 0.73 | 12.57 |
| Total protein | 2.5 | 5.03 | 8.03 | 0.63 | 8.26 |
| Sodium | 1.1 | 1.91 | 1.2 | 1.61 | 3.25 |
| Potassium | 1.04 | 8.65 | 7.92 | 1.1 | 12.81 |
| Calcium | 1.2 | 3.81 | 6.34 | 0.6 | 5.88 |
| Inorganic phosphate | 2.12 | 14.51 | 17.4 | 0.85 | 21.57 |
RCV was calculated as 14.5% for creatinine, 16.5% for urea, 13.7% for urate, 12.57% for albumin, and 8.26% for total protein (all at 85% confidence interval). Additionally, RCV for sodium, potassium, calcium, and inorganic phosphate were calculated as 3.25%, 12.81%, 5.88%, and 21.57%, respectively (Table 1).
Table 2 shows the BV components of posttransplant patients versus healthy subjects. The data of healthy subjects were obtained from BV database 13.
Table 2.
Biological Variation Components in Renal Posttransplant Patients Versus Healthy Subjects; Data of Healthy Subjects Were Taken From Biological Variation Database (13)
| Healthy | Transplanted | Healthy | Transplanted | |||||
|---|---|---|---|---|---|---|---|---|
| CVw | CVw | CVg | CVg | Healthy | Transplanted | |||
| Analyte | (%) | (%) | 95% Confidence intervals | (%) | (%) | 95% Confidence intervals | II | II |
| Urea | 12.3 | 12.9 | (10.6–15.2) | 18.3 | 17.3 | (6.65–31.35) | 0.67 | 0.74 |
| Creatinine | 5.3 | 9.2 | (8.02–10.38) | 14.2 | 17.2 | (7.22–27.18) | 0.37 | 0.53 |
| Urate | 9 | 9.2 | (8.06–10.34) | 17.6 | 18.4 | (7.03–29.77) | 0.51 | 0.5 |
| Albumin | 3.1 | 8.4 | (7.44–9.36) | 4.2 | 11.5 | (6.96–16.04) | 0.74 | 0.73 |
| Total protein | 2.7 | 5.03 | (4.67–5.39) | 4 | 8.03 | (5.83–10.23) | 0.68 | 0.63 |
| Sodium | 0.7 | 1.91 | (1.87–1.97) | 1 | 1.2 | (1.14–1.26) | 0.7 | 1.61 |
| Potassium | 4.8 | 8.65 | (7.64–9.66) | 5.6 | 7.92 | (5.66–10.18) | 0.86 | 1.1 |
| Calcium | 1.9 | 3.81 | (3.61–4.01) | 2.8 | 6.34 | (4.98–7.7) | 0.68 | 0.6 |
| Inorganic phosphate | 8.5 | 14.51 | (11.67–17.35) | 9.4 | 17.4 | (6.92–27.16) | 0.9 | 0.85 |
DISCUSSION
It has become more common to use RCVs of analytes estimated from healthy subjects to detect significant changes in the status of patients. However, the underlying pathology may modify the set‐point in diseased patients and, more importantly, the variation around that set‐point. If this is true, the use of RCVs from healthy subjects may not be the most appropriate strategy for this task. CVw estimated from individuals with a specific disease may be more suitable for calculation of RCV that could be of value in the interpretation of serial results in patients with that particular disease. The changes in serum concentrations of creatinine, uric acid, urea, sodium, potassium, calcium, inorganic phosphate, total protein, and albumin seen in this population can be secondary to various causes (e.g., dehydration, or the use of diuretics or other medications). However, this would more likely be related to problems with renal function. Thus, this study was designed to clarify the significance of variations in the results of routine biochemical analyses and to assess the predictive power of these analytes in various causes, which could occur simultaneously in renal posttransplant patients.
According to the results for each of the nine analytes, we observed that CVw of the renal transplant patients was higher than the healthy controls. Seven of these analytes including creatinine, uric acid, urea, calcium, inorganic phosphate, total protein, albumin, which had II of <1, are suitable for using RCVs and monitoring the early indicators of negative evolution in stable renal posttransplant patients. Variations less than the RCVs defined in this study (14.5% for creatinine, 16.5% for urea, 13.7% for urate, 12.57% for albumin, 8.26% for total protein, 5.88% for calcium, and 21.57% for inorganic phosphate) between serial determinations within a normal clinical context (no changes) usually do not prompt a modification of the posterior follow‐up in a patient with clinical and analytical assessment. Simultaneous changes in creatinine, urea, urate, albumin, total protein, calcium, and inorganic phosphate above these RCVs would justify immediate clinical evaluation of the cause of these changes.
BV data in pathological conditions have been the subject of various studies in recent years 14, 15, 16, 17, 18, 19, 20. Ricos et al. compiled the data of previous studies and generated a database for the BV in pathological conditions. This database contained information from 66 quantities estimated in 34 diseases, which were obtained from 45 papers published in 15 scientific journals. The subjects studied were from several countries and continents 24. As can be seen in this database, a preliminary work by Biosca et al. has been performed in renal posttransplant patients with the aim of early detection of rejection and other causes. They reported that CVw was higher in renal posttransplant patients than in the healthy population, being most evident in the creatinine, potassium, urate, and urea results and they found no differences in the CVg of the analytes except potassium 25, 26. In addition to six analytes studied in Biosca et al.'s study, in this study BV of albumin, total protein, calcium, and inorganic phosphate were also calculated. Although CVw of albumin, total protein, and calcium were calculated in some chronic diseases (e.g., chronic renal failure) and can be seen in this database, there do not exist CVw data of these analytes for renal posttransplant patients. Furthermore any CVw data for inorganic phosphate do not exist for any pathologic condition 24. To our knowledge, BV of albumin, total protein, calcium, and inorganic phosphate for renal posttransplant patients were calculated in this study for the first time.
RCVs of urate and creatinine in renal posttransplant patients were first calculated in 2001 by Biosca et al. (RCV were found 18.1% for creatinine and 19.8% for urate) 23. On the other hand the RCVs were calculated as 14.5% for creatinine and 13.7% for urate in our study. Differences between the two studies may be because of the selected renal posttransplant populations. In the study of Biosca et al. the patient group consisted of both clinically stable renal posttransplant patients and patients with acute rejection. Therefore in our study the patient group consisted of only long‐term clinically stable renal posttransplant patients. In another study of Biosca et al., it was mentioned that BV components for creatinine and urate were similar in short‐ and long‐term posttransplant patients and that independence maintained implies that the short‐term posttransplant RCVs of urate and creatinine can also be applied in long‐term posttransplant patients 14. In addition to RCVs of urate and creatinine calculated in renal posttransplant patients in Biosca et al.'s study, in our study RCVs of urea, albumin, total protein, calcium, and inorganic phosphate were also calculated.
Collaboration between the laboratory and clinician is becoming more necessary as the complexity of the diagnostic processes is growing. Using of RCV model could be valuable for follow‐up transplant patients and does not include extra cost for the laboratory or discomfort of the patient. It only requires a laboratory information managment system in which a reliable algorithm can be programmed to relate the diagnosis, patient's prior results, and the present values 27, 28. When the assigned percentages (RCVs) for analytes exceed simultaneously, a warning message can be created by the laboratory information system to inform the clinician that the clinical status of the patient may be undergoing toward an unfavorable change. Laboratories can use this model as a routine application for improving the interpretation of laboratory reports 28.
In conclusion, for laboratories to give reliable results all possible BVs must be considered and it is thought that calculated RCV values of different ongoing clinical conditions can be helpful for clinicians. In such situations, using disease‐specific RCVs may be more valuable than using reference intervals or RCV derived from healthy individuals to monitor the patient's status. The RCV for predicting clinical status in renal posttransplant patients represents an optimization of laboratory reporting and could be a valuable tool for clinical decision.
REFERENCES
- 1. Gwinner W. Renal transplant rejection markers. World J Urol 2007;25:445–455. [DOI] [PubMed] [Google Scholar]
- 2. Ricos C, Cava F, Garcia‐Lario JV, et al. The reference change value: A proposal to interpret laboratory reports in serial testing based on biological variation. Scand J Clin Lab Invest 2004;64:175–184. [DOI] [PubMed] [Google Scholar]
- 3. Omar F, van der Watt GF, Pillay TS. Reference change values: How useful are they? J Clin Pathol 2008;61:426–427. [DOI] [PubMed] [Google Scholar]
- 4. Fraser CG. Biological Variation: From Principles to Practice, Washington, DC: AACC Press; 2001. [Google Scholar]
- 5. Fraser CG, Harris EK. Generation and application of data on biological variation in clinical chemistry. Crit Rev Clin Lab Sci 1989;27:409–437. [DOI] [PubMed] [Google Scholar]
- 6. Fraser CG. Data on biological variation: Essential prerequisites for introducing new procedures? [Editorial]. Clin Chem 1994;40:1671–1673. [PubMed] [Google Scholar]
- 7. Fraser CG, Petersen PH. Analytical performance characteristics should be judged against objective quality specifications [Editorial]. Clin Chem 1999;45:321–323. [PubMed] [Google Scholar]
- 8. Fraser CG. Inherent biological variation and reference values. Clin Chem Lab Med 2004;42:758–764. [DOI] [PubMed] [Google Scholar]
- 9. Hyltoff Petersen P, Fraser CG, Sandberg S, Goldschmidt H. The index of individuality is often misinterpreted quantity characteristic. Clin Chem Lab Med 1999;37:655–661. [DOI] [PubMed] [Google Scholar]
- 10. Petersen PH, Fraser CG, Sandberg S, Goldschmidt H. The index of individuality is often a misinterpreted quantity characteristic. Clin Chem Lab Med 1999;37:655–661. [DOI] [PubMed] [Google Scholar]
- 11. Ricos C, Perich C, Minchinela J, et al. Application of biological variation: A review. Biochemia Medica 2009;19:250–259. [Google Scholar]
- 12. Ricos C, Alvarez V, Cava F, et al. Current databases on biological variation: Pros, cons and progress. Scand J Clin Lab Invest 1999;59:491–500. [DOI] [PubMed] [Google Scholar]
- 13. Biological Variation Database. 2010. http://westgard.com/biodatabase1.htm. Accessed 15 May 2012.
- 14. Biosca C, Ricos C, Lauzurica R, Petersen PH. Biological variation at long‐term renal post‐transplantation. Clinica Chimica Acta 2006;368:188–191. [DOI] [PubMed] [Google Scholar]
- 15. Trape J, Botargues JM, Porta F, et al. Reference change value for a‐fetoprotein and its application in early detection of hepatocellular carcinoma in patients with hepatic disease. Clin Chem 2003;49:1209–1211. [DOI] [PubMed] [Google Scholar]
- 16. Alvarez L, Ricos C, Peris P, et al. Components of biological variation of biochemical markers of bone turnover in Paget's bone disease. Bone 2000;26:571–576. [DOI] [PubMed] [Google Scholar]
- 17. Bruins S, Fokkema MR, Romer JW, et al. High intraindividuial variation of brain natriuretic peptide (BNP) and amino‐terminal proBNP in patients with stable chronic heart failure. Clin Chem 2004;50:2052–2058. [DOI] [PubMed] [Google Scholar]
- 18. Hernandez C, Francisco G, Chacon P, Mesa J, Simo R. Biological variation of Lipoprotein(a) in a diabetic population. Analysis of the causes and clinical implications. Clin Chem Lab Med 2003;41:1075–1080. [DOI] [PubMed] [Google Scholar]
- 19. Sambasivan AS, Lepage N, Filler G. Cystatin C intrapatient variability in children with chronic kidney disease is less than in serum creatinine. Clin Chem 2005;51:2215–2216. [DOI] [PubMed] [Google Scholar]
- 20. Trape J, Aliart M, Brunet M, Dern E, Abadal E, Queralto JM. Reference change value for HbA1c in patients with Type 2 Diabetes Mellitus. Clin Chem Lab Med 2000;38:1283–1287. [DOI] [PubMed] [Google Scholar]
- 21. Røraas T, Petersen PH, Sandberg S. Confidence intervals and power calculations for within‐person biological variation: Effect of analytical imprecision, number of replicates, number of samples, and number of individuals. Clin Chem 2012;58:1306–1313. [DOI] [PubMed] [Google Scholar]
- 22. Smellie WSA. What is a significant difference between sequential laboratory results? J Clin Pathol 2008;61:419–425. [DOI] [PubMed] [Google Scholar]
- 23. Biosca C, Ricós C, Lauzurica R, Galimany R, Hyltoft Petersen P. Reference change value concept combining two delta values to predict crises in renal posttransplantation. Clin Chem 2001;47:2146–2148. [PubMed] [Google Scholar]
- 24. Ricos C, Iglesias N, Garcia‐Lario JV, et al. Within‐subject biological variation in disease: Collated data and clinical consequences. Ann Clin Biochem 2007;44:343–352. [DOI] [PubMed] [Google Scholar]
- 25. Biosca C, Ricos C, Jiménez CV, Lauzurica R, Galimany R. Model for establishing biological variation in non‐healthy situations: Renal posttransplantation data. Clin Chem 1997;43:2206–2208. [PubMed] [Google Scholar]
- 26. Biosca C, Ricos C, Jiménez CV, Lauzurica R, Galimany R. Are equally spaced specimen collections necessary to assess biological variation? Evidence from renal transplant recipients. Clin Chim Acta 2000;30:79–85. [DOI] [PubMed] [Google Scholar]
- 27. Fraser CG. Reference change values. Clin Chem Lab Med 2011;50:807–812. [DOI] [PubMed] [Google Scholar]
- 28. Ozturk OG. Using biological variation data for reference change values in clinical laboratories. Biochem Anal Biochem 2012;1:e106 DOI: 10.4172/2161-1009.1000e106 [DOI] [Google Scholar]


