Abstract
Background
We first describe a patient who developed urosepsis from an ordinary urinary tract infection. In this case, the new hematological parameters of immature leukocytes, that is, the high‐fluorescence lymphocyte cell (HFLC) and immature granulocyte (IG) counts peaked early, whereas the established infection parameters, that is, C‐reactive protein (CRP) and total white blood cell count showed less dynamic regarding infection and therapy.
Methods
To investigate this phenomenon in greater detail, the novel parameters HFLC and IG counts are investigated retrospectively in a cohort of 38 patients who were admitted to the anesthesia intensive care unit. Three groups of patients have been analyzed and compared: patients without signs of infection, patients with limited infections, and patients with sepsis. Data were collected with a Sysmex XE‐5000 hematological analyzer.
Results
In patients (n = 22) without any signs of infection, both values are very low. In patients with limited local infections (n = 10), moderate elevations of the IG and HFLC counts are seen. In patients with sepsis (n = 6), the IG and HFLC counts are significantly higher.
Conclusion
The total IG count seems to be useful for distinguishing a septic patient from a nonseptic (P < 0.004). Hematological parameters have the advantage of being measured easily during routine blood cell analysis.
Keywords: anesthesiology, hematology, flow cytometry
INTRODUCTION
Sepsis is a common and severe disease in intensive care units (ICUs), which is difficult to monitor and treat 1. The identification of immature granulocytes (IGs) and high‐fluorescence lymphocyte cell (HFLC) with modern hematological analyzers in cases of sepsis offers a new possibility of monitoring sepsis. An increase in the IG count in this setting has also been reported by Ansari et al. 2. Tests to measure parameters similar to the IGs are also offered by other manufacturers 3. This topic is of special importance given by the fact that sepsis is a commonly critical and potentially curable disease in ICUs due to which many patients continue to die 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15.
An immature leukocyte count obtained by using a modern hematological analyzer could provide a useful sepsis marker. The principles of measuring immature leukocytes are described in the literature 16, 17, 18, 19, 20, 21, 22, 23, 24. However, these parameters have not been measured during sepsis until now except for a study conducted on newborns by Nigro et al. 18. State of the art is still the measurement of C‐reactive protein (CRP) and procalcitonine (PCT) for monitoring patients with severe infections and sepsis 25, 26.
METHODS
First, a case description of a patient with sepsis is given and then a cohort of total 38 patients has been analyzed retrospectively. Three different groups of patients have been analyzed: patients without signs of infection, patients with local infections, and patients with sepsis. Sample collection was done in the anesthesia ICU (ANE‐ICU) under routine conditions in the morning 7–8 a.m. at the first day after transfer of the patient to the ICU. Measurements were done on a Sysmex XE 5000 (Sysmex, Kobe, Japan) hematological analyzer in the laboratory. The evaluation was done retrospectively since the parameters investigated here are not routine up until now. The study was approved by the local ethics committee.
STUDY POPULATION
Case Description
The patient in the first case presented with the clinical picture of sepsis was admitted to the ANE‐ICU. This patient developed a urosepsis secondary to an ordinary urinary tract infection. For the different parameters of this patient, please see Figure 1. The patient came to the hospital for standard surgery (tumor operation of a colon carcinoma) that was performed without any complications.
Figure 1.
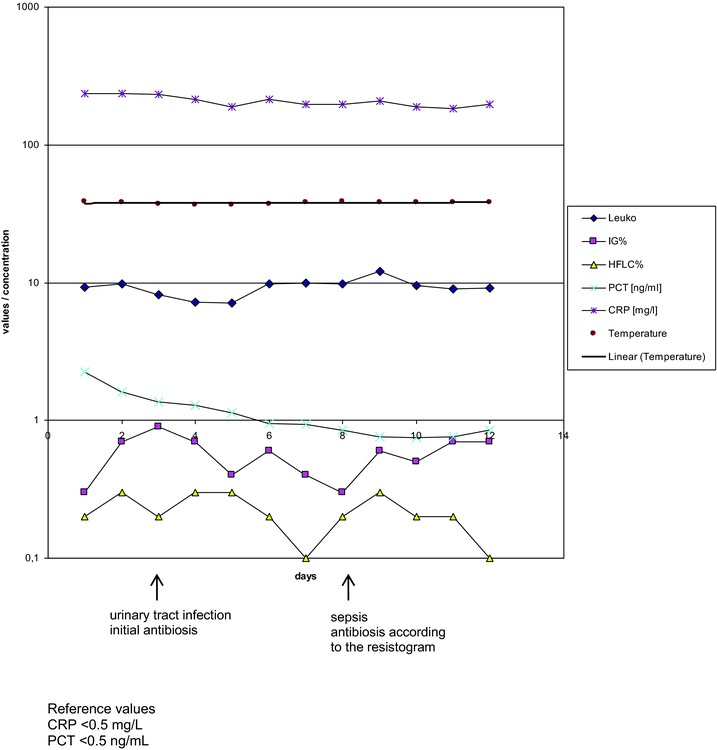
Case study of a patient who developed urosepsis from an ordinary urinary tract infection. Although the conventional parameters CRP (∼200 mg/l), leukocytes (∼10 109/l), and PCT (∼1 ng/l) did not show any change in response to the onset of sepsis, the new parameters derived from blood cell counts, percentage of HFLC and IG, were significantly increased from day 7 onwards and dynamically reacted in response to antibiotic therapy. CRP in milligram per liter reference range <0.5mg/l; PCT in nanogram per milliliter reference range <0.5 ng/l; leukocytes in 109/l, reference range 3.8–9.8·109/l.
Based on microbiological evidence of sepsis, it was concluded that the patient was infected with several bacterial pathogens. These pathogens were later identified in the microbiological laboratory as Pseudomonas aeruginosa and extended spectrum beta‐lactamase (ESBL) positive Escherichia coli. As part of the immune response, the patient also started to develop a proliferation of neutrophil granulocytes in the sense of a left shift, including immature and adult leukocytes. These increases in immature blood cell populations were compared to the classical inflammatory serum parameters, CRP, and PCT, as well as to the total white blood cell (WBC) count. All cell counts are measured on a Sysmex XE 5000 hematological analyzer.
Study Collective
To determine the clinical relevance of the new extended blood cell count parameters in greater detail, HFLCs and IGs were measured in the whole blood of 38 patients from the ANE‐ICU following surgical interventions, together with routine laboratory measurements. The results were collected and statistically analyzed retrospectively. Systemic inflammatory host response (SIRS) was defined according to the ACCP/SCCM consensus criteria 27 with the presence of two or more of the following symptoms:
Fever, with a body temperature of <38°C, or hypothermia, with a temperature <36°C.
Tachycardia, with a heart rate of <90 beats/min.
Tachypnea, with a respiratory frequency of <20, or hyperventilation (pCO2 < 4.3 kPa). and/or
Leukocytosis (>12 Gpt/l) or leukopenia (<4 Gpt/l) or <10% bands or left shift.
SIRS is a precondition for the diagnosis of sepsis. Sepsis was defined as the presence of SIRS (condition 1) and an additional proven microbiological etiology (condition 2). Blood samples for the analysis of complete blood cell count were obtained by venipuncture within the first 24 hr of ICU admission. The blood samples were drawn into ethylene diamine tetra acetic acid tubes (Sarstedt, Nümbrecht, Germany) and were transported to the chemical and hematological laboratory department. All analyses were performed within 45–60 min after blood sampling.
Statistical Methods
Statistical calculations were performed with SPSS Statistics software (IBM, 2011) and with R‐project software. An unpaired Student's t‐test was used to test for statistical differences.
RESULTS
Including the patient with urosepsis, the changes within the cellular parameters in detail, the HFLC and IG counts were present before any changes in the cytokine levels were measurable. Therefore, it is possible that cellular proliferation occurs more quickly than any serum changes. Figure 1 gives the different inflammatory values as CRP and PCT and the different cell counts in comparison for the patient with urosepsis.
Figure 2 provides an overview of the results from this study. In patients without any signs of an additional infection (n = 22), the HFLC and IG counts were very low. The reference range for both the HFLC and IG counts is <0.01 Gpt/l (<0.2%). For patients with local limited infections (n = 10), such as urinary tract infections and infected wounds, these values increased slightly. In contrast, IG values (absolute number and %) were greatly increased in all of the patients with sepsis (n = 6), two of whom had HFLC values that were also clearly increased. The changes in IG values between the patients without signs of an infection and the patients with sepsis were statistically significant (P < 0.01). These results suggest that the parameters of the newly extended blood cell count could become the basis of novel approaches for diagnosing and treating sepsis. The changes became more extensive with the degree of disease. Patients with sepsis demonstrated higher values than patients with moderate local infections who, in turn, had higher values than did those patients without any signs of infection. Table 1 gives an overview of the three different patient groups investigated here: patients without signs of infection, patients with local infections, and patients with sepsis.
Figure 2.
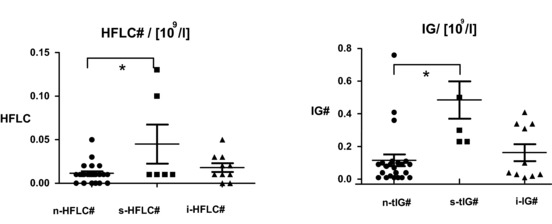
This figure gives the key findings and demonstrates it in absolute cell counts. Differences in absolute amounts of HFLCs and absolute amounts of IGs among patients with no infections (n, N = 22), patients with local infections (i, N = 10), and patients with sepsis (s, N = 6). All of the patients with sepsis had elevated IG‐cell counts, and two septic patients had increased HFLC counts. *Significant difference based on the unpaired t‐test (P < 0.01).
Table 1.
Clinical Data to the Patients
| Group 1 | Group 2 | Group 3 | |
|---|---|---|---|
| Number | 10 | 22 | 6 |
| Age [years] | 56 | 58 | 63 |
| Gender | |||
| Male | 6 | 15 | 3 |
| Female | 4 | 7 | 3 |
| Previous diseases with relation to the actual hospitalization | Yes, patients are postoperation | Yes, patients are postoperation and/or with traumata | Yes, patients are postoperation, and/or with traumata, and/or liver cirrhoses, and/or other organ failures, and/or multiorgan failures |
| Clinical status | Stable | Stable | Critical |
| Immature lymphocytesa | n‐HFLC | i‐HFLC | s‐HFLC |
| Immature granulocytesa | n‐IG | i‐IG | s‐IG |
Group 1: Patients without signs of infections.
Group 2: Patients with local infections.
Group 3: Patients with sepsis.
n‐HFLC, i‐HFLC, s‐HFLC, n‐IG, i‐IG, s‐IG, labeling according to Figure 2.
DISCUSSION
Discussion of the Case Description: A Patient with Urosepsis
In this patient, the new parameters derived from the extended total blood cell count, that is, the immature cells (IGs and HFLCs), responded better to the therapy, in contrast to the established serum markers. The IG count was high over the entire clinical course (0.5–1 Gpt/l); however, this parameter was responsive, as was the HFLC count. The HFLC count reacted quickly once antibiotic therapy was initiated. In the example case, the routine determination of immature cells in the whole blood of the patient would have had significant advantages compared to the measurement of the established serum markers CRP and PCT. Because the number of immature cells (IGs, HFLCs) increased very rapidly in whole blood, the diagnosis of the reonset of the urinary tract infection and urosepsis would have been possible much sooner using these novel parameters. Furthermore, it would have been possible to treat this septic patient much earlier with adequate antibiotics. In this specific case, the new parameters were beneficial, although the immature cell count differed significantly from the established inflammatory markers CRP and PCT. Of note, the novel parameters were slower to normalize following the initiation of antibiotic therapy, in contrast to the rapid decrease in the serum markers of CRP and PCT. Even after the patient was discharged from the ICU to the regular floor, the immature cell count levels still had not decreased, whereas the inflammatory marker PCT had already normalized. The present case revealed that the immature cells demonstrated a faster increase in number compared to the older, more established cells; however, the immature cells seemed to normalize much more slowly.
Discussion of the Group Analysis
In the present study, we determined that the increase in the number of IGs, as measured by an XE 5000 hematological analyzer, which reflects the number of circulating granulocyte precursors, was correlated with the onset of severe infection and sepsis. Main observation of the present study is the finding of significantly higher levels of IG and HFLC in septic patients. Both IGs and HFLCs are elevated in patients with sepsis compared to patients in the ICU without any signs of infection (reference ranges for IG and HFLC counts: 0–0.01 Gpt/l). However, IGs seem to be the more effective marker because their receiver operating curve has a larger area under it.
This finding seems logical because first these precursor cells must form and then mature into adult cells, which in turn can produce inflammatory markers. Once the cells are formed, they will remain in circulation and will be slower to disappear than cytokines. In contrast, the cytokine concentrations can decrease again much more quickly and return to normal serum values.
The increase in the number of IGs due to sepsis was more significant than the increase in the number of HFLCs. This result might have occurred because most severe sepsis cases are caused by bacteria and granulocytes and therefore also IGs are more sensitive to bacteria, whereas HFLCs should be more specific to viruses. According to the ACCP/SCCM guidelines 27, pathogen identification is a necessary step in the diagnosis of sepsis, and the identification of bacteria is easier and the tests are more commonly used than the identification of viruses and can be performed before cell counts can normalize.
In summary, the determination of immature cell count numbers has the potential of identifying infections more quickly than conventional methods. The advantage of counting immature cells versus mature cells is the possibility of differentiating diseases that result from defects in regeneration, such as bone marrow defects, from those that are caused by increased cellular consumption, such as autoimmune diseases (e.g., autoimmune hemolysis, autoimmune thrombopenia). Particularly in severe infections and sepsis, a fast and reliable method of detecting exacerbation and spreading of infection is necessary. According to the present study, by counting immature cells, it is possible to detect an infection earlier than by examining mature cell populations or by analyzing serum markers.
LIMITATIONS OF THE STUDY
The present study has several limitations. These limitations of the current study are given by the fact that the study is retrospective. Then only a limited number of patients have been included in the present study. Furthermore, the patients have been recruited retrospectively by diagnosis.
CONCLUSION
In this retrospective study, elevated IG and HFLC levels have been detected within septic patients. Thus, the hypothesis that these parameters might be useful in future clinical cases of sepsis patients can be generated. This hypothesis has to be proved in larger future studies.
The newly extended blood cell count parameters described here might be useful for monitoring patients with sepsis and for monitoring the effectiveness of their treatment. This has to be investigated in future studies in more detail.
Supporting information
Disclaimer: Supplementary materials have been peer‐reviewed but not copyedited.
supplementary material
REFERENCES
- 1. Alberti C, Brun‐Buisson C, Goodman SV, et al. European Sepsis Group influence of systemic inflammatory response syndrome and sepsis on outcome of critically ill infected patients. Am J Respir Crit Care Med 2003;168(1):77–84. [DOI] [PubMed] [Google Scholar]
- 2. Ansari‐Lari M, Kickler T, Borowitz M. Immature granulocyte measurement using the Sysmex XE‐2100. Relationship to infection and sepsis. Am J Clin Pathol 2003;120(5):795–799. [DOI] [PubMed] [Google Scholar]
- 3. Nahm C, Choi J, Lee J. Delta neutrophil index in automated immature granulocyte counts for assessing disease severity of patients with sepsis. Ann Clin Lab Sci 2008;38(3):241–246. [PubMed] [Google Scholar]
- 4. Schottmüller H, Bingold K. Die Septische Erkrankung Handbuch der Inneren Medizin. 22. Auflage (Bd. Part 1, 2). Springer Verlag; 1937. p 776–778. [Google Scholar]
- 5. Schottmüller H. Über Wesen und Therapie der Sepsis. D. Kongress f. Inner. Medizin, Wiesbaden, Voss; 1914. p 1–10.
- 6. Schottmüller H. Das Problem der Sepsis Festschrift des Eppendorfer Krankenhauses zur Feier seines 25jährigen Bestehens, Leipzig‐ Hamburg Leipzig u. Hamburg, Voss; 1914. p 914–149. [Google Scholar]
- 7. Bone R, Fisher C, Clemmer T, Slotman G, Metz C, Balk R. Sepsis syndrome: A valid clinical entity. Methylprednisolone Severe Sepsis Study Group. Crit Care Med 1989;17(5):389–393. [PubMed] [Google Scholar]
- 8. American College of Chest Physicians/Society of Critical Care Medicine . Consensus Conference: Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. Crit Care Med 1992;20(6):864–874. [PubMed] [Google Scholar]
- 9. Bone R, Sibbald W, Sprung C. The ACCP‐SCCM consensus conference on sepsis and organ failure. Chest 1992;101(6):1481–1483. [DOI] [PubMed] [Google Scholar]
- 10. Levy M, Fink M, Marshall J, et al. International Sepsis Definitions Conference, 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Intensive Care Med 2003;29(4):530–538. [DOI] [PubMed] [Google Scholar]
- 11. Martin G, Mannino D, Eaton S, Moss M. The epidemiology of sepsis in the United States from 1979 through 2000. N Engl J Med 2003;348:1546–1554. [DOI] [PubMed] [Google Scholar]
- 12. Alberti C, Brun‐Buisson C, Burchardi H, et al. Epidemiology of sepsis and infection in ICU patients from an international multicentre cohort study. Intensive Care Med 2002;28:108–121. [DOI] [PubMed] [Google Scholar]
- 13. Brun‐Buisson C, Doyon F, Carlet J. Bacteremia and severe sepsis in adults: A multicenter prospective survey in ICUs and wards of 24 hospitals. French Bacteremia‐Sepsis Study Group. Am J Respir Crit Care Med 1996;154:617–624. [DOI] [PubMed] [Google Scholar]
- 14. Brun‐Buisson C, Doyon F, Carlet J, et al. Incidence, risk factors, and outcome of severe sepsis and septic shock in adults. A multicenter prospective study in intensive care units. French ICU Group for Severe Sepsis. JAMA 1995;274:968–974. [PubMed] [Google Scholar]
- 15. Martin G, Mannino D, Moss M. The effect of age on the development and outcome of adult sepsis. Crit Care Med 2006;34(1):15–21. [DOI] [PubMed] [Google Scholar]
- 16. Nahm C, Choi J, Lee J. Delta neutrophil index in automated immature granulocyte counts for assessing disease severity of patients with sepsis. Ann Clin Lab Sci 2008;38:241–246. [PubMed] [Google Scholar]
- 17. Shiga S, Fujimoto H, Mori Y, et al. Immature granulocyte count after liver transplantation. Clin Chem Lab Med 2002;40:775–780. [DOI] [PubMed] [Google Scholar]
- 18. Nigro K, O'Riordan M, Molloy E, Walsh M, Sandhaus L. Performance of an automated immature granulocyte count as a predictor of neonatal sepsis. Am J Clin Pathol 2005;123:618–624. [DOI] [PubMed] [Google Scholar]
- 19. Davis H, Bigelow N. Comparison of neutrophil CD64 expression, manual myeloid immaturity counts, and automated hematology analyzer flags as indicators of infection or sepsis. Lab Hematol 2005;11(2):137–147. [PubMed] [Google Scholar]
- 20. Field D, Taube E, Heumann S. Performance evaluation of the immature granulocyte parameter on the Sysmex XE‐2100 automated hematology analyzer. Lab Hematol 2006;12(1):11–14. [PubMed] [Google Scholar]
- 21. Fernandes B, Hamaguchi Y. Automated enumeration of immature granulocytes. Am J Clin Pathol 2007;128(3):454–463. [DOI] [PubMed] [Google Scholar]
- 22. Briggs C, Harrison P, Grant D, Staves J, Machin S. New quantitative parameters on a recently introduced automated blood cell counter—The XE 2100. Clin Lab Haematol 2000;22(6):345–350. [DOI] [PubMed] [Google Scholar]
- 23. Groner W, Kanter R. Optical technology in blood cell counting. Sysmex J. Int. 1999;22:29–42. [Google Scholar]
- 24. Sakata T. Regent Characteristics in the XE‐2100 NRBC channel. Sysmex J. Int. 2000;10:41–46. [Google Scholar]
- 25. Yentis S, Soni N, Sheldon J. C‐reactive protein as an indicator of resolution of sepsis in the intensive care unit. Intensive Care Med 1995;21:602–605. [DOI] [PubMed] [Google Scholar]
- 26. Wacker C, Prkno A, Brunkhorst F, Schlattmann P. Procalcitonin as a diagnostic marker for sepsis: A systematic review and meta‐analysis. Lancet Infect Dis 2013;1:1–10. [DOI] [PubMed] [Google Scholar]
- 27. ACCP/SCCM Consensus Conference Committee . Definition for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. Crit Care Med 1992;20:864–874. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Disclaimer: Supplementary materials have been peer‐reviewed but not copyedited.
supplementary material


