Abstract
Background
Noninvasive laboratory tests have been widely used in the diagnosis of liver fibrosis. This study was designed to evaluate the diagnostic value of the four serum markers detected on a JETLIA‐962 chemiluminescence analyzer and the FibroTest index for chronic hepatitis B (CHB) related liver fibrosis. J. Clin. Lab. Anal. 27:5–11, 2013. © 2012 Wiley Periodicals, Inc.
Methods
A JETLIA‐962 chemiluminescence analyzer was used to measure the hyaluronic acid (HA), laminin (LN), procollagen III N‐terminal peptide (PIIINP), and type IV collagen (CIV) contents in the sera of CHB patients and controls.
Results
In our research, it was found that serum HA, LN, PIIINP, and CIV concentrations and the FibroTest index were correlated with fibrosis stage. The FibroTest index had the largest area under the receiver operating characteristic (ROC) curve for fibrosis and cirrhosis (0.80 and 0.776, respectively). The five indices together were able to exclude fibrosis with a negative likelihood ratio <0.1. The logistic regression equations for diagnosing liver fibrosis and early cirrhosis were separately established.
Conclusions
We found that the JETLIA‐962 chemiluminescence analyzer was of great value in diagnosis of liver fibrosis. Combining five indices improved the diagnostic efficiency and reduced the incidences of unnecessary liver biopsies. Two logistic regression equations established may be helpful in diagnosing liver fibrosis and early cirrhosis, which need to be further evaluated.
Keywords: chemiluminescence, chronic hepatitis B, diagnosis, liver fibrosis, receiver operating characteristic curve
INTRODUCTION
China has a high infection rate of hepatitis B virus, and correspondingly, chronic hepatitis B (CHB) induced liver fibrosis is a serious problem 1. Studies have clearly shown that liver fibrosis, especially mild fibrosis, is a reversible process, whereas cirrhosis is not reversible. Therefore, new diagnostic methods of CHB‐induced liver fibrosis are needed to accurately determine the stage of fibrosis, which is very important for initiating early treatment interventions.
The gold standard for the diagnosis of liver fibrosis has been the pathological examination of a liver biopsy 2, which has played an important role in the diagnosis, determination of the degree of inflammation and fibrosis, prediction of drug efficacy, and prognosis. Liver biopsies also have some shortcomings, however, such as the invasive nature of the examination and potential complications. In addition, a liver biopsy does not allow for persistent observation. Therefore, researchers and clinicians have been searching for a simple, noninvasive diagnostic method that can dynamically monitor liver fibrosis.
Radioimmunoassay (RIA) and enzyme‐linked immunosorbent assay (ELISA) are often used for the detection of fibrosis‐associated serum biochemical indices, including direct and indirect indices. Direct indices include hyaluronic acid (HA), the N‐terminal peptide of type III collagen (PIIINP), type IV collagen (CIV), laminin (LN), matrix metalloproteinases, and tissue inhibitors of metalloproteinases . Indirect indices include platelet (PLT), alanine transaminase, α2‐macroglobulin (α2M), glutamyltransferase, haptoglobin (HP), total bilirubin, and apolipoprotein A1. The ELISA method is complicated, and several factors can affect the detection process. In addition, the reproducibility of the assay is poor. The RIA method also has limitations and is affected by numerous factors, such as marker stability, the measurement range, radioactive contamination, and a long detection cycle. Many researchers have tried to establish mathematical models to represent the severity of liver fibrosis by combining multiple indices, including the FibroTest index 3, Forns’ index 4, the aspartate aminotransferase‐to‐platelet ratio index 5, FIBROSpect II 6, and the FibroMeter 7; however, these models have primarily been established by foreign scholars based on the information of patients with chronic hepatitis C virus infection or alcoholic liver disease. The diagnostic value of these models for CHB patients with liver fibrosis has not been effectively verified.
In recent years, China has successfully developed the JETLIA‐962 chemiluminescence analyzer (Beijing Yuande Biological Engineering Company, Beijing, China), for the measurement of serum markers such as HA. This analyzer has several advantages over current methods, such as a high level of sensitivity, simple operation and automation, a short test cycle, good reproducibility of the results, and a wide linear range. Although the JETLIA‐962 chemiluminescence analyzer appears to have potential, its diagnostic value and performance have not been evaluated.
The present study evaluated the diagnostic value of the JETLIA‐962 chemiluminescence analyzer for the detection of HA, PIIINP, CIV, and LN for CHB‐related liver fibrosis. We investigated the use of single serum markers and a combination of the serum markers and the FibroTest index on the diagnostic value of CHB‐related liver fibrosis. In addition, we established a mathematical diagnostic model of Hepatitis B liver fibrosis and early cirrhosis based on Chinese CHB patient data.
MATERIALS AND METHODS
Patient Group
Data were collected from 108 hospitalized CHB patients at The First Affiliated Hospital of Fujian Medical University from December 2009 to November 2010. There were 88 male patients and 20 female patients. The ages of the patients ranged from 17 to 63 years, and the mean age was 36.44 ± 10.68 years. Twenty‐five of the patients had liver steatosis. All patients underwent a liver biopsy pathological examination and met the established CHB diagnostic criteria 1. The liver tissue biopsy pathological diagnostic criteria were based on the “The programme of prevention and cure for viral hepatitis” 8, and the results of the specific grade and stage of each biopsy are shown in Table 1. A stage ≥ S1 was defined as liver fibrosis, and S4 was defined as cirrhosis. A grade ≥ G1 was defined as inflammation.
Table 1.
Comparison of the Serum Fibrosis Direct Indices and the FibroTest Index in the Patient and Control Groups
| Patient group | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Inflammation grade (n = 108) | Fibrosis stage (n = 108) | Control group | |||||||||
| Index | n = 108 Mean | G1 (n=16) | G2 (n=21) | G3 (n=51) | G4 (n=20) | S0 (n=12) | S1 (n=18) | S2 (n=21) | S3 (n=20) | S4 (n=37) | n = 53 Mean |
| HA (ng/ml) | 63.95a | 40.55 | 35.77 | 76.63 | 104.68c | 56.49 | 47.30 | 47.99 | 66.31 | 88.78 | 34.72b |
| CIV (ng/ml) | 67.32a | 40.66 | 35.49 | 74.25 | 144.35c | 49.27 | 28.46 | 53.32 | 86.86 | 112.74 | 23.31b |
| LN (ng/ml) | 78.39a | 32.03 | 22.72 | 120.38 | 174.20c | 30.89 | 21.22 | 75.86 | 130.27 | 154.84 | 30.67b |
| PIIINP (ng/ml) | 9.26a | 5.95 | 6.13 | 10.01 | 15.95c | 9.77 | 4.88 | 9.86 | 8.73 | 12.36 | 3.90b |
| FibroTest index | 0.41 | −0.24 | −0.27 | 0.34 | 1.74 | −0.90 | −0.24 | 0.23 | 0.62 | 1.15 | −0.84 |
| ±1.55a | ±1.84 | ±1.26 | ±1.20 | ±1.53c | ±1.06 | ±1.39 | ±1.28 | ±1.29 | ±1.63c | ±1.15b | |
Comparison between the patient and control groups, P < 0.05.
In comparison to the G3, G4/S3, and S4 patients, P < 0.001.
The indices of the G4 patients were significantly higher than those of the G1 and G2 patients, and the FibroTest index of the S4 patients was significantly higher than those of the S1 and S2 patients, P < 0.01.
In the table, the HA, CIV, LN, and PIIINP values represent the geometric mean values, whereas the value of FibroTest index is the arithmetic mean ± standard deviation.
Control Group
The present study included 30 healthy individuals who came to our hospital for a physical examination in November of 2010 and 23 patients with diseases other than CHB. The healthy individuals have the characteristics: 25 males and 5 females; aged from 22 to 65 years with the mean age 39.00 ± 7.80 years; HBsAg and HBV DNA tests were negative; nothing abnormal in the serum biochemical indices; and the physical examination concluded to be normal. And the patients with diseases other than CHB have the characteristics: 18 males and 5 females; aged from 19 to 62 years with the mean age 34.23 ± 9.84 years; 4 cases of hepatolith, 2 cases of hepatic hemangioma, 12 cases of cholecystolithiasis or choledocholithiasis, and 5 cases of gallbladder polyps.
Specimen Collection
Five milliliters of venous blood were collected from all of the patients and controls in the morning after a fasting period. Serum was separated by centrifugation and stored at –20°C. Within 3 days after the blood collection, all 108 patients underwent a color Doppler ultrasound‐guided (ASPEN system, Siemens, Malvern, PA) liver biopsy. A 16‐G fine biopsy needle (Doctor Japan Co., Saitama, Japan) was used to conduct a 1‐sec rapid liver puncture. The lengths of the sampled liver tissues were > 1.6 cm, and the number of portal areas was > 15. The liver samples were immediately fixed in 4% neutral formaldehyde solution, embedded in paraffin, and serially sectioned for hematoxylin and eosin, Masson's, and reticular fiber staining. The pathological diagnosis of each liver biopsy tissue was determined from the inflammation grade and fibrosis staging after a double‐blind inspection by two specialists in the Pathological Diagnostic Center at Fujian Medical University.
Chemical Reagents
HA, PIIINP, CIV, and LN detection kits were purchased from Beijing Yuande Biological Engineering Company (Beijing, China), and α2M and HP detection kits were purchased from Siemens (Marburg, Germany) and tested on a BN‐II specific protein analyzer (Siemens, Deerfield, Illinois). Total bilirubin detection kits were purchased from the Wako Pure Chemical Industries, Ltd. (Osaka, Japan), and apolipoprotein A1 detection kits were purchased from Randox Laboratories (Crumlin, UK). Albumin detection kits were purchased from Sekisui Chemical Co., Ltd. (Tokyo, Japan). Alanine transaminase test kits, aspartate aminotransferase test kits, 5‐nucleotidase assay kits, and adenosine deaminase assay kits were purchased from the Ningbo MeiKang Company (Ningbo, China). Cholinesterase detection kits were purchased from the Shanghai KeHua Bio‐engineering Co. Ltd. (Shanghai, China), and automated detection was performed on an AU2700 analyzer (Olympus, Tokyo, Japan). Platelet detection (Siemens, Tarrytown, NY) was performed using an ADVIA 2120 hematology analyzer (Siemens, Tarrytown, NY). Prothrombin time test kits and fibrinogen assay kits were purchased from Diagnostica Stago (Asnieres, France) and detected with a STA‐R automated coagulation analyzer (Stago, Asnieres, France).
Statistical Analysis
The SPSS 13.0 software package (SPSS Inc., Chicago, IL) was used for the data analysis. The geometric means of four indices (HA, PIIINP, CIV, and LN) were used for the statistical analysis, wherein we performed logarithmic transformations followed by a t‐test or analysis of variance. The FibroTest index was calculated according to a previously published formula 9 and presented as the mean ± standard deviation. A t‐test or analysis of variance was then used as the statistical test, and a P < 0.05 was considered to be statistically significant. Spearman rank correlation was applied to all of the measured induces for correlation analysis. The sensitivity, specificity, positive predictive value, negative predictive value, positive likelihood ratio, and negative likelihood ratio of the indices were calculated, and receiver operator characteristic (ROC) curves were sketched to evaluate the diagnostic capacity of each index. Univariate/multivariate unconditional logistic regression analysis was used to analyze the relationship of the indices with CHB liver fibrosis and cirrhosis. Indices associated with liver fibrosis and cirrhosis were screened, and logistic regression equations were established.
RESULTS
Comparison of Five Indices between the Patient and Control Groups
The mean levels of the four direct indices and the FibroTest index were all lower in the control group in comparison to the patient group (Table 1). In addition, the values for the indices were highest for patients who were classified as G4 or S4. The correlation analysis showed that the five indices (four direct indices and the FibroTest index) were significantly correlated with inflammation (r = 0.318–0.577, P < 0.001) and fibrosis severity (r = 0.265–0.56, P < 0.001). We also found significant differences when we compared the five indices among the patients with different inflammation grades (F = 6.033–15.026, P < 0.001). The least significant difference (LSD) test was used to perform pairwise comparisons between the groups. We found that the five indices in the control group (noninflammatory group) were lower than the G3 and G4 patients (P < 0.001), and the five indices in the G4 patients were significantly higher than the G1 and G2 patients (P < 0.01). In addition, the results indicate that there were significant differences in the five indices among the different fibrosis stages in the patient group (F = 3.290–11.868, P < 0.01). An LSD test was also used for pairwise comparisons between stages. We found that the five indices in the control group (no fibrosis) were lower than those in the S3 and S4 patients (P < 0.001). Moreover, the FibroTest index in the S4 patients was significantly higher than those in the S1 and S2 patients (P < 0.05).
Comparisons of Five Indices in Subgroups of the Patient Group (Combined with Liver Steatosis and Not Combined with Liver Steatosis)
The patient group is divided into two subgroups: combined with liver steatosis and not combined with liver steatosis. In our research, there were no statistically significant differences in the five indices between the subgroups (P > 0.05; Table 2)
Table 2.
Comparison of Five Indices in the Subgroups of the Patient Group (Combined with Liver Steatosis and Not Combined with Liver Steatosis)
| Cases | HA (ng/ml) | CIV (ng/ml) | LN (ng/ml) | PIIINP (ng/ml) | FibroTest index | |
|---|---|---|---|---|---|---|
| Combined with liver steatosis | 25 | 62.99 | 70.62 | 75.70 | 9.05 | 0.42 ± 1.50 |
| Not combined with liver steatosis | 83 | 67.01 | 57.51 | 87.88 | 9.98 | 0.38 ± 1.72 |
| T | −0.214 | 0.673 | −0.338 | −0.368 | 0.102 | |
| P | 0.831 | 0.502 | 0.736 | 0.713 | 0.919 |
ROC Curves for the Diagnosis of Liver Inflammation (≥G1) via Five Indices
We drew ROC curves for the diagnosis of liver inflammation via the five indices (≥G1; Fig. 1.). The results demonstrate that the negative likelihood ratio of the five combined indices was <0.1, which indicated that the indices could reliably exclude liver inflammation. In addition, the positive likelihood ratio of LN was >10, which indicated that LN was a very reliable marker for the diagnosis of liver inflammation (Table 3).
Figure 1.
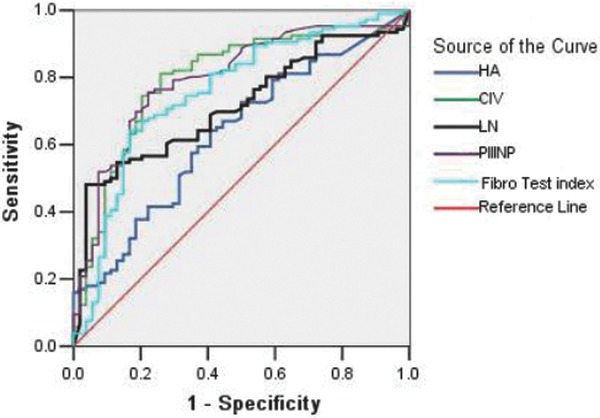
ROC curves for the diagnosis of liver inflammation (≥G1) via five indices. The AUCs of the inflammation diagnostic indices were ranked in the following order: PIIINP (0.793) > CIV (0.787) > FibroTest index (0.753) > LN (0.715) > HA (0.631), and the boundary values that were estimated by the ROC curves to indicate liver inflammation were 5.65 ng/ml, 36.2 ng/ml, –0.28, 88.65 ng/ml, and 43.5 ng/ml, respectively.
Table 3.
The Diagnostic Ability of Five Indices to Detect Liver Inflammation (≥G1)
| AUC | 95% CI of AUC | Sensitivity (%) | Specificity (%) | Youden index | Positive predictive value (%) | Negative predictive value (%) | Positive likelihood ratio | Negative likelihood ratio | |
|---|---|---|---|---|---|---|---|---|---|
| HA | 0.631 | 0.542−0.720 | 63.55 | 59.26 | 0.23 | 75.56 | 45.07 | 1.56 | 0.62 |
| CIV | 0.787 | 0.711–0.863 | 80.37 | 74.07 | 0.54 | 86.00 | 65.60 | 3.10 | 0.27 |
| LN | 0.715 | 0.636–0.794 | 47.66 | 96.30 | 0.44 | 96.20 | 48.10 | 12.88 | 0.54 |
| PIIINP | 0.793 | 0.720–0.866 | 74.77 | 77.78 | 0.53 | 87.00 | 60.90 | 3.36 | 0.32 |
| FibroTest | 0.753 | 0.671–0.835 | 63.60 | 83.30 | 0.47 | 88.30 | 53.60 | 3.81 | 0.44 |
| Four indices combined | ‐ | ‐ | 89.81 | 52.83 | 0.43 | 79.51 | 71.79 | 1.90 | 0.19 |
| Five indices combined | ‐ | ‐ | 95.30 | 50.00 | 0.45 | 79.10 | 84.40 | 1.91 | 0.094 |
ROC Curves for the Diagnosis of Liver Fibrosis (≥S1) via Five Indices
We drew ROC curves for the diagnosis of liver fibrosis via the five indices (≥S1; Fig. 2). The results demonstrate that the negative likelihood ratio of the five combined indices was <0.1, which indicated that the combination of the indices could reliably exclude liver fibrosis. In addition, the positive likelihood ratio of LN was >10, which indicated that LN was a very reliable marker for the diagnosis of liver fibrosis (Table 4).
Figure 2.
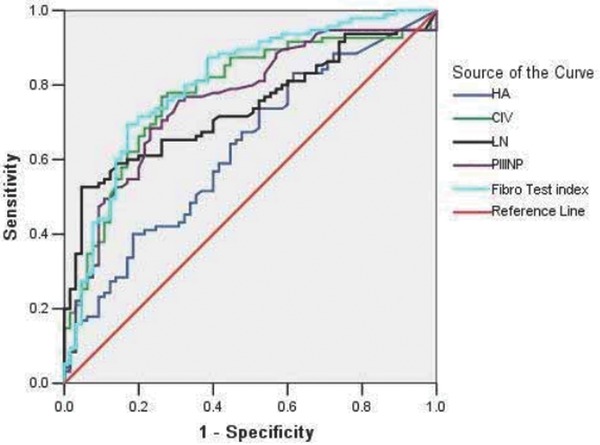
ROCcurves for the diagnosis of liver fibrosis (≥S1) via five indices. The AUCs of the fibrosis diagnostic indices were ranked in the following order: FibroTest (0.80) > CIV (0.771) > PIIINP (0.755) > LN (0.738) > HA (0.626), and the boundary values that were estimated by the ROC curves to indicate liver fibrosis were −0.28, 42.05 ng/ml, 6.75 ng/ml, 88.65 ng/ml, and 22.1 ng/ml, respectively.
Table 4.
The Diagnostic Ability of Five Indices to Detect Liver Fibrosis (≥S1)
| AUC | 95% CI of AUC | Sensitivity (%) | Specificity (%) | Youden index | Positive predictive value (%) | Negative predictive value (%) | Positive likelihood ratio | Negative likelihood ratio | |
|---|---|---|---|---|---|---|---|---|---|
| HA | 0.626 | 0.539‐0.713 | 83.33 | 38.46 | 0.22 | 66.70 | 61.00 | 1.35 | 0.43 |
| CIV | 0.771 | 0.696‐0.845 | 76.04 | 73.85 | 0.50 | 81.10 | 67.60 | 2.91 | 0.32 |
| LN | 0.738 | 0.663‐0.814 | 52.08 | 95.38 | 0.47 | 94.30 | 57.40 | 11.27 | 0.50 |
| PIIINP | 0.735 | 0.678‐0.831 | 67.71 | 76.92 | 0.45 | 81.30 | 61.70 | 2.93 | 0.42 |
| FibroTest | 0.80 | 0.729‐0.871 | 68.75 | 83.08 | 0.52 | 85.70 | 64.30 | 4.06 | 0.38 |
| Four indices combined | ‐ | ‐ | 93.75 | 30.77 | 0.25 | 66.67 | 76.92 | 1.35 | 0.20 |
| Five indices combined | ‐ | ‐ | 99.00 | 29.20 | 0.28 | 67.40 | 95.00 | 1.40 | 0.03 |
The Diagnostic Ability of Five Indices in Cirrhosis (S4)
We drew ROC curves for the diagnosis of liver cirrhosis via the five indices (S4; Fig. 3.). In regards to the diagnosis of cirrhosis, the greatest negative predictive value was observed when we combined the five indices (98.1%).
Figure 3.
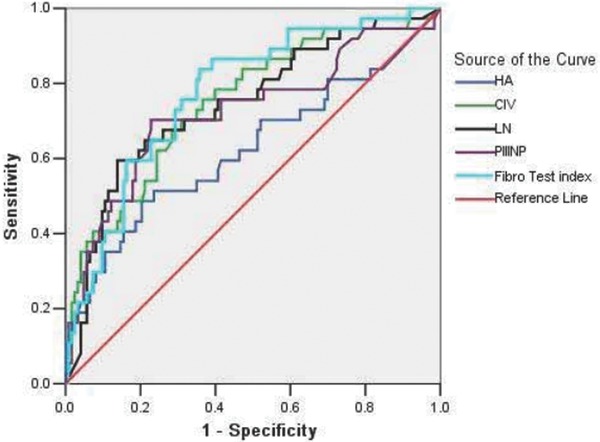
ROC curves for the diagnosis of cirrhosis (S4) via five indices. The AUCs of the ROC curves were ranked in the following order: FibroTest (0.776) > CIV (0.752) > LN (0.745) > PIIINP (0.725) > HA (0.625). In addition, the boundary values that were estimated by the ROC curves to indicate cirrhosis were −0.22, 60.6 ng/ml, 126.5 ng/ml, 9.9 ng/ml, and 91.15 ng/ml, respectively.
The Logistic Regression Equation for CHB‐Related Liver Fibrosis and Early Cirrhosis
Liver fibrosis was set as the dependent variable, and gender, age, the four direct indices, the FibroTest index, albumin, alanine transaminase, aspartate aminotransferase, cholinesterase, 5′‐nucleotidase (5‐NT), adenosine deaminase, PLT, prothrombin time, and fibrinogen (FIB) were set as independent variables. Univariate unconditional logistic regression analysis was conducted, and the statistically significant variables were set as independent variables. The “Enter” method was used to conduct a multivariate unconditional logistic regression analysis. The results demonstrate that the risk of liver fibrosis in males was much higher than in females. In addition, increases in CIV and α2M increased the risk of fibrosis. Interestingly, increases in PLT and HP decreased the risk of fibrosis, whereas FibroTest index had a weak relationship with fibrosis. The results of the logistic regression analysis were used to create a regression equation for the diagnosis of CHB‐related liver fibrosis: logitP = −2.739 + (3.270 × Gender) + (0.003 × CIV) + (1.959 × α2M) – (0.032 × PLT) – (2.625 × HP). Similarly, cirrhosis was set as the dependent variable to establish the logistic regression equation of CHB‐related early‐stage cirrhosis. The results show that the risk of cirrhosis was much higher for males than females. In addition, increases in PLT and FIB decreased the risk of liver cirrhosis, whereas HA and CIV had a weak relationship with liver cirrhosis. The regression equation for the diagnosis of CHB‐related early‐stage liver cirrhosis was logitP = 16.388 + (2.85 × gender) + (0.004 × CIV) – (0.037 × PLT) – (1.694 × FIB).
DISCUSSION
Although the diagnostic value of the indices used in this study for liver fibrosis have been documented, the traditional detection method is not convenient, the linear range is not wide enough, and automation cannot be implemented. To overcome these methodological limitations, China has successfully developed a new generation JETLIA‐962 chemiluminescence analyzer; however, its diagnostic value is unknown. The present article evaluated and affirmed that this chemiluminescence analyzer is beneficial in the diagnosis of CHB‐related liver fibrosis.
Four direct serum indices (HA, LN, PIIINP, and CIV) and the FibroTest index level were significantly related to the CHB inflammation grade and fibrosis stage. The values of the indices in G3 and G4 patients were significantly higher than those in G1 and G2 patients, and the values in S3 and S4 patients were significantly higher than those in control patients (no fibrosis), which indicated that these indices were suitable for monitoring the progression of inflammation and fibrosis in CHB patients. Our results also demonstrate that there was not a correlation between the occurrence of liver steatosis and fibrosis stage. Indeed, the diagnosis of fibrosis by all of the indices was not affected by steatosis.
An early diagnosis of inflammation is very important in liver fibrosis cases. ROC curve analysis demonstrates that serum PIIINP best predicted early inflammation and reflected the activity and extent of CHB, which is consistent with a report by Liu et al. 10. When we examined the relationships between liver fibrosis and the indices, we found that the combination of the five indices could exclude inflammation and fibrosis. When the indices were separately examined, LN was able to diagnose inflammation and fibrosis. Taking the most commonly used diagnostic procedure in liver fibrosis reported in 2008 into account 11, we think that analysis of LN and combination of five indices could avoid unnecessary liver biopsies, which would reduce the risks and suffering of patients.
For the diagnosis of cirrhosis, the FibroTest index had the largest AUC (0.776) of the indices; however, the value was slightly lower than the value reported by Gui 12 in China. Interestingly, the FibroTest index was found to be better for the diagnosis of cirrhosis in comparison to the combined indices. Therefore, we believe that the FibroTest index can achieve better diagnostic efficiency without the necessity to combine all of the indices when diagnosing early‐stage hepatitis B cirrhosis.
A comparison of the results of the four direct indices detected by the JETLIA‐962 chemiluminescence analyzer and the results detected from the conventional ELISA and RIA methods demonstrate that the sensitivity and specificity of CIV detected by the JETLIA‐962 analyzer were slightly lower than the values obtained by conventional methods in the diagnosis of early‐stage fibrosis and cirrhosis. In contrast, PIIINP and LN had much better diagnostic values when detected with the JETLIA‐962 in comparison to detection by ELISA and RIA 10, 13. Interestingly, LN did not have any diagnostic value when detected with the ELISA and RIA methods. Moreover, we found that the sensitivity and specificity of HA as detected by the JETLIA‐962 chemiluminescence analyzer were much lower than those detected via the RIA method (the AUC of HA was minimal in inflammation, fibrosis, and cirrhosis; <0.70; 10. Interestingly, The HA results were not consistent with most previous reports. The inconsistency may have resulted because HA was detected by the competitive chemiluminescence method, which involves more impact factors during the operation. Thus, the reliability of the results needs to be improved.
In recent years, scholars in China have established a number of diagnostic models for CHB liver fibrosis, including the Zeng model 14, the Hui model 15, the S index 16, and the FibroIndex model 17. Because of different indices and calculations, the models cannot be applied in all laboratories. In the present study, the establishment of a simple feasible mathematical diagnostic model of fibrosis and early‐stage liver cirrhosis was based on the routine serological indices of our laboratory. Although there were certain diagnostic values of fibrosis and early‐stage liver cirrhosis for some individual serum indices (e.g. LN and PIIINP), they were not included in the regression equation because a single index did not have a sufficiently strong impact on the dependent variable (in this study, the relative risks and odds ratios of all of the indices were closer to 1, which indicated an invalid value). When carrying out multivariate logistic regression analysis, the impact of multiple independent variables was considered. Because certain influences might exist between independent variables, the impact of some independent variables on the dependent variables was further weakened and became meaningless. Therefore, they were not included in the equation.
The results of the present study demonstrated that the four direct indices of liver fibrosis detected by the domestically produced JETLIA‐962 chemiluminescence analyzer and the FibroTest index could be used to monitor the course of CHB. Indeed, the combination of the five indices helped to rule out inflammation and fibrosis, whereas LN helped to confirm inflammation and fibrosis. Taken together, the results of the present study show that application of JETLIA‐962 can help reduce unnecessary clinical liver biopsies. The diagnostic capabilities of LN and PIIINP on CHB liver fibrosis as detected by this instrument were superior to the ELISA and RIA methods. The present study also established the mathematical diagnostic models for CHB‐related liver fibrosis and early‐stage liver cirrhosis, which may be helpful in the noninvasive diagnosis of CHB liver fibrosis and need to be further evaluated.
ACKNOWLEDGMENTS
The authors thank Dr. Jing Chen for his support in liver biopsy. The authors who have taken part in this study declared that we do not have anything to disclose regarding funding or conflict of interest with respect to this article.
REFERENCES
- 1. Chinese Society of Hepatology, Chinese Medical Association, Chinese Society of Infectious Diseases, Chinese Medical Association. The guideline of prevention and treatment for chronic hepatitis B (2010 version). Zhonghua Liu Xing Bing Xue Za Zhi 2011;32:405–415. [PubMed] [Google Scholar]
- 2. Rockey DC, Bissell DM. Noninvasive measures of liver fibrosis. Hepatology 2006;43:S113–S120. [DOI] [PubMed] [Google Scholar]
- 3. Imbert‐Bismut F, Ratziu V, Pieroni L, et al. Biochemical markers of liver fibrosis in patients with hepatitis C virus infection: A prospective study. Lancet 2001;357:1069–1075. [DOI] [PubMed] [Google Scholar]
- 4. Forns X, Ampurdanès S, Llovet JM, et al. Identification of chronic hepatitis C patients without hepatic fibrosis by a simple predictive model. Hepatology 2002;36:986–992. [DOI] [PubMed] [Google Scholar]
- 5. Wai CT, Greenson JK, Fontana RJ, et al. A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C . Hepatology 2003;38:518–526. [DOI] [PubMed] [Google Scholar]
- 6. Patel K, Gordon SC, Jacobson I, et al. Evaluation of a panel of non‐invasive serum markers to differentiate mild from moderate‐to‐advanced liver fibrosis in chronic hepatitis C patients. J Hepatol 2004;41:935–942. [DOI] [PubMed] [Google Scholar]
- 7. Calès P, Oberti F, Michalak S, et al. A novel panel of blood markers to assess the degree of liver fibrosis. Hepatology 2005;42:1373–1381. [DOI] [PubMed] [Google Scholar]
- 8. Chinese Society of Infectious Diseases and Parasitology and Chinese Society of Hepatology, Chinese Medical Association . The programme of prevention and cure for viral hepatitis. Zhonghua Gan Zang Bing Za Zhi 2000;8:324–329. [Google Scholar]
- 9. Poynard T. Diagnosis method of inflammatory, fibrotic or cancerous disease using the biochemical markers. United States: Patent No US 6,631,330 B1, 2003.
- 10. Liu J, Wang JY, Lu Y. Serum fibrosis markers in diagnosing liver fibrosis. Zhonghua Nei Ke Za Zhi 2006;45:475–477. [PubMed] [Google Scholar]
- 11. Manning DS, Afdhal NH. Diagnosis and quantitation of fibrosis. Gastroenterology 2008;134:1670–1681. [DOI] [PubMed] [Google Scholar]
- 12. Gui HL, Xie Q, Wang H. FibroTest‐ActiTest for predicting liver fibrosis and inflammatory activity in Chinese patients with chronichepatitis B . Zhonghua Gan Zang Bing Za Zhi 2008;16:897–901. [PubMed] [Google Scholar]
- 13. Li Q, Ou XL, Li J, Jia JD, Wang BE. The contrast study of serum liver fibrosis indexes and liver pathology. J Clin Hepatol (Chinese) 2004;20:155–156. [Google Scholar]
- 14. Zeng MD, Lu LG, Mao YM, et al. Prediction of significant fibrosis in HBeAg‐positive patients with chronic hepatitis B by a noninvasive model. Hepatology 2005;42:1437–1445. [DOI] [PubMed] [Google Scholar]
- 15. Hui AY, Chan HL, Wong VW, et al. Identification of chronic hepatitis B patients without significant liver fibrosis by a simple noninvasive predictive mode1. Am J Gastroenterol 2005;100:616–623. [DOI] [PubMed] [Google Scholar]
- 16. Zhou K, Zheng RD, Xian JC, et. al. Building a noninvasive diagnostic model based on conventional laboratory markers to predict liver fibrosis.Chin Hepatol 2008;13(5):362–367. [Google Scholar]
- 17. Zhang WS, Wang BE, Wang TL, et al. Noninvasive assessment of liver fibrosis in chronic hepatitis B using a predictive model. Zhonghua Gan Zang Bing Za Zhi 2006;14:169–173. [PubMed] [Google Scholar]


