Abstract
Purpose
Recently, role of adipokin in the pathogenesis of nonalcoholic fatty liver disease (NAFLD) has been suggested. Among adipokins, role of leptin and adiponectin is rather well known; however, there are only a few data concerning visfatin.
Material and Methods
NAFLD is confirmed in 30 patients by ultrasonography. As a control group, patients without fatty liver or other liver diseases were included. Viral hepatitis, metabolic liver diseases, and autoimmune hepatitis and consumption of alcohol were excluded in all patients. Fasting serum level of visfatin was determined by ELISA method.
Results
Serum visfatin concentration in the NAFLD group (14.7 ± 8.1 ng/ml) was significantly higher than in controls (9.4 ± 1.6 ng/ml) (P < 0.001). There were no correlations between visfatin and anthropometric parameters, transaminases, lipids, and homeostasis model assessment‐estimated insulin resistance (HOMA‐IR).
Conclusion
Serum visfatin concentration increases in patients with NAFLD.
Keywords: visfatin, nonalcoholic fatty liver disease
INTRODUCTION
Nonalcoholic fatty liver disease (NAFLD), the most common liver disorder in the world, is a clinicohistopathological entity in which excessive triglyceride accumulation in the liver occurs. NAFLD represents the necroinflammatory form, which can lead to advance liver fibrosis, cirrhosis, and hepatocellular carcinoma. The pathogenesis of NAFLD is complex but increased visceral adiposity plus insulin resistance with increased free fatty acids release play an initial key role for the onset and perpetuation of liver steatosis 1. Several factors have roles in histopathological changes of NAFLD 2, 3, 4, 5, 6, 7, 8, 9, 10, 11.
Visfatin was recently identified as a protein expressed in visceral adipose tissue visfatin, an adipokine isolated by Fukuhara et al. (2005), corresponds to a protein identified previously as pre‐B cell colony‐enhancing factor, a 52 kDa cytokine expressed, and secreted by lymphocytes. The biological role of visfatin is not entirely understood, but several studies indicated glucose lowering and insulin‐mimicking/‐sensitizing effects of visfatin 12. Visfatin is proposed as important proinflammatory mediators; however, conflicting results have been reported on the potential link between NAFLD and visfatin. Some authors argued visfatin was proinflammatuar cytokine, while others suggested that it was protective cytokine 3, 4, 5, 6, 7, 8. The purpose of the present study was to investigate the serum visfatin levels in NAFLD.
Material and Methods
This prospective cohort study was conducted in adults attending the NAFLD in a hospital in 2010. Thirty NAFLD patients admitted to the gastroenterology outpatient clinic because of increased liver enzymes were evaluated together with 27 healthy control subjects in terms of fasting blood glucose, lipid profile, insulin resistance with homeostasis model assessment‐estimated insulin resistance (HOMA‐IR), alanine aminotransferase (ALT), aspartate aminotransferase (AST), and visfatin.
NAFLD was diagnosed and its grade was assessed with the findings of liver ultrasound scan performed by a single experienced sonographist, who was blind to all biochemical characteristics of the participants. Patients with grade 2 and 3 steatosis described by Hamaguchi et al. were included in this study 13. Ultrasonographic findings scored in this study included hepatorenal echo contrast, liver brightness, deep attenuation, and vascular blurring. Scores ranged from 1 to 3, with 1 representing either hepatorenal contrast or that bright liver was present, 2 representing that both were present, and a score 3 indicating in addition to hepatorenal echo contrast, bright liver was severe.
Control group included persons who admitted to gastroenterology outpatient clinic with complaint of dyspepsia. None had fatty liver disease on sonographic imaging and without any systemic diseases. Also, all had normal biochemical tests including serum liver enzymes. For control and NAFLD groups, the exclusion criteria were hepatitis B, C, cytomegalovirus, Epstein‐Barr infections, monogram‐specific auto antibodies, alcohol consumption, diabetes mellitus, intolerance fasting glucose, medication (and diabetic drugs, blood‐pressure‐lowering medication, and statins), and hereditary defects (iron and copper storage diseases and alpha 1‐antitrypsin deficiency). The study was approved by an institutional ethics committee.
All patients’ serums were collected and ultrasound scanning was performed with patient consent after ethics committee approved this study. All the participants’ height and weight values were measured and their body mass index (BMI) was calculated. Glucose, creatinin, urea, ALT, AST, total cholesterol, triglyceride, and high density lipoprotein (HDL) cholesterol levels were studied with Roche Diagnostic kits (Boehringer‐Mannheim Diagnostics, GmbH, Germany) in a Roche/Hitachi D2400 auto analyzer. Insulin levels were measured with Elecsys 1010 auto analyzer (Roche Diagnostics GmbH, Mannheim, Germany). Low‐density lipoprotein (LDL) cholesterol and very LDL (VLDL) cholesterol levels were calculated by Friedewald formula 14. VLDL (mg/dl) = triglyceride (mg/dl)/5, LDL (mg/dl) = total cholesterol (mg/dl) − (HDL [mg/dl] + VLDL [mg/dl]). Insulin resistance was calculated by HOMA‐IR formula (=fasting insulin value × fasting blood glucose/22.5) 15.
Visfatin was analyzed using a commercially available ELISA kit (Phoenix Peptides, Belmont, CA). Assay sensitivity was 2 ng/ml and interassay and intraassay coefficients of variation were less than 10 and 5%, respectively.
Statistical Analysis
The results were expressed as mean ± standard deviation. The distribution of variables was analyzed with Kolmogorov–Smirnov test. Quantitative variables with normal distribution were analyzed with a two‐tailed, unpaired Student's t‐test. Nonparametric variables were analyzed with the Mann–Whitney U‐test. Qualitative variables were analyzed with the chi‐square test P‐value under 0.05 was considered statistically significant. SPSS 15.0 software (IL) was used.
RESULTS
Age and gender were similar in NAFLD and control groups (Table 1). Waist circumference, BMI, AST, ALT, glutamyltransferase (GGT), insulin, and HOMA‐IR were higher in patients with NAFLD than in the control group. Alkaline phosphatase (ALP), lactate dehydrogenase (LDH), triglyceride, total cholesterol, LDL cholesterol, and HDL cholesterol levels were comparable among NAFLD and control groups (Table 1).
Table 1.
Demographics and Laboratory Findings are of NAFLD and Control Group
| Variables | Control group | NAFLD group | P‐value |
|---|---|---|---|
| Age (year) | 43.6 ± 10.2 | 41.1 ± 9.1 | 0.539 |
| Gender, male, (%) | 45 | 52 | 0.435 |
| BMI (kg/m2) | 25.2 ± 3.6 | 30.5 ± 5.4 | 0.005 |
| Waist circumference (cm) | 89.0 ± 9.8 | 101.1 ± 15.9 | 0.01 |
| Triglyceride (mg/dl) | 166.4 ± 86.2 | 191.2 ± 103.4 | 0.540 |
| Cholesterol (mg/dl) | 196.6 ± 43.6 | 202.0 ± 45.5 | 0.396 |
| LDL cholesterol (mg/dl) | 109.8 ± 41.2 | 120.4 ± 32.9 | 0.391 |
| HDL cholesterol (mg/dl) | 46.2 ± 8.9 | 43.8 ± 6.2 | 0.391 |
| Glucose (mg/dl) | 94.8 ± 9.3 | 103.1 ± 22.3 | 0.224 |
| AST (IU/l) | 22.8 ± 6.0 | 62.7 ± 24.5 | 0.01 |
| ALT (IU/l) | 24.1 ± 9.9 | 76.9 ± 19.1 | 0.006 |
| GGT (IU/l) | 22.5 ± 8.7 | 67.7 ± 14.0 | 0.034 |
| ALP (IU/l) | 110 ± 21.4 | 114.02 ± 24.2 | 0.198 |
| LDH (IU/l) | 184.4 ± 69.0 | 188.1 ± 64.9 | 0.311 |
| Total bilirubine (mg/dl) | 0.6 ± 0.3 | 0.7 ± 0.3 | 0.198 |
| Insulin (mU) | 8.0 ± 4.3 | 15.2 ± 10.1 | 0.008 |
| HOMA‐IR | 2.2 ± 1.9 | 4.5 ± 3.2 | 0.005 |
| Visfatin (ng/ml) | 9.4 ± 1.6 | 14.7 ± 8.1 | <0.001 |
A statistically significant increase in visfatin levels was noted in patients with NAFLD (14.7 ± 8.1 ng/ml) compared with healthy controls (9.4 ± 1.6 ng/ml) (P < 0.001; Table 1 and Fig. 1. There was no correlation between visfatin and BMI, waist circumference, insulin, and HOMA‐IR (P = 0.777, r = 0.06, P = 0.323, r = −0.255, P = 0.666, r = 0.113, and P = 0.956, r = 0.01, respectively).
Figure 1.
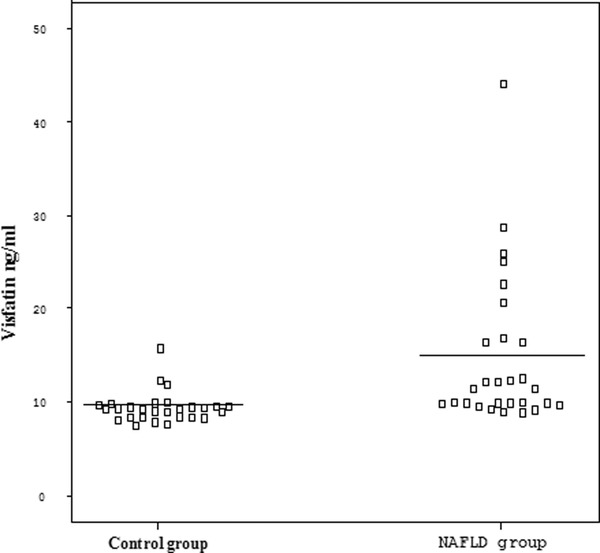
Serum visfatin levels in NAFLD and control group.
DISCUSSION
In the present study, we found that the patients with NAFLD had significantly higher serum visfatin levels than the healthy control group. In addition in this study, we have found no relationship between serum visfatin levels, HOMA‐IR, insulin levels, and BMI.
Visfatin is a novel adipokine predominantly expressed in visceral adipose tissue and is involved in inflammatory and immunological processes. Several studies have shown that the expression and secretion of visfatin are upregulated during inflammation and in response to proinflammatory cytokines 4, 16, 17. In the current literature, there are few studies that have assessed the association between visfatin and NALFD and the results of these studies are controversial.
Romanowska et al. revealed that serum visfatin concentration in the NAFLD had been significantly higher than in controls. In that study, they found a positive correlation between visfatin and proinflamatuar cytokine 6. Aller et al. demonstrated a correlation between portal inflammation and visfatin concentration. Moreover, they claimed that visfatin plasma concentrations could predict the presence of portal inflammation in NAFLD patients 10. In another study, Jarrar et al. revealed that visfatin elevated in NAFLD. However, visfatin was lower in non alcoholic steatohepatitis patients than patients with simple steatosis and obese controls. A study claimed the protective effect of visfatin in NAFLD and reported that visceral visfatin level declined significantly in NAFLD 11. Visfatin expression had decreased in hepatic cells and that in vitro studies showed that visfatin exerts antiapoptotic effects in these cells 9.
It has been claimed that adipokines such as adiponectin and resistin and several cytokines including TNF‐alpha and IL‐6 have roles in the pathogenesis of NAFLD. However, the role of visfatin is not clear 11, 14. One study demonstrates the relationship between visfatin and IL6 in NAFLD 8. Visfatin upregulates the production of proinflammatory cytokines including IL‐1β, TNF‐α, IL‐6, IL‐10 and enhances the surface expression of the costimulatory molecules 16, 17, 18, 19. Our results also support the direct inflammatory role of visfatin, hence it may be a proinflammatuary cytokine.
Insulin resistance is the key mechanism leading to hepatic steatosis, and perhaps also to steatohepatitis. Visfatin is controverisal whether are related to changes in body weight and insulin resistance. Visfatin exerts insulin‐mimetic effects and lowers plasma glucose level throughout binding and activating the insulin receptor in animal model. However, studies in humans’ subjects reported conflicting results in regard to its relation with adiposity, insulin resistance, dyslipidemia, suggesting that the role of this protein in the development of obesity and insulin resistance is unclear 17, 18, 19, 20. As previous report, the present study demonstrates no correlations between visfatin and anthropometric parameters, transaminases, lipids, or HOMA‐IR in NAFLD. However, recent study revealed that LDL cholesterol and C‐reactive protein levels were positively correlated, while weight was negatively correlated with visfatin levels 21. Other study revealed that monounsaturated fat intake remained as an independent predictor of visfatin levels. Visfatin concentration decreased monounsaturated fat intake 22. The massive weight reduction 1 year after biliopancreatic diversion was not associated with a significant change in the circulating visfatin levels in morbidly obese women 23. In this study, we found no relationship between serum, HOMA‐IR, insulin levels, BMI, and visfatin levels. The nonsignificant results related to the differences between groups are probably due to a type II error because of the small sample size.
The major limitation of our study was the absence of liver biopsy. Taking into account for the ethical concerns, we could not perform liver biopsy to our patients. We used scoring system described in the “Material and methods” section for a long time in our clinic. Similar to other reports, the diagnosis of NAFLD in our study was based on ultrasonography and the exclusion of known causes of chronic liver disease, not confirmed by liver biopsy 24, 25. Ultrasonography is the commonest way of diagnosing NAFLD in clinical practice and has acceptable sensitivity and specificity in detecting moderate and severe steatosis in patients with biopsy proven disease 13, 26. Therefore, we apporoved only grade 2 and 3 NAFLD in this study. The other hand, in the light of clinical and laboratory parameters, the diagnosis of NAFLD was clearly demonstrated.
In conclusion, serum visfatin levels elevated in NAFLD. However, the relationship between visfatin and NAFLD requires further investigation.
Sources of Funding: No commercial party having a direct financial support, any conflicts interest in the results of the research supporting this article has or will confer a benefit on the authors or on any organization with which the authors are associated.
REFERENCES
- 1. Neuschwander‐Tetri BA, Clark JM, Bass NM, Van Natta ML, Unalp‐Arida A, Tonascia J, Zein CO, Brunt EM, Kleiner DE, McCullough AJ, Sanyal AJ, Diehl AM, Lavine JE, Chalasani N, Kowdley KV. NASH Clinical Research Network. Clinical, laboratory and histological associations in adults with nonalcoholic fatty liver disease. Hepatology 2010;52:913–924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Fitzpatrick E, Mitry RR, Quaglia A, Hussain MJ, DeBruyne R, Dhawan A. Serum levels of CK18 M30 and leptin are useful predictors of steatohepatitis and fibrosis in paediatric NAFLD. J Pediatr Gastroenterol Nutr 2010;51:500–506. [DOI] [PubMed] [Google Scholar]
- 3. Bertolani C, Sancho‐Bru P, Failli P, Bataller R, Aleffi S, DeFranco R, Mazzinghi B, Romagnani P, Milani S, Ginés P, Colmenero J, Parola M, Gelmini S, Tarquini R, Laffi G, Pinzani M, Marra F. Resistin as an intrahepatic cytokine: overexpression during chronic injury and induction of proinflammatory actions in hepatic stellate cells. Am J Pathol 2006;169:2042–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Tilg H. Adipocytokines in nonalcoholic fatty liver disease: key players regulating steatosis, inflammation and fibrosis. Curr Pharm Des 2010;16:1893–1895. [DOI] [PubMed] [Google Scholar]
- 5. Marra F, Bertolani C. Adipokines in liver diseases. Hepatology 2009;50:957–969. [DOI] [PubMed] [Google Scholar]
- 6. Romanowska A, Lebensztejn DM. Evaluation of serum visfatin concentrations in children with nonalcoholic fatty liver disease. Pol Merkur Lekarski 2010;28:459–461. [PubMed] [Google Scholar]
- 7. Kukla M, Ciupińska‐Kajor M, Kajor M, Wylezoł M, Zwirska‐Korczala K, Hartleb M, Berdowska A, Mazur W. Liver visfatin expression in morbidly obese patients with nonalcoholic fatty liver disease undergoing bariatric surgery. Pol J Pathol 2010;61:147–153. [PubMed] [Google Scholar]
- 8. Gaddipati R, Sasikala M, Padaki N, Mukherjee RM, Sekaran A, Jayaraj‐Mansard M, Rabella P, Rao‐Guduru V, Reddy‐Duvvuru N. Visceral adipose tissue visfatin in nonalcoholic fatty liver disease. Ann Hepatol 2010;9:266–270. [PubMed] [Google Scholar]
- 9. Dahl TB, Haukeland JW, Yndestad A, Ranheim T, Gladhaug IP, Damås JK, Haaland T, Løberg EM, Arntsen B, Birkeland K, Bjøro K, Ulven SM, Konopski Z,Nebb HI, Aukrust P, Halvorsen B. Intracellular nicotinamide phosphoribosyltransferase protects against hepatocyte apoptosis and is down‐regulated in nonalcoholic fatty liver disease. J Clin Endocrinol Metab 2010;95:3039–3047. [DOI] [PubMed] [Google Scholar]
- 10. Aller R, de Luis DA, Izaola O, Sagrado MG, Conde R, Velasco MC, Alvarez T, Pacheco D, González JM. Influence of visfatin on histopathological changes of non‐alcoholic fatty liver disease. Dig Dis Sci 2009;54:1772–1777. [DOI] [PubMed] [Google Scholar]
- 11. Jarrar MH, Baranova A, Collantes R, Ranard B, Stepanova M, Bennett C, Fang Y, Elariny H, Goodman Z, Chandhoke V, Younossi ZM. Adipokines and cytokines in non‐alcoholic fatty liver disease. Aliment Pharmacol Ther 2008;27:412–421. [DOI] [PubMed] [Google Scholar]
- 12. Fukuhara A, Matsuda M, Nishizawa M, Segawa K, Tanaka M, Kishimoto K, Matsuki Y, Murakami M, Ichisaka T, Murakami H, Watanabe E, Takagi T, Akiyoshi M,Ohtsubo T, Kihara S, Yamashita S, Makishima M, Funahashi T, Yamanaka S, Hiramatsu R, Matsuzawa Y, Shimomura I. Visfatin: a protein secreted by visceral fat that mimics the effects of insulin. Science 2005;307:426–430. [DOI] [PubMed] [Google Scholar]
- 13. Hamaguchi M, Kojima T, Itoh Y, Harano Y, Fujii K, Nakajima T, Kato T, Takeda N, Okuda J, Ida K, Kawahito Y, Yoshikawa T, Okanoue T. The severity of ultrasonographic findings in nonalcoholic fatty liver disease reflects the metabolic syndrome and visceral fat accumulation. Am J Gastroenterol 2007;12:2708–2715. [DOI] [PubMed] [Google Scholar]
- 14. Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low‐density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem 1972;18:499–502. [PubMed] [Google Scholar]
- 15. Wallace TM, Levy JC, Matthews DR. Use and abuse of HOMA modeling. Diabetes Care 2004;27:1487—1495. [DOI] [PubMed] [Google Scholar]
- 16. Stofkova A. Resistin and visfatin: regulators of insulin sensitivity, inflammation and immunity. Endocr Regul 2010;44:25–36. [DOI] [PubMed] [Google Scholar]
- 17. Kang YS, Song HK, Lee MH, Ko GJ, Cha DR. Plasma concentration of visfatin is a new surrogate marker of systemic inflammation in type 2 diabetic patients. Diabetes Res Clin Pract 2010;89:141–149. [DOI] [PubMed] [Google Scholar]
- 18. Pagano C, Pilon C, Olivieri M, Mason P, Fabris R, Serra R, Milan G, Rossato M, Federspil G, Vettor R. Reduced plasma visfatin/pre B‐cell colony enhancing factor in obesity is not related to insulin resistance in humans. J Clin Endocrinol Metab 2006;91:3165–3170. [DOI] [PubMed] [Google Scholar]
- 19. Moschen AR, Gerner RR, Tilg H. Pre‐B cell colony enhancing factor/NAMPT/visfatin in inflammation and obesity‐related disorders. Curr Pharm Des 2010;16:1913–1920. [DOI] [PubMed] [Google Scholar]
- 20. Arner P. Visfatin–a true or false trail to type 2 diabetes mellitus. J Clin Endocrinol Metab 2006;91:28–30. [DOI] [PubMed] [Google Scholar]
- 21. de Luis DA, Ballesteros M, Ruiz E, Muñoz C, Penacho A, Iglesias P, Guzmán AL, Abreu C, Maldonado A, Delgado M, Martín LS, Puigdevall V, Romero E, Sagrado MG, Izaola O, Conde R. Mar Cordero y Rocio Aller, Grupo de Nutrición, Sociedad Castellano Leonesa de Endocrinología, diabetes y nutrición. Visfatin in obese patients, relation with cardiovascular risk factors, a cross sectional study. Med Clin (Barc) 2011;137:199–203. [DOI] [PubMed] [Google Scholar]
- 22. de Luis DA, Aller R, Gonzalez Sagrado M, Conde R, Izaola O, Perez Castrillon JL, Romero E. Serum visfatin concentrations are related to dietary intake in obese patients. Ann Nutr Metab 2010;57:265–270. [DOI] [PubMed] [Google Scholar]
- 23. de Luis DA, Izaola O, Conde R, Primo D, Sagrado MG, Aller R. Visfatin levels in female, morbid, nondiabetic obese patients after biliopancreatic diversion surgery. Surg Obes Relat Dis 2011; 7:195–198. [DOI] [PubMed] [Google Scholar]
- 24. Babalı A, Cakal E, Purnak T, Bıyıkoğlu I, Cakal B, Yüksel O, Köklü S. Serum alpha‐fetoprotein levels in liver steatosis. Hepatol Int 2009; 3:551–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Liang RJ, Wang HH, Lee WJ, Liew PL, Lin JT, Wu MS. Diagnostic value of ultrasonographic examination for nonalcoholic steatohepatitis in morbidly obese patients undergoing laparoscopic bariatric surgery. Obes Surg 2007;17:45–56. [DOI] [PubMed] [Google Scholar]
- 26. Mishra P, Younossi ZM. Abdominal ultrasound for diagnosis of nonalcoholic fatty liver disease (NAFLD) Am J Gastroenterl 2007;12:2716–2717. [DOI] [PubMed] [Google Scholar]


