Abstract
Background
The aim of this study was to investigate the differential plasma levels of lipocalin 2 (LCN2) and its complex with MMP‐9 (where MMP is matrix metalloproteinase) before and after antibiotic treatment in hospitalized adult patients with community‐acquired pneumonia (CAP).
Method
Plasma LCN2 and LCN2/MMP‐9 complex levels were measured in 61 adult patients with CAP and 60 healthy controls using commercial enzyme‐linked immunosorbent assay (ELISA).
Results
A decrease in the number of white blood cells (WBCs) and neutrophils and decreases in the levels of C‐reactive protein (CRP), LCN2, and LCN2/MMP‐9 complex were observed after antibiotic treatment. The plasma level of LCN2, but not that of CRP, was correlated with the severity of CAP based on the Pneumonia Severity Index (PSI; r = 0.333, P = 0.009), confusion, urea, respiratory rate and blood pressure (CURB)‐65 (r = 0.288, P = 0.024), and Acute Physiology And Chronic Health Evaluation II (APACHE II) scores (r = 0.328, P = 0.010). LCN2 levels were also significantly correlated with LCN2/MMP‐9 levels and the numbers of WBCs or neutrophils.
Conclusions
Plasma levels of LCN2 and the LCN2/MMP‐9 complex can act as adjuvant diagnostic biomarkers for CAP. Plasma LCN2 might play a further role in the clinical assessment of the severity of CAP, which could potentially guide the development of future treatment strategies.
Keywords: community‐acquired pneumonia, Pneumonia Severity Index, lipocalin 2, matrix metalloprotease‐9
INTRODUCTION
Community‐acquired pneumonia (CAP) is a major cause of severe morbidity and mortality. It is the sixth most common cause of death in the Unitedw States, and it is estimated that four million cases of CAP occur annually 1. In Taiwan, CAP is the sixth leading cause of death 2, and there has been a worldwide increase in the number of hospitalizations due to CAP in the general population 3, 4. Timely and optimized antibiotic treatment can reduce morbidity and mortality; therefore, early diagnosis and recognition of the disease severity are necessary for the optimal care of CAP patients 5, 6.
Some inflammatory cells secrete matrix metalloproteinases (MMPs), which are critical for pulmonary morphogenesis and remodeling and the repair and regeneration of damaged lung tissue 7. Of the MMP family, MMP‐9 (92‐kDa type IV collagenase, gelatinase B) has been reported to be associated with the pathogenesis of lung injury 8, 9. In addition, various studies have shown that MMP‐9 is implicated in the pathogenesis of other pulmonary inflammatory diseases, such as acute respiratory distress syndrome 10, interstitial lung disease 11, and chronic obstructive pulmonary disease 12, 13.
Lipocalin 2 (LCN2), also known as neutrophil gelatinase‐associated lipocalin, neu‐related lipocalin, oncogene 24p3, and uterocalin, is a 25‐kDa protein, which is stored in the granules of human neutrophils 14. It belongs to the lipocalin family, which consists of more than 50 members, all of which are characterized by the ability to bind and transport small lipophilic substances 15. For example, LCN2 is a siderophore‐binding antimicrobial protein that participates in an iron‐depletion strategy exploited by the immune defense against bacterial pathogens 16. Moreover, LCN2 has been reported to be upregulated in epithelial tissues during inflammation, and it seems to play an important role in this process 17. This protein is also upregulated in several pathological conditions, including cancers 18, 19, inflammatory bowel disease 20, acute kidney injury (AKI; 21), autoimmune myocarditis 22, arthritis 23, pelvic inflammatory disease (PID; 24), bacterial pneumonia 25, 26, sepsis 27, and pancreatitis 28. Several studies have shown LCN2 to be a useful biomarker for the early detection of AKI in postcardiac surgery, nephritis, and radiocontrast exposure 21.
It has been established that LCN2 forms a complex with MMP‐9, thereby preventing the autodegradation of MMP‐9 and increasing its activity in vitro 29. Recent findings have suggested that detection of the LCN2/MMP‐9 complex might represent a predictor of disease status or of therapeutic response in several types of cancer, including breast 30, brain 31, gastric 32, and oral 19 cancers. Moreover, LCN2/MMP‐9 complex was also proved to act as a diagnostic biomarker for PID 24.
LCN2 and MMP‐9 are stored in specific granules in the neutrophils, while MMP‐9 is also found in gelatinase granules. It was suggested that MMP‐9 and LCN2 are mainly secreted into the blood by neutrophils infiltrating injured tissues tissue, and they are subsequently excreted in the urine 29. Although detecting LCN2 and its complex with MMP‐9 in the systemic circulation seems reasonable, few studies on LCN2 and LCN2/MMP‐9 in plasma are currently available 19, 24, 33. To the best of our knowledge, no study has investigated the prognostic value of LCN2 and LCN2/MMP‐9 complex in a cohort of patients with CAP. Herein, we measured the plasma levels of the LCN2 and LCN2/MMP‐9 complex in a group of patients with CAP and healthy control subjects to evaluate whether LCN2 or LCN2/MMP‐9 could be a useful biochemical marker. We also purposed to assessment of relationship between LCN2, LCN2/MMP‐9 complex, and severity of CAP.
MATERIALS AND METHODS
Subjects and Diagnosis
The Chung Shan Medical University Hospital (CSMUH) is a tertiary care university hospital in Taichung in west‐central Taiwan. This prospective study was conducted during the period from January 2009 to December 2009 by the Department of Medical Research and the Departments of Infectious Diseases and Chest Medicine, CSMUH. The study was approved by the Institutional Review Board of CSMUH (IRB No. CS11237). All of the subjects provided informed consent for the use of their blood samples. The inclusion criteria required that patients be >20 years old, admitted for the treatment of CAP, and diagnosed in the emergency room or by the outpatient department. Demographic characteristics, comorbidities, symptoms and signs of pneumonia, laboratory results, and previous antibiotic treatment were recorded upon admission. The diagnostic criteria for CAP were based on the guidelines of the Infectious Diseases Society of America (IDSA)/American Thoracic Society (ATS; 33). The guidelines for diagnosing CAP include typical infiltrative changes on chest X‐ray films within 1 day of symptom occurrence and at least one clinical manifestation, such as cough, yellow thick sputum, or high fever (>37.8°C) or at least two minor criteria, including tachypnea, dyspnea, pleural pain, chest pain, confusion or disorientation, lung consolidation, or a white blood cell (WBC) count of >12,000 cells/ml. The exclusion criteria included being an outpatient; having been transferred from another hospital or having had a separate hospital admission within the previous 3 weeks with another acute condition, such as pulmonary edema, pulmonary embolism, or malignancy appearing during follow‐up; pneumonia caused by tuberculosis or malignancy and severe immunocompromise, including severe neutropenia (a WBC count of <109 cells/l); and having an organ or bone marrow transplant or human immunodeficiency virus (HIV) infection. Pneumonia severity was assessed using the Pneumonia Severity Index (PSI; the Acute Physiology And Chronic Health Evaluation II (APACHE II; 36), and CURB‐65 37 tests.
Subjects and Blood Sample Collection
We consecutively enrolled 61 CAP patients and 60 healthy people to serve as a control group. All CAP patients were treated with empirical antimicrobial agents (e.g., moxifloxacin, levofloxacin, amoxicillin). We collected blood samples to measure WBCs, neutrophil cells, C‐reactive protein (CRP) levels, and plasma levels of LCN2 and LCN2/MMP‐9 complex before and after antibiotic treatment of the CAP patients. Blood samples were also collected from the control subjects and tested. The obtained blood samples were placed in tubes containing EDTA and were immediately centrifuged and stored at −80°C.
Measurements of WBCs, Neutrophil Cells, and CRP Levels
WBCs, neutrophils, and CRP levels were measured by clinical laboratory staff members, who were unaware of the sources of the samples (blinded to the study).
Measurements of Plasma LCN2 and LCN2/MMP‐9 Complex
Enzyme‐linked immunosorbent assay (ELISA) was used to measure the plasma levels of LCN2 and LCN2/MMP‐9 complex in the blood samples (R&D Systems, Abingdon, UK). From each plasma sample, 100 μl was directly transferred to the microtest strip wells of the ELISA plate and then was assayed according to the manufacturer's instructions. The absorbance was measured at 450 nm in a microtest plate spectrophotometer, and LCN2 and LCN2/MMP‐9 complex levels were quantified with a calibration curve using human LCN2 and LCN2/MMP‐9 complex as the standards.
Statistical Analysis
Statistical analyses were performed using SPSS statistics software (SPSS Inc., Chicago, IL), version 15.0. All continuous variables are expressed as the means ± SE, and n is expressed with percentages for the categorical variables. For comparisons between untreated patients and healthy individuals, the Mann–Whitney U test was performed for continuous variables not following a parametric distribution, and the Wilcoxon signed ranks test was used to compare untreated and treated patients for categorical variables. Linear regression analysis was applied for the correlation of LCN2 and LCN2/MMP‐9 with all of the clinical and laboratory variables of the CAP patients. Statistical significance was defined as P < 0.05 in a two‐tailed test.
RESULTS
The clinical characteristics of the subjects are summarized in Table 1. In total, 121 subjects were included in the analysis, and their ages and sexes did not significantly differ between the CAP patients and the control group (age, P = 0.963; sex, P = 0.941). Among the 61 CAP patients, the mean scores on the PSI, CURB‐65, and APACHE II were 79.03 ± 5.92, 0.88 ± 0.11, and 9.22 ± 0.72, respectively. Moreover, the CAP patients had significantly higher CRP levels (median 8.63 vs. 0.30, P < 0.001), WBCs (median 10,890 vs. 5,860, P < 0.001), and neutrophils (median 8,673 vs. 3,530, P < 0.001) compared to the control subjects (Table 1), and there were significant decreases in CRP levels (untreated: median 8.63; treated: median 0.94; P < 0.001), WBCs (untreated: median 10,890; treated: median 8,450; P < 0.001), and neutrophils (untreated: median 8,673; treated: median 5,484; P < 0.001) after antibiotic treatment (Table 1).
Table 1.
The Laboratory Data of Both Controls and Patients With Community‐Acquired Pneumonia (CAP) Before and After They Received Treatmenta
| Controls (n = 60), | Pretreatment (n = 61), | Posttreatment (n = 61), | |||
|---|---|---|---|---|---|
| Clinical variables | median (range) | median (range) | median (range) | P value UT/Cb | P value UT/Tc |
| Age | 59.38 ± 1.48e | 59.52 ± 2.62e | P = 0.963 | ||
| Gender | |||||
| Male | 36 (60%) | 37 (60.7%) | P = 0.941 | ||
| Female | 24 (40%) | 24 (39.3%) | |||
| Cigarette smoking | |||||
| Yes | 16 (26.7%) | 19 (31.2%) | P = 0.587 | ||
| No | 44 (73.3%) | 42 (68.8%) | |||
| LCN2 (ng/ml) | 17.85 | 50.68 | 31.46 | P < 0.001 | P = 0.057 |
| (5.00–107.50) | (2.07–305.62) | (3.26–305.62) | |||
| LCN2/MMP‐9 complex (ng/ml) | 15.25 | 58.11 | 18.95 | P < 0.001 | P < 0.001 |
| (2.81–75.79) | (6.59–244.08) | (3.41–264.56) | |||
| CRP (mg/dl) | 0.30 | 8.63 | 0.94 | P < 0.001 | P < 0.001 |
| (0.02–1.65) | (0.69–27.40) | (0.30–11.30) | |||
| WBC (/mm3) | 5,860 | 10,890 | 8,450 | P < 0.001 | P < 0.001 |
| (3,110–10,190) | (3,560–32,480) | (3,460–22,340) | |||
| Neutrophils (/mm3) | 3,530 | 8,673 | 5,484 | P < 0.001 | P < 0.001 |
| (1,738–6,046) | (1,032–29,686) | (1,518–21,155) | |||
| PSI score | 79.03 ± 5.92e | ||||
| CURB‐65 scored | 0.88 ± 0.11e | ||||
| APACHE II score | 9.22 ± 0.72e | ||||
CRP, C‐reactive protein; WBC, white blood cell; C, controls; UT, patients with CAP before they received treatment; T, patients with CAP after they received treatment; PSI, Pneumonia Severity Index; APACHE II, Acute Physiology and Chronic Health Evaluation II.
p value < 0.05 was considered significant.
The difference was analyzed using the Mann–Whitney U test.
The difference was analyzed using the Wilcoxon signed ranks test.
A 6‐point score, one point each for confusion, blood urea nitrogen >19 mg/dl, respiratory rate >30/min, low systolic (<90 mmHg) or diastolic (<60 mmHg) blood pressure, and aged >65 years.
Mean ± SE.
Figure 1 shows the median levels of plasma LCN2 and LCN2/MMP‐9 in the CAP patients before and after antibiotic treatment and in the control subjects. The CAP patients had presented with significantly higher plasma levels of LCN2, compared to the control subjects (controls: 17.85 ng/ml; patients: 50.68 ng/ml; P < 0.001; Table 1 and Fig. 1A). After the CAP patients received antibiotic treatment, the level of LCN2 was obviously decreased, and the P value was close to 0.05 (untreated: 50.68 ng/ml; treated: 31.46 ng/ml; P = 0.057; Table 1 and Fig. 1A). Furthermore, the level of LCN2/MMP‐9 complex in the pretreatment plasma of the patients with CAP was also significantly higher than that in the plasma of the controls (controls: 15.25 ng/ml; patients: 58.11 ng/ml; P < 0.001; Table 1 and Fig. 1B). The level of LCN2/MMP‐9 complex in the posttreatment plasma of the patients with CAP was significantly decreased compared to that in the pretreatment plasma (untreated: 58.11 ng/ml; treated: 18.95 ng/ml; P < 0.001; Table 1 and Fig. 1B).
Figure 1.
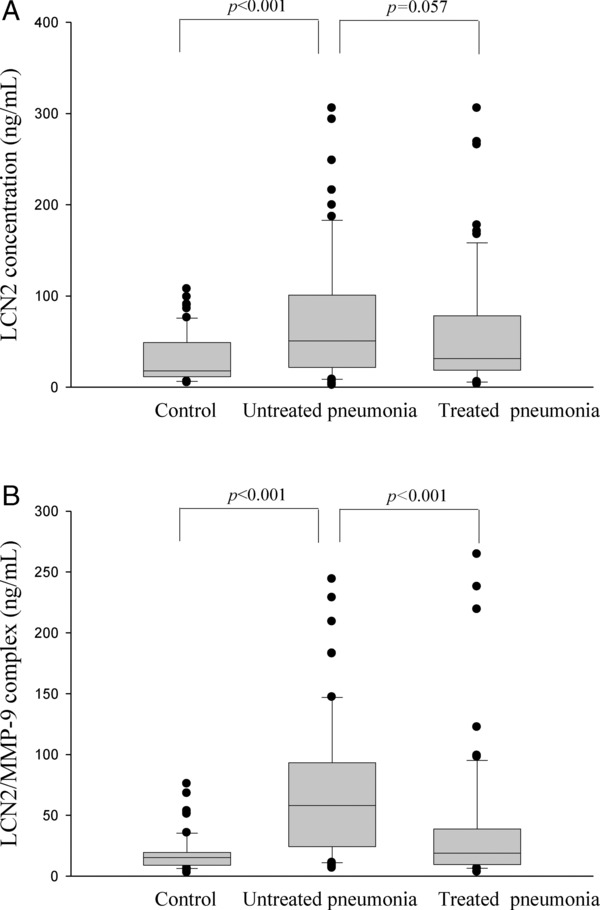
Levels of plasma (A) LCN2 and (B) LCN2/MMP‐9 complex in 61 patients with community‐acquired pneumonia (CAP) and 60 control subjects, as measured by an ELISA analysis before and after antibiotic treatment. (A) The plasma LCN2 level was significantly elevated in patients with CAP before they received treatment compared to the controls (P < 0.001). (B) The plasma LCN2/MMP‐9 complex level was significantly elevated in patients with CAP before they received treatment compared to the controls (P < 0.001) and significantly decreased after the CAP patients received treatment (P < 0.001).
There was a significant correlation between LCN2 levels and WBCs (Spearman's correlation coefficients r = 0.432, P = 0.001, n = 61; Fig. 2A), neutrophils counts (Spearman's correlation coefficients r = 0.411, P = 0.001, n = 61; Fig. 2B), and LCN2/MMP9 complex levels (Spearman's correlation coefficients r = 0.348, P = 0.006, n = 61; Fig. 2C) in the CAP patients. However, no significant correlation was observed between the pretreatment plasma levels of LCN2 and CRP (Spearman's correlation coefficients r = 0.108, P = 0.407, n = 61; Fig. 2D).
Figure 2.
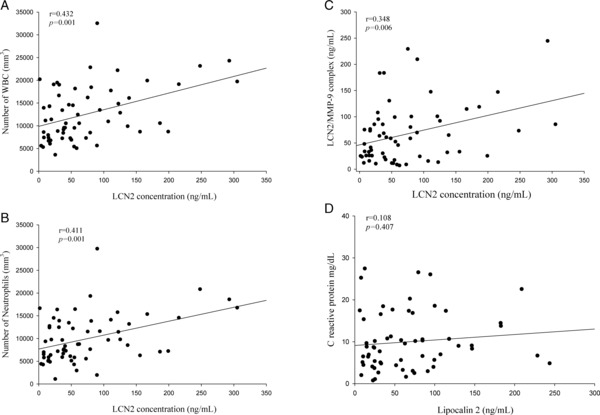
Correlations of plasma LCN2 with WBCs, neutrophils, LCN2/MMP9 complex in 61 patients with community‐acquired pneumonia (CAP). (A) There was a significant correlation between LCN2 levels and WBCs (Spearman's correlation coefficients r = 0.432, P = 0.001, n = 61). (B) There was a significant correlation between LCN2 levels and neutrophils counts (Spearman's correlation coefficients r = 0.411, P = 0.001, n = 61). (C) There was a significant correlation between LCN2 levels and LCN2/MMP9 complex levels (Spearman's correlation coefficients r = 0.348, P = 0.006, n = 61). (D) There was no significant correlation was observed between the pretreatment plasma levels of LCN2 and CRP (Spearman's correlation coefficients r = 0.108, P = 0.407, n = 61).
To further investigate the correlation between LCN2 levels and the severity of CAP, we used PSI, CURB‐65, and APACHE II scores as PSIs. The correlations among PSI, CURB‐65, and APACHE II scores and LCN2 levels in the CAP patients before they received treatment are shown in Figure 3. There were significant correlations between LCN2 and PSI (Spearman's correlation coefficient r = 0.333, P = 0.009, n = 61; Fig. 3A), CURB‐65 (Spearman's correlation coefficient r = 0.288, P = 0.024, n = 61; Fig. 3B), and APACHE II (Spearman's correlation coefficient r = 0.328, P = 0.010, n = 61; Fig. 3C). In contrast to LCN2 levels, the PSI, CURB‐65, and APACHE II scores were not significantly correlated with LCN2/MMP‐9 complex levels in the CAP patients before they received treatment (P = 0.444, 0.381, and 0.972, respectively). Furthermore, the PSI, CURB‐65, and APACHE II scores were also not significantly correlated with CRP levels in the CAP patients before they received treatment (P = 0.726, 0.758, and 0.804, respectively).
Figure 3.
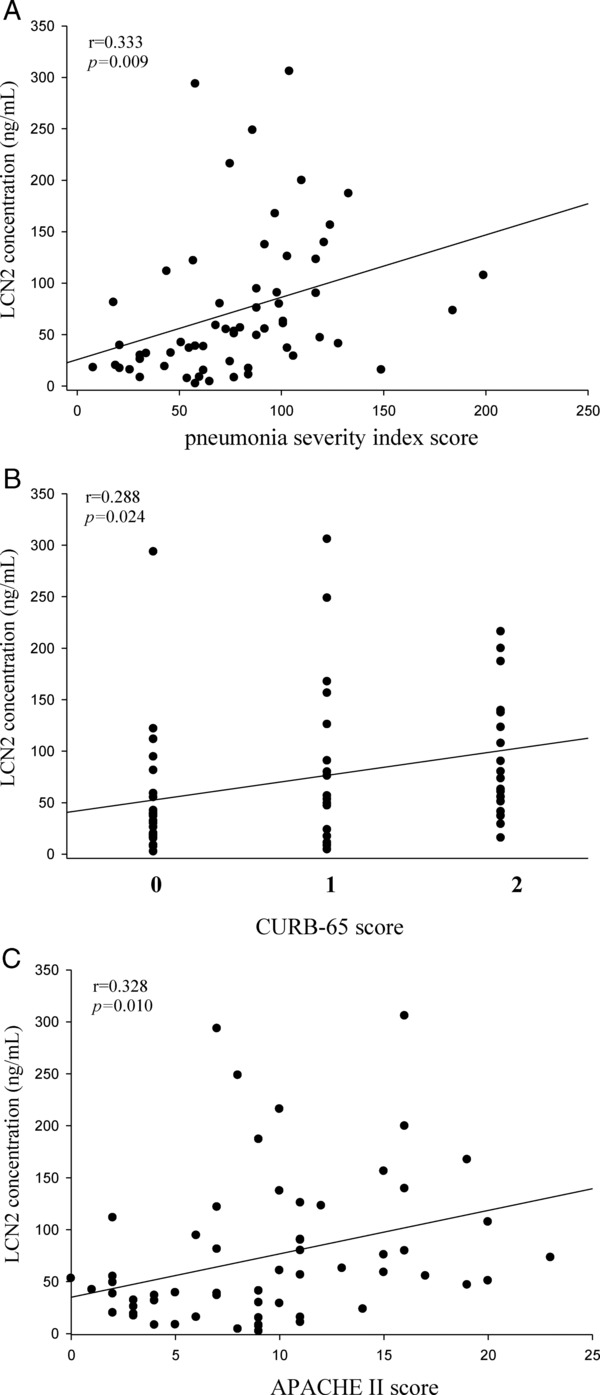
Correlations of plasma LCN2 with PSI, CURB‐65, APACHE II scores in 61 patients with community‐acquired pneumonia (CAP). (A) There was a significant positive correlation between plasma LCN2 levels and PSI scores (Spearman correlation coefficients r = 0.333, P = 0.009). (B) There was a significant positive correlation between plasma LCN2 levels and CURB‐65 scores (Spearman correlation coefficients r = 0.288, P = 0.024). (C) There was a positive correlation between plasma LCN2 levels and APACHE II scores (Spearman correlation coefficients r = 0.328, P = 0.010).
DISCUSSION
The plasma LCN2 levels in patients with CAP decreased after the antibiotic treatment, however, the difference was not statistically significant (P = 0.057), this might be caused by the relatively smaller sample size. Cruz et al. reported that plasma LCN2 was a good predictor of AKI, and they found that the levels of plasma LCN2 were higher in septic patients, compared to nonseptic patients, with AKI 38. This finding implies, though in the same disease, that LCN2 might be highly and differently expressed in severe states of infection. Similarly, we found that high LCN2 levels were associated with several variables (PSI, CURB‐65, and APACHE II scores) that are indicative of CAP disease severity. The present study also showed significantly higher values for CRP levels, WBCs, and neutrophils in CAP patients before treatment, compared to control subjects, as well as significant decreases in those parameters in the same patients after they received antibiotic treatment. Although WBCs, CRP, and other biomarkers, such as procalcitonin (PCT), and N‐terminal pro‐brain natriuretic peptide (NT‐proBND), have been applied as severity‐prediction tools for CAP 39, 40, 41, in our recruited CAP patients, we found no significant correlation between CRP level and the CAP‐severity indices (PSI, CURB‐65, and APACHE II). This discrepancy might have been due to racial differences or the smaller sample size. We suggest that LCN2 might act as a more specific marker than CRP for the diagnosis and clinical assessment of the severity of CAP in Taiwanese populations.
The level of plasma LCN2/MMP‐9 complex has a significant correlation with the level of LCN2, based on our findings. It was demonstrated that LCN2 forms a complex with MMP‐9, and this binding can modify the functionality of its bound ligands 29. Significantly, LCN2 has been shown to protect MMP‐9 from degradation in a dose‐dependent manner, thereby preserving its enzymatic activity and promoting its activity in vitro. Thus, it triggers enhancement of the enzymatic activity of MMP‐9. Therefore, LCN2/MMP‐9 complex level should be directly proportional to LCN2 level. Indeed, in this and our previous 42 studies, we also found that the levels of plasma LCN2/MMP‐9 complex and MMP‐9 are significantly increased in the CAP process, and they are decreased after antibiotic treatment.
PSI and CURB‐65 scores have been widely used to evaluate CAP and to predict mortality 43, 44, 45, 46. Among the disadvantages of both the PSI and CURB‐65 scores are the need to use the most routine clinical parameters and laboratory data, which requires timely clinical assessments of patients with the symptoms and signs of CAP. In addition, there are similar clinical manifestations caused by other infectious pulmonary diseases, such as acute bronchitis, by the acute exacerbation of chronic obstructive pulmonary disease, and by noninfectious diseases, such as congestive heart failure 47. Thus, clinicians cannot use such manifestations as easily in clinical practice. In addition, the diagnostic accuracies of PSI and CURB‐65 scores for the severity assessment of CAP have been debated 48. Use of the APACHE II score to assess the severity of CAP was also reported, and it resulted in greater accuracy than the PSI and CURB‐65 scores. However, similar to the PSI score, it is somewhat difficult to calculate and to use in clinical practice 40.
In this study, CAP patients had significantly higher CRP levels, but the levels were not correlated with the severity of CAP. This finding suggests that CRP is a useful marker of bacterial infection, but it might be less specific than LCN2 for the severity of CAP. The major advantage of LCN2 is its simplicity; it provides serial measurements to evaluate therapeutic response, and it is more sensitive than CRP for evaluating CAP severity. Its weaknesses are that it is relatively expensive, it is nonspecific for severity assessment in CAP patients, and it is not routinely utilized in clinical practice 49. Furthermore, the limitation of this study is the lack of microbial data; different pathogens may have different impacts on the severity of CAP.
In conclusion, plasma LCN2 levels can be used in Taiwanese populations to diagnose the severity of CAP with greater sensitivity than CRP. Plasma LCN2 and its complex with MMP‐9 can be applied to distinguish patients with CAP from healthy subjects and evaluate the effects of antibiotic treatment on CAP patients. In this study, we showed that measuring the plasma levels of LCN2 and LCN2/MMP‐9 complex can be useful in the clinical management of CAP.
CONFLICT OF INTEREST STATEMENT
None declared.
Grant sponsor: Shin Kong Wu Ho‐Su Memorial Hospital; Grant number SKH‐TMU‐101–05 and Taoyuan Armed Forces General Hospital (TAFGH‐99‐11 & 99‐12).
REFERENCES
- 1. Pinner RW, Teutsch SM, Simonsen L, et al. Trends in infectious diseases mortality in the United States. JAMA 1996;275:189–193. [PubMed] [Google Scholar]
- 2. Lee YT, Chen SC, Shyu LY, et al. Significant elevation of plasma cathepsin B and cystatin C in patients with community‐acquired pneumonia. Clin Chim Acta 2012;413:630–635. [DOI] [PubMed] [Google Scholar]
- 3. Fry AM, Shay DK, Holman RC, Curns AT, Anderson LJ. Trends in hospitalizations for pneumonia among persons aged 65 years or older in the United States, 1988–2002. JAMA 2005;294:2712–2719. [DOI] [PubMed] [Google Scholar]
- 4. Thomsen RW, Riis A, Norgaard M, et al. Rising incidence and persistently high mortality of hospitalized pneumonia: A 10‐year population‐based study in Denmark. J Intern Med 2006;259:410–417. [DOI] [PubMed] [Google Scholar]
- 5. Houck PM, Bratzler DW, Nsa W, Ma A, Bartlett JG. Timing of antibiotic administration and outcomes for Medicare patients hospitalized with community‐acquired pneumonia. Arch Intern Med 2004;164:637–644. [DOI] [PubMed] [Google Scholar]
- 6. Kanwar M, Brar N, Khatib R, Fakih MG. Misdiagnosis of community‐acquired pneumonia and inappropriate utilization of antibiotics: Side effects of the 4‐h antibiotic administration rule. Chest 2007;131:1865–1869. [DOI] [PubMed] [Google Scholar]
- 7. Tetley TD. New perspectives on basic mechanisms in lung disease. 6. Proteinase imbalance: Its role in lung disease. Thorax 1993;48:560–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Atkinson JJ, Senior RM. Matrix metalloproteinase‐9 in lung remodeling. Am J Respir Cell Mol Biol 2003;28:12–24. [DOI] [PubMed] [Google Scholar]
- 9. Lukkarinen H, Hogmalm A, Lappalainen U, Bry K. Matrix metalloproteinase‐9 deficiency worsens lung injury in a model of bronchopulmonary dysplasia. Am J Respir Cell Mol Biol 2009;41:59–68. [DOI] [PubMed] [Google Scholar]
- 10. Torii K, Iida K, Miyazaki Y, et al. Higher concentrations of matrix metalloproteinases in bronchoalveolar lavage fluid of patients with adult respiratory distress syndrome. Am J Respir Crit Care Med 1997;155:43–46. [DOI] [PubMed] [Google Scholar]
- 11. Choi KH, Lee HB, Jeong MY, et al. The role of matrix metalloproteinase‐9 and tissue inhibitor of metalloproteinase‐1 in cryptogenic organizing pneumonia. Chest 2002;121:1478–485. [DOI] [PubMed] [Google Scholar]
- 12. Segura‐Valdez L, Pardo A, Gaxiola M, Uhal BD, Becerril C, Selman M. Upregulation of gelatinases A and B, collagenases 1 and 2, and increased parenchymal cell death in COPD. Chest 2000;117:684–694. [DOI] [PubMed] [Google Scholar]
- 13. Piesiak P, Brzecka A, Kosacka M, Passowicz‐Muszynska E, Dyla T, Jankowska R. Concentrations of matrix metalloproteinase‐9 and tissue inhibitor of metalloproteinases‐1 in serum of patients with chronic obstructive pulmonary disease. Pol Merkur Lekarski 2011;31:270–273. [PubMed] [Google Scholar]
- 14. Cowland JB, Borregaard N. Molecular characterization and pattern of tissue expression of the gene for neutrophil gelatinase‐associated lipocalin from humans. Genomics 1997;45:17–23. [DOI] [PubMed] [Google Scholar]
- 15. Bratt T. Lipocalins and cancer. Biochim Biophys Acta 2000;1482:318–326. [DOI] [PubMed] [Google Scholar]
- 16. Saiga H, Nishimura J, Kuwata H, et al. Lipocalin 2‐dependent inhibition of mycobacterial growth in alveolar epithelium. J Immunol 2008;181:8521–8527. [DOI] [PubMed] [Google Scholar]
- 17. Devarajan P. Neutrophil gelatinase‐associated lipocalin (NGAL): A new marker of kidney disease. Scand J Clin Lab Invest Suppl 2008;241:89–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bolignano D, Donato V, Lacquaniti A, et al. Neutrophil gelatinase‐associated lipocalin (NGAL) in human neoplasias: A new protein enters the scene. Cancer Lett 2010;288:10–16. [DOI] [PubMed] [Google Scholar]
- 19. Lin CW, Tseng SW, Yang SF, et al. Role of lipocalin 2 and its complex with matrix metalloproteinase‐9 in oral cancer. Oral Dis 2012;18:734–740. [DOI] [PubMed] [Google Scholar]
- 20. Bolignano D, Della Torre A, Lacquaniti A, Costantino G, Fries W, Buemi M. Neutrophil gelatinase‐associated lipocalin levels in patients with crohn disease undergoing treatment with infliximab. J Investig Med 2010;58:569–571. [DOI] [PubMed] [Google Scholar]
- 21. Devarajan P. NGAL in acute kidney injury: From serendipity to utility. Am J Kidney Dis 2008;52:395–399. [DOI] [PubMed] [Google Scholar]
- 22. Ding L, Hanawa H, Ota Y, et al. Lipocalin‐2/neutrophil gelatinase‐B associated lipocalin is strongly induced in hearts of rats with autoimmune myocarditis and in human myocarditis. Circ J 2010;74:523–530. [DOI] [PubMed] [Google Scholar]
- 23. Gupta K, Shukla M, Cowland JB, Malemud CJ, Haqqi TM. Neutrophil gelatinase‐associated lipocalin is expressed in osteoarthritis and forms a complex with matrix metalloproteinase 9. Arthritis Rheum 2007;56:3326–3335. [DOI] [PubMed] [Google Scholar]
- 24. Tsai HT, Su PH, Lee TH, et al. Significant elevation and correlation of plasma neutrophil gelatinase associated lipocalin and its complex with matrix metalloproteinase‐9 in patients with pelvic inflammatory disease. Clin Chim Acta 2011;412:1252–1256. [DOI] [PubMed] [Google Scholar]
- 25. Chan YR, Liu JS, Pociask DA, et al., Lipocalin 2 is required for pulmonary host defense against Klebsiella infection. J Immunol 2009;182:4947–4956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Gupta N, Krasnodembskaya A, Kapetanaki M, et al., Mesenchymal stem cells enhance survival and bacterial clearance in murine Escherichia coli pneumonia. Thorax 2012;67:533–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Srinivasan G, Aitken JD, Zhang B, et al., Lipocalin 2 deficiency dysregulates iron homeostasis and exacerbates endotoxin‐induced sepsis. J Immunol 2012;189:1911–1919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Chakraborty S, Kaur S, Muddana V, et al. Elevated serum neutrophil gelatinase‐associated lipocalin is an early predictor of severity and outcome in acute pancreatitis. Am J Gastroenterol 2010;105:2050–2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Yan L, Borregaard N, Kjeldsen L, Moses MA. The high molecular weight urinary matrix metalloproteinase (MMP) activity is a complex of gelatinase B/MMP‐9 and neutrophil gelatinase‐associated lipocalin (NGAL). Modulation of MMP‐9 activity by NGAL. J Biol Chem 2001;276:37258–37265. [DOI] [PubMed] [Google Scholar]
- 30. Fernandez CA, Yan L, Louis G, Yang J, Kutok JL, Moses MA. The matrix metalloproteinase‐9/neutrophil gelatinase‐associated lipocalin complex plays a role in breast tumor growth and is present in the urine of breast cancer patients. Clin Cancer Res 2005;11:5390–5395. [DOI] [PubMed] [Google Scholar]
- 31. Smith ER, Zurakowski D, Saad A, Scott RM, Moses MA. Urinary biomarkers predict brain tumor presence and response to therapy. Clin Cancer Res 2008;14:2378–2386. [DOI] [PubMed] [Google Scholar]
- 32. Kubben FJ, Sier CF, Hawinkels LJ, et al. Clinical evidence for a protective role of lipocalin‐2 against MMP‐9 autodegradation and the impact for gastric cancer. Eur J Cancer 2007;43:1869–1876. [DOI] [PubMed] [Google Scholar]
- 33. Haase‐Fielitz A, Bellomo R, Devarajan P, et al. The predictive performance of plasma neutrophil gelatinase‐associated lipocalin (NGAL) increases with grade of acute kidney injury. Nephrol Dial Transplant 2009;24:3349–3354. [DOI] [PubMed] [Google Scholar]
- 34. Mandell LA, Wunderink RG, Anzueto A, et al. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community‐acquired pneumonia in adults. Clin Infect Dis 2007;44(Suppl 2):S27–S72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Fine MJ, Auble TE, Yealy DM, et al. A prediction rule to identify low‐risk patients with community‐acquired pneumonia. N Engl J Med 1997;336:243–250. [DOI] [PubMed] [Google Scholar]
- 36. Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: A severity of disease classification system. Crit Care Med 1985;13:818–829. [PubMed] [Google Scholar]
- 37. Lim WS, van der Eerden MM, Laing R, et al. Defining community acquired pneumonia severity on presentation to hospital: An international derivation and validation study. Thorax 2003;58:377–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Cruz DN, de Cal M, Garzotto F, et al. Plasma neutrophil gelatinase‐associated lipocalin is an early biomarker for acute kidney injury in an adult ICU population. Intensive Care Med 2010;36:444–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Chalmers JD, Singanayagam A, Hill AT. C‐reactive protein is an independent predictor of severity in community‐acquired pneumonia. Am J Med 2008;121:219–225. [DOI] [PubMed] [Google Scholar]
- 40. Jeong KY, Kim K, Kim TY, et al. Prognostic value of N‐terminal pro‐brain natriuretic peptide in hospitalised patients with community‐acquired pneumonia. Emerg Med J 2011;28:122–127. [DOI] [PubMed] [Google Scholar]
- 41. Muller F, Christ‐Crain M, Bregenzer T, et al. Procalcitonin levels predict bacteremia in patients with community‐acquired pneumonia: A prospective cohort trial. Chest 2010;138:121–129. [DOI] [PubMed] [Google Scholar]
- 42. Chiang TY, Shyu LY, Tsao TC, et al. Elevated plasma matrix metalloproteinase‐9 protein and its gene polymorphism in patients with community‐acquired pneumonia. Clin Chem Lab Med 2012;50:449–454. [DOI] [PubMed] [Google Scholar]
- 43. Arnold FW, Brock GN, Peyrani P, et al. Predictive accuracy of the pneumonia severity index vs CRB‐65 for time to clinical stability: Results from the Community‐Acquired Pneumonia Organization (CAPO) International Cohort Study. Respir Med 2010;104:1736–1743. [DOI] [PubMed] [Google Scholar]
- 44. Cilloniz C, Ewig S, Polverino E, et al. Microbial aetiology of community‐acquired pneumonia and its relation to severity. Thorax 2011;66:340–346. [DOI] [PubMed] [Google Scholar]
- 45. Kollef KE, Reichley RM, Micek ST, Kollef MH. The modified APACHE II score outperforms CURB65 pneumonia severity score as a predictor of 30‐day mortality in patients with methicillin‐resistant Staphylococcus aureus pneumonia. Chest 2008;133:363–369. [DOI] [PubMed] [Google Scholar]
- 46. Richards G, Levy H, Laterre PF, et al. CURB‐65, PSI, and APACHE II to assess mortality risk in patients with severe sepsis and community acquired pneumonia in PROWESS. J Intens Care Med 2011;26:34–40. [DOI] [PubMed] [Google Scholar]
- 47. Muller B, Harbarth S, Stolz D, et al. Diagnostic and prognostic accuracy of clinical and laboratory parameters in community‐acquired pneumonia. BMC Infect Dis 2007;7:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Niederman MS, Feldman C, Richards GA. Combining information from prognostic scoring tools for CAP: An American view on how to get the best of all worlds. Eur Respir J 2006;27:9–11. [DOI] [PubMed] [Google Scholar]
- 49. Singanayagam A, Chalmers JD, Hill AT. Severity assessment in community‐acquired pneumonia: A review. QJM 2009;102:379–388. [DOI] [PubMed] [Google Scholar]
- 50. Polzin A, Pletz M, Erbes R, et al. Procalcitonin as a diagnostic tool in lower respiratory tract infections and tuberculosis. Eur Respir J 2003;21:939–943. [DOI] [PubMed] [Google Scholar]
- 51. Tateda K, Kusano E, Matsumoto T, et al. Semi‐quantitative analysis of Streptococcus pneumoniae urinary antigen: Kinetics of antigen titers and severity of diseases. Scand J Infect Dis 2006;38:166–171. [DOI] [PubMed] [Google Scholar]


