Abstract
Background
The aim of this study was to evaluate the changing of TK1 (where TK is thymidine kinase) activity before and after adjuvant chemotherapy in patients with breast and colorectal cancer.
Methods
The study included 16 breast cancer, 25 colorectal cancer, and 38 healthy volunteers as the control group. Blood samples were taken twice from each patient; first at the beginning of the chemotherapy and second after six cycles of chemotherapy. TK1 activity was measured enzyme immunoassay method.
Results
The mean TK1 activity in the breast and colorectal cancer was significantly higher than the controls. TK1 activity in the colorectal cancer was higher than the breast cancer but this difference was not significant. TK1 activity after six doses of chemotherapy was lower than baseline TK1 activity before the start of chemotherapy in breast and colorectal cancer. TK1 activity was positively correlated with CA15–3, before and after chemotherapy in patients with breast cancer. TK1 activity in the colorectal cancer was also positively correlated with CA19–9, before and after chemotherapy. The values for the cutoff point, sensitivity, specificity, and the area under curve were determined for TK1 as >44.36 Du/L, 68.29%, 100% and 0.819, respectively in all subjects.
Conclusion
Our results showed that serum TK1 activity in patients with breast and colorectal cancer was significantly higher than that of the healthy controls. Moreover, after the completion of chemotherapy the values were lower than baseline. Pretreatment TK1 activity should be considered as a useful marker for assessment tumor cell proliferation in breast and colorectal cancer. Further work is needed to understand TK1 activity better in large populations of patients with solid tumor.
Keywords: thymidine kinase activity, breast cancer, colorectal cancer, chemotherapy
INTRODUCTION
Cancer is a leading cause of death in the world, and the number of affected individuals is increasing, although different methods of treatment for cancer, for example, surgery, radiotherapy, chemotherapy, and endocrine therapy have been improved tremendously in recent years. Cancer can be treated effectively, if discovered at an early stage. Diagnostic and prognostic markers play a key role in classifying tumors and determining the best treatment plan for a patient 1.
Thymidine kinase (TK) is a cellular enzyme involved in a salvage pathway for DNA synthesis. There are two isoforms of this enzyme: TK1 and TK2. TK1 is found in the cytoplasm of dividing cells and is absent in resting cells. TK2 is located in the mitochondria of resting cells. TK1 is a soluble biomarker associated with DNA synthesis which has been used for prognosis and monitoring of treatment of lymphoma and leukemia since 1980, and also to some extent in patients with solid tumors 1, 2, 3, 4, 5, 6. TK1 is an enzyme linked to DNA synthesis, and thus expressed in proliferating cells, both nonmalignant and malignant. The concentration and activity of TK1 in blood serum of cancer patients are elevated, while TK1 in serum of healthy people is low or undetectable. However, in some healthy people showing acute illness (infection, inflammation) or exhibiting other physiological changes (menstruation, blood donor, surgery), TK1 could increase transiently 1.
Recently, among the available serum markers, TK1 has been the most widely tested in solid tumors, including gastric cancer, particularly for monitoring the effect of tumor therapies, prognosis, and follow‐up. Yet, its role as a marker of treatment efficacy is still contradictory to date 7. The serum TK1 assay is commonly used to follow systemic chemotherapy in human and animals 7, 8, 9, 10, 11, 12. The effect of treatments that do not in themselves influence the TK1 value can be concluded from values obtained before and after a treatment cycle: if the TK1 level decreases, therapy can be concluded to be effective, while increasing TK1 levels indicate that the therapy is inefficient.
TK1 is still unclear whether they can be used to predict the response to treatment. The aim of the present study was to investigate the changing of the serum TK1 activity before and after chemotherapy treatment as a surrogate marker and the relationship between these values and tumor markers was also evaluated in patients with breast and colorectal cancer who received adjuvant chemotherapy.
MATERIALS AND METHODS
A prospective, cross‐sectional study was performed on 16 breast cancer patients, consecutive 25 colorectal cancer patients whom were seen at outpatient clinic of the oncology department of Cerrahpasa Medical Faculty, Istanbul. Complete history, physical and laboratory examinations were obtained from all patients. All patients were assessed by the same medical oncologist for received chemotherapy. The study was approved by the Ethics Committee of Cerrahpasa Medical Faculty, and every patient and healthy volunteers gave informed consent.
We evaluated clinicopathological features (histology, menopausal status, estrogen receptor (ER), and progesterone receptor (PR) status, number of axillary lymph nodes involved, grade, tumor size, and stage according to the American Joint Committee on Cancer (AJCC) staging system) in patients with breast cancer. All patients underwent standard treatment protocol for adjuvant chemotherapy. We used the following adjuvant chemotherapies: epirubicin (100 mg/m2) plus cyclophosphamide (600 mg/m2) plus 5‐fluorouracil (5‐FU) (600 mg/m2).
Twenty five patients with newly diagnosed and histologically confirmed primary colorectal cancer were included in this prospective study. Tumor staging was performed according to AJCC, Tumor, Lymph nodes, Metastasis (TNM) staging classification. All patients underwent standard treatment protocol for adjuvant chemotherapy. We used the following adjuvant chemotherapies: oxaliplatin (85 mg/m2) plus folic acid (30 mg/m2) plus 5‐FU (500 mg/m2).
We also included 38 healthy volunteers as the control group. These volunteers were tested for TK1 and also blood and urine tests; imaging examination and physical examination were held on them. Volunteers with systemic infections, inflammatory diseases, or history of recent surgery were excluded.
Sample Collection and Preparation
For all persons, clinical parameters including routine biochemical parameters were measured using the standard protocols. Blood samples were taken twice from each patient; first about 1 month after surgery at the beginning of the chemotherapy and second 1 month after six doses of chemotherapy in anticoagulant‐free tubes after an overnight fasting. Serum were separated immediately and stored at −80°C until analysis.
MEASUREMENT OF SERUM TK1 ACTIVITY
Serum TK1 activity was measured in duplicate aliquots, using a human enzyme‐linked immunosorbent assay (ELISA, DiviTum assay) in accordance with the manufacturer's instructions (BIOVICA, Uppsala, Sweden). This assay uses bromo‐deoxyuridine which is phosphorylated to its monophosphate as substrate. In order to immobilize and remove the monophosphate produced from the solution it is further phosphorylated to the tri‐phosphate by kinases present in the reaction solution. The tri‐phosphate is immobilized by DNA synthesis in which a polyA strand covalently bound to the microtiter plate well, acts as template, reverse transcriptase as the catalyst, and odT as the primer. After the TK1 activity incubation is completed, the plate is washed and incubated with an antibromodeoxyuridine antibody conjugated to alkaline phosphatase followed by a second wash. The alkaline phosphatase correlates with the TK1 activity in the sample. This amount of alkaline phosphatase is evaluated using a chromogenic substrate, p‐nitrophenyl phosphate. The coefficients of intra‐ and interassay variations were 4.3% (n = 10) and 5.0% (n = 10), respectively.
The other tumor markers were measured by chemiluminescence immunassay with Abbott I200 analyzer (Chicago, IL).
Statistical Analysis
Data are presented as the mean ± SEM. The unpaired Student's t or Mann–Whitney U tests were used to compare continuous variables between the two groups. Correlations analysis was tested using Pearson's correlation. Differences were considered statistically significant when P < 0.05. To assess the diagnostic accuracy, we performed receiver operating characteristic (ROC) curve analysis. The area under the ROC curve (AUC) was then estimated. Data were analyzed by statistical software (SPSS for Windows 15.0; SPSS, Chicago, IL).
RESULTS
The demographic features and tumor markers of all groups are presented in the Table 1. Age was not different among the groups. The mean TK1 activity in the breast and colorectal cancer was significantly higher than the controls (83.73 ± 76.44 Du/L; 95.93 ± 73.11 Du/L; 25.29 ± 11.84 Du/L, P < 0.001; P < 0.001, respectively). TK1 activity in the colorectal cancer was higher than the breast cancer but this difference was not significant. TK1 activity after six doses of chemotherapy (48.17 ± 41.61 Du/L and 41.09 ± 26.59 Du/L, respectively) was lower than baseline TK1 activity before the start of chemotherapy in breast and colorectal cancer (Fig. 1). CA 19–9 levels and TK1 activities were not found to be significantly different between colorectal cancer patients with metastase and without metastase.
Table 1.
Demographic Features and Tumor Markers in Control and Patient Groups
| Control, | Breast | Colorectal | |
|---|---|---|---|
| n: 38 | cancer, n:16 | cancer, n: 25 | |
| Age (year) | 50.42 ± 11.34 | 49.63 ± 10.63 | 55.56 ± 9.23 |
| Sex (F/M) | 16/22 | 16/0 | 18/7 |
| CA19–9 (U/mL) | – | – | 41.88±91.36 |
| CA15–3 (U/mL) | – | 25.96±15.03 | – |
| TK1 (Du/L) | 25.29 ± 11.84 | 83.73 ± 76.44a, ** | 95.93 ± 73.11a, *** |
Statistical difference vs control.
**P < 0.01, ***P < 0.001
Figure 1.
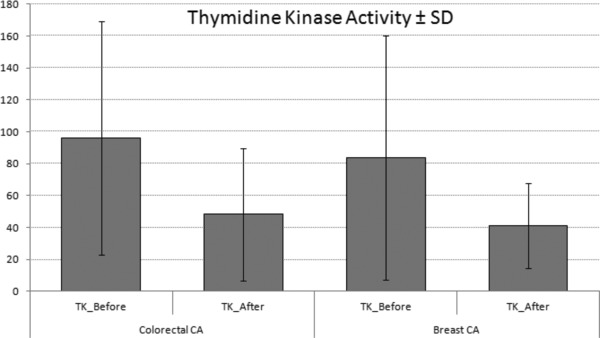
TK1 activity in cancer patients before and after chemotherapy.
There was positive correlation between serum TK1 activity before and after chemotherapy in patients with breast cancer (r = 0.704; P = 0.002; Fig. 2A). There was also established positive correlation between serum TK1 activity before and after chemotherapy in patients with colorectal cancer (r = 0.788; P < 0.001; Fig. 2B). TK1 activity was positively correlated with CA15–3 levels before chemotherapy in patients with breast cancer(r = 0.689; P = 0.003; Fig. 2C). TK1 activity in the colorectal cancer was also positively correlated with CA19–9 (P < 0.001) before chemotherapy (r = 0.711; P < 0.001; Fig. 2D).
Figure 2.
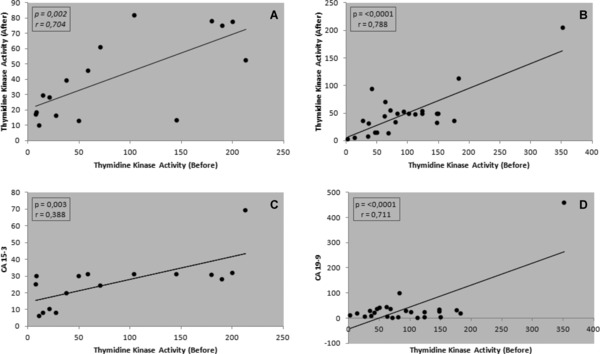
(A) Positive correlation between serum TK1 activity in patients with breast cancer before and after chemotherapy. (B) Positive correlation between serum TK1 activity in patients with colorectal cancer before and after chemotherapy. (C) Positive correlation between serum TK1 activity and CA15–3 levels in patients with breast cancer. (D) Positive correlation between serum TK1 activity and CA19–9 in patients with colorectal cancer.
A comparison of ROC characteristics for TK1 in all subjects is shown in Figure 3. The values for the cutoff point, sensitivity, specificity, and the area under curve were determined for TK1 as >44.36 Du/L, 68.29%, 100%, and 0.819, respectively.
Figure 3.
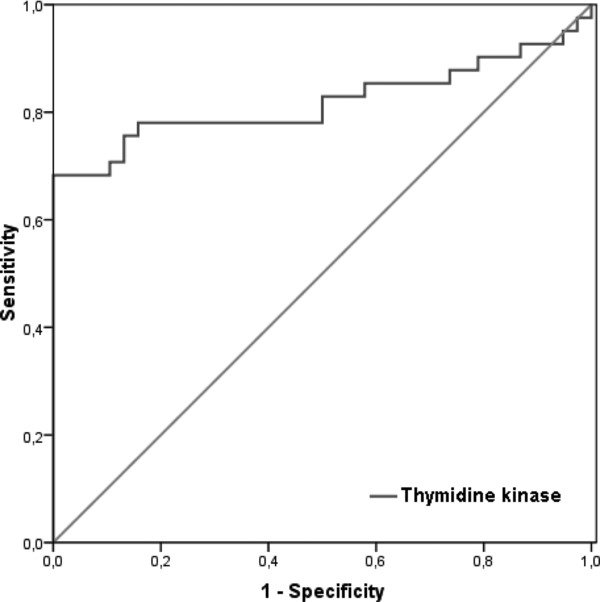
Comparison of ROC characteristics for TK1 in all subjects.
DISCUSSION
TK1 plays an essential role in the processing of thymidine within the cell and therefore it is an important marker of proliferation, particularly in tumor cells. Serum TK1 activity assays have found their major clinical application for the prognosis and the monitoring of therapy in blood malignancies. The assay used in the present study is a nonradioactive immunological alternative to the traditional TK1 radioimmunoassay. This novel patented procedure has no product feed‐back inhibition, the enzyme works under conditions of substrate saturation and has been found to give at least a ten times higher sensitivity for detection of growing tumor mass than previous TK1 assays. This would facilitate the use of TK1 as a tumor marker 13. Benjamin et al. 14 compared two recently developed immunoassays for serum TK1 activity: one manual assay (DiviTum, Biovica®) and one fully automated assay (Liaison, Diasorin®). In spite of differences observed between TK1 activity measured by the DiviTum and Liaison assays, both of them may be used for recurrence prediction in preoperative evaluation of patients with primary breast cancer. Serum TK1 activity was measured by the DiviTum in this study.
Similar to our study, serum TK1 activity is significantly elevated in patients with malignancies, as compared with levels in patients with benign tumors and those in healthy individuals 15, 16, 17, 18, 19, 20, 21, 22. However, in some healthy people showing acute illness (infection, inflammation) or exhibiting other physiological changes (menstruation, blood donor, surgery), TK1 could increase transiently. There was about 30 times higher risk to develop new malignancies in the elevated TK1 group compared to the normal TK1 group. STK1 assay can distinguish between people with low or high risk of developing malignancies, before any indication of visible tumors. The reason(s) behind the differences in the TK1 levels of healthy people and people with malignancies are still not fully understood 1. Li et al. 23 demonstrated that transient increases of TK1 in serum after surgery of patients with carcinoma. The transiently elevated TK1 values postoperation might be due to surgery‐induced complications, such as anemia and infection/inflammation, but also to operation execution times and age of patients. TK1 should not be used within 1 week postoperation, but before surgery and after 1 month to avoid nontumor‐related increases in TK1, and thus misleading results. Zhang et al. 24 reported that TK1 gradually declined, being 66% lower after 1 week. TK1 reached the level of healthy controls at 1 month and remained there for at least 6 months postoperatively until this study ended. Surgery could lead to a transient increase of TK1 activity resulting from postoperative wound healing.
The regimens used in adjuvant treatment of breast and colon cancer included 5‐FU, which is an irreversible inhibitor of thymidylate synthase (TS). Inhibition of TS causes the activation of TK1 as a salvage way for synthesis of thymidine monophophate (TMP), so‐called “flare response” 25, which was observed by Topolcan et al. 26 during adjuvant and palliative chemotherapy in colorectal cancer after every chemotherapy cycle. 5‐FU treatment leads to TK1 induction. Liu et al. 7 reported that after 2 and 4 months of chemotherapy, the TK1 values decreased significantly in the patients without recurrence, while in patients with recurrence, the STK1 values increased significantly after 2 months of chemotherapy compared with that before chemotherapy. Particularly, after 1 month of chemotherapy, the corresponding TK1 values started to decline in the patients without recurrence and to increase in the patients with recurrence. Therefore, its role as a marker of treatment efficacy is still paradoxical to date. Although the number of patients was limited in this study, the TK1 activity before chemotherapy was also high in serum, but a decrease was observed 1 month following therapy in patients with breast and colorectal cancer. Serum TK1 activity might possess an important reference value in the evaluation of treatment and prognosis of breast and colorectal cancer following chemotherapy. Increased TK1 activity at the beginning of the therapy may improve the ability to predict changes in the tumor burden. The level of serum TK1 is not only a sensitive marker of tumor, but also reflects tumor burden and delivers prognostic information in breast and colorectal cancer. Results of our study is enough to confirm this in cancer patients 6, 8, 13, 17, 18, 20, 21, 22, 23, 24, 25, 26, 27. Nisman et al. 27 suggested that elevated serum TK1 is an important risk factor indicating a high proliferation potential of tumors at the time of excision. In multivariate analysis, TK1 activity was found to be an independent prognostic factor in terms of recurrence‐free survival in breast cancer patients.
CA 15–3 (also known as MUC1) is the most widely used serum marker and one of the first circulating prognostic factors in breast cancer. In multivariate analysis CA 15–3 as a prognostic marker was independent of both tumor size and nodal status. The most important clinical application of CA 15–3 is in monitoring therapy in patients with advanced breast cancer that is not assessable by existing clinical or radiologic procedures 28, 29. TK1 activity was positively correlated with CA15–3 before and after chemotherapy in patients with breast cancer in our study. Elfagieh et al. 30 showed that the combined data set of the CEA, CA 15.3, and TK1 concentrations’ data from three markers increased the diagnostic sensitivity to 90%. The serum marker analysis for CEA, CA 15.3, and TK1 concentrations is shown to be a useful tool for identification of malignant cases in breast cancer population and for the prognostic evaluation of patients with primary breast cancer. Increased concentrations of the markers were also observed to be higher in patients with advanced tumors and indicative of the development of distant metastasis.
CA 19–9 was the marker with the highest sensitivity in colorectal cancer 31. Lin et al. 32 demonstrated that CA19–9 may be a prognostic factor for colorectal cancer patients with normal CEA levels. CA 19–9 are also well‐established oncological biomarker used in the follow‐up care and prognosis of colorectal cancer patients 33. On the other hand, elevated CA 19–9 is related to an unfavorable prognosis, in terms of cancer recurrence and mortality rate. TK1 activity was positively correlated with CA19–9 in patients with colorectal cancer in our study. According to our hypothesis, TK1 activity should increase in colorectal cancer patients with metastase. However, CA 19–9 levels and TK1 activities were not found to be significantly different between colorectal cancer patients with metastase and without metastase. This indicates a more complex regulation of the serum TK1 activity of some patients. It may also be possible that these patients had a metastase but not had come up yet. On the other hand if populations of patients with metastase (n:12) was higher, we could have found a different result.
In conclusion, the present study shows that TK1 activity can be a potential tumor marker in breast and colorectal cancer. A very important use of a laboratory test for oncological applications is to predict the outcome of patient treatment. TK1 assay is fast, sensitive, economical, and easily performed for measuring serum TK1 activity. These findings obtained by ELISA could provide the basis for future studies of the clinical utility of the assay. Our results show that the test is also used in particular to monitor the effect of chemotherapy because it has a short biological half‐life and participates in DNA synthesis 26. Therefore, this enzyme may be served as a tumor marker in future. Further work is needed to understand TK1 activity better in longer period of follow‐up and a larger population of patients with solid tumor.
CONFLICT OF INTEREST
The authors declare that they have no conflicts of interest related to the publication of this article.
REFERENCES
- 1. Chen ZH, Huang SQ, Wang Y, et al. Serological thymidine kinase 1 is a biomarker for early detection of tumours—a health screening study on 35,365 people, using a sensitive chemiluminescent dot blot assay. Sensors (Basel) 2011;11:11064–11080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kallander CF, Simonsson B, Hagberg H, Gronowitz JS. Serum deoxythymidine kinase gives prognostic information in chronic lymphocytic leukemia. Cancer 1984;54:2450–2455. [DOI] [PubMed] [Google Scholar]
- 3. Hallek M, Wanders L, Strohmeyer S, Emmerich B. Thymidine kinase: A tumor marker with prognostic value for non‐Hodgkin's lymphoma and a broad range of potential clinical applications. Ann Hematol 1992;65:1–5. [DOI] [PubMed] [Google Scholar]
- 4. O'Neill KL Buckwalter MR, Murray BK. Thymidine kinase: Diagnostic and prognostic potential. Expert Rev Mol Diagn 2001;1:428–433. [DOI] [PubMed] [Google Scholar]
- 5. Topolcan O, Holubec L, Jr . The role of thymidine kinase in cancer diseases. Expert Opin Med Diagn 2008;2:129–141. [DOI] [PubMed] [Google Scholar]
- 6. Broet P, Romain S, Daver A, et al. Thymidine kinase as a proliferative marker: Clinical relevance in 1,692 primary breast cancer patients. J Clin Oncol 2001;19:2778–2787. [DOI] [PubMed] [Google Scholar]
- 7. Liu Y, Ling Y, Qi Q, et al. Changes in serum thymidine kinase 1 levels during chemotherapy correlate with objective response in patients with advanced gastric cancer. Exp Ther Med 2011;2:1177–1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Huang ZH, Tian XS, Li R, et al. Elevated thymidine kinase 1 in serum following neoadjuvant chemotherapy predicts poor outcome for patients with locally advanced breast cancer. Exp Ther Med 2012;3:331–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gowda A, Byrd JC. Use of prognostic factors in risk stratification at diagnosis and time of treatment of patients with chronic lymphocytic leukemia. Curr Opin Hematol 2006;13:266–272. [DOI] [PubMed] [Google Scholar]
- 10. Xu XH, Zhang YM, Shu XH, et al. Serum thymidine kinase 1 reflects the progression of pre‐malignant and malignant tumors during therapy. Mol Med Report 2008;1:705–711. [DOI] [PubMed] [Google Scholar]
- 11. von Euler H, Einarsson R, Olsson U, Lagerstedt AS, Eriksson S. Serum thymidine kinase activity in dogs with malignant lymphoma: A potent marker for prognosis and monitoring the disease. J Vet Intern Med 2004;18:696–702. [DOI] [PubMed] [Google Scholar]
- 12. Sharif H, von Euler H, Westberg S, He E, Wang L, Eriksson S. A sensitive and kinetically defined radiochemical assay for canine and human serum thymidine kinase 1 (TK1) to monitor canine malignant lymphoma. Vet J 2012;194:40–47. [DOI] [PubMed] [Google Scholar]
- 13. Carlsson L, Larsson A, Lindman H. Elevated levels of thymidine kinase 1 peptide in serum from patients with breast cancer. Ups J Med Sci 2009;114:116–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Nisman B, Allweis T, Kadouri L, et al. Comparison of diagnostic and prognostic performance of two assays measuring thymidine kinase 1 activity in serum of breast cancer patients. Clin Chem Lab 2012;51:439–447. [DOI] [PubMed] [Google Scholar]
- 15. Luo P, He E, Eriksson S, et al. Thymidine kinase activity in serum of renal cell carcinoma patients is a useful prognostic marker. Eur J Cancer Prev 2009;18:220–224. [DOI] [PubMed] [Google Scholar]
- 16. Pan ZL, Ji XY, Shi YM, Zhou J, He E, Skog S. Serum thymidine kinase 1 concentration as a prognostic factor of chemotherapy‐treated non‐Hodgkin's lymphoma patients. J Cancer Res Clin Oncol 2010;136:1193–1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chen Y, Ying M, Chen Y, et al. Serum thymidine kinase 1 correlates to clinical stages and clinical reactions and monitors the outcome of therapy of 1,247 cancer patients in routine clinical settings. Int J Clin Oncol 2010;15:359–368. [DOI] [PubMed] [Google Scholar]
- 18. He E, Xu XH, Guan H, et al. Thymidine kinase 1 is a potential marker for prognosis and monitoring the response to treatment of patients with breast, lung, and esophageal cancer and non‐Hodgkin's lymphoma. Nucleosides Nucleotides Nucleic Acids 2010;29:352–358. [DOI] [PubMed] [Google Scholar]
- 19. Zou L, Zhang PG, Zou S, Li Y, He Q. The half‐life of thymidine kinase 1 in serum measured by ECL dot blot: A potential marker for monitoring the response to surgery of patients with gastric cancer. Int J Biol Markers 2002;17:135–140. [DOI] [PubMed] [Google Scholar]
- 20. He Q, Zhang P, Zou L, et al. Concentration of thymidine kinase 1 in serum (S‐TK1) is a more sensitive proliferation marker in human solid tumors than its activity. Oncol Rep 2005;14:1013–1019. [PubMed] [Google Scholar]
- 21. Foekens JA, Romain S, Look MP, Martin PM, Klijn JG. Thymidine kinase and thymidylate synthase in advanced breast cancer: Response to tamoxifen and chemotherapy. Cancer Res 2001;61:1421–1425. [PubMed] [Google Scholar]
- 22. Svobodova S, Topolcan O, Holubec L, et al. Prognostic importance of thymidine kinase in colorectal and breast cancer. Anticancer Res 2007;27:1907–1909. [PubMed] [Google Scholar]
- 23. Li Z, Wang Y, Ma J, et al. Transient increase in serum thymidine kinase 1 within one week after surgery of patients with carcinoma. Anticancer Res 2010;30:1295–1299. [PubMed] [Google Scholar]
- 24. Zhang J, Jia Q, Zou S, et al. Thymidine kinase 1: A proliferation marker for determining prognosis and monitoring the surgical outcome of primary bladder carcinoma patients. Oncol Rep 2006;15:455–461. [PubMed] [Google Scholar]
- 25. Lee SJ, Kim SY, Chung JH, et al. Induction of thymidine kinase 1 after 5‐fluorouracil as a mechanism for 3’‐deoxy‐3’‐[18F]fluorothymidine flare. Biochem Pharmacol 2010;80:1528–1536. [DOI] [PubMed] [Google Scholar]
- 26. Topolcan O, Holubec L, Jr , Finek J, et al. Changes of thymidine kinase (TK) during adjuvant and palliative chemotherapy. Anticancer Res 2005;25:1831–1833. [PubMed] [Google Scholar]
- 27. Nisman B, Allweis T, Kaduri L, et al. Serum thymidine kinase 1 activity in breast cancer. Cancer Biomark 2010;7:65–72. [DOI] [PubMed] [Google Scholar]
- 28. Duffy MJ, Shering S, Sherry F, McDermott E, O'Higgins N. CA 15–3: A prognostic marker in breast cancer. Int J Biol Markers 2000;15:330–333. [DOI] [PubMed] [Google Scholar]
- 29. Duffy MJ. Serum tumor markers in breast cancer: Are they of clinical value? Clin Chem 2006;52:345–351. [DOI] [PubMed] [Google Scholar]
- 30. Elfagieh M, Abdalla F, Gliwan A, Boder J, Nichols W, Buhmeida A. Serum tumour markers as a diagnostic and prognostic tool in Libyan breast cancer. Tumour Biol 2012;33:2371–2377. [DOI] [PubMed] [Google Scholar]
- 31. Mourtzikou A, Stamouli M, Kroupis C, et al. Evaluation of carcinoembryonic antigen (CEA), epidermal growth factor receptor (EGFR), epithelial cell adhesion molecule EpCAM (GA733–2), and carbohydrate antigen 19–9 (CA 19–9) levels in colorectal cancer patients and correlation with clinicopathological characteristics. Clin Lab 2012;58:441–448. [PubMed] [Google Scholar]
- 32. Lin PC, Lin JK, Lin CC, et al. Carbohydrate antigen 19–9 is a valuable prognostic factor in colorectal cancer patients with normal levels of carcinoembryonic antigen and may help predict lung metastasis. Int J Colorectal Dis 2012;27:1333–1338. [DOI] [PubMed] [Google Scholar]
- 33. Fahmueller YN, Nagel D, Hoffmann RT, et al. Predictive and prognostic value of circulating nucleosomes and serum biomarkers in patients with metastasized colorectal cancer undergoing. Selective Internal Radiation Therapy. BMC Cancer 2012;12:5. [DOI] [PMC free article] [PubMed] [Google Scholar]


