Abstract
Objective
Ischemia‐modified albumin (IMA) is a novel marker for diagnosis of myocardial ischemia and it is considered as a serum marker. The aim of the study was to evaluate salivary IMA levels in patients with acute myocardial infarction (AMI) and to determine the relation between serum and salivary IMA levels.
Methods
A total of 60 patients with AMI and 40 control subjects who are age and sex matched with AMI group were included in our study. The diagnosis of AMI was based on the WHO classification criteria. All patients underwent the clinical assessment, consisting of electrocardiography, and serum cardiac markers. Serum and salivary IMA levels were measured at the first and second days of AMI by using a colorimetric method.
Results
Serum IMA levels were significantly higher in the first and second day of AMI patients, however, salivary IMA levels were significantly higher in the first day of AMI patients compared to the control (P < 0.05). There was a positive correlation between salivary IMA levels and serum IMA levels both in the first and second day of AMI patients (r = 0.298, P < 0.05; r = 0.319, P < 0.05, respectively).
Conclusion
We concluded that salivary IMA levels at the first day of AMI could be used as an alternative marker to serum IMA levels for diagnosis of AMI. J. Clin. Lab. Anal. 27:99–104, 2013. © 2013 Wiley Periodicals, Inc.
Keywords: ischemia‐modified albumin, acute myocardial infarction, saliva
INTRODUCTION
Acute myocardial infarction (AMI) is the major cause of death throughout the world. The well‐known symptom of AMI is central substernal chest discomfort that may radiate to the neck, back, or arms. The discomfort is persistent (unrelieved by rest or nitrates); is frequently associated with diaphoresis, nausea, dyspnea, and fear of impending death; and usually achieves maximum intensity over several minutes 1. It occurs when profound and prolonged ischemia leads to irreversible myocardial cell damage and necrosis that is accompanied by the release of structural proteins and other intracellular macromolecules 2. These are myoglobin, cardiac troponins T and I (cTnT and cTnI), creatine kinase (CK), lactate dehydrogenase, as well as many others. In the clinical practice, an elevation in levels of sensitive and specific biomarkers, such as cTn and CK‐MB, is recognized as AMI 3. Although these usual biomarkers are sensitive and specific for the detection of myocardial necrosis, an increase occurs approximately 3 to 6 hr after the onset of myocardial cell injury. Therefore, patients could wait before they are diagnosed and treated in the emergency department 4, 5.
New biomarkers have been assessed for acute coronary syndrome (ACS) diagnosis such as heart‐type fatty acid‐binding protein (h‐FABP) 6 and ischemia‐modified albumin (IMA) 7. Myocardial ischemia gives rise to change in the structure of the N‐terminus of serum albumin such that it reduces its binding capacity for transitional metals such as cobalt. This alteration can be measured by the albumin cobalt binding (ACB) test 7, 8. There are conflicting results about IMA levels and its specifity in AMI. Some studies have shown that IMA is an early sensitive marker for the diagnosis of reversible ischemia 9, 10. And it was also reported that IMA when measured within 24 hr is a strong and independent predictor of cardiac outcome for 1 year and may be beneficial to identify those requiring more aggressive medical management 11. On the other hand, some authors found frequent false‐positive results and a low specificity with this test 12. Moreover, on a large cohort of patients admitted to an emergency department for chest pain, it was concluded that IMA and h‐FABP did not provide valuable information for ACS diagnosis 13.
Saliva might offer an alternative to serum as a biological fluid analyzed for diagnostic purposes . Saliva is very easy to collect and offers a cost‐effective approach for screening of large populations. It may also be an alternative for patients whose blood is difficult to obtain or especially when compliance is a problem 14. There is a growing number of markers measured in saliva. As a diagnostic fluid, saliva can give the same information as serum testing and also additional or new information that cannot be obtained from serum 15. Recently, it was shown that both salivary CK and CK‐MB isoform (CK‐MB) levels could be used as an alternative to those of serum levels in patients with AMI 16, 17. The present study was aimed to determine the relationship between serum and salivary IMA levels and compare them between healthy subjects and patients with AMI.
METHODS
Study Population
The protocol of this study was approved by the Medical Ethics Committee of our institution and written informed consent was obtained from all participants. The diagnosis of AMI was based on fulfilling any two of the following criteria 18: (i) Chest pain of less than 12 hr duration, (ii) ST‐segment elevation more than 1 mm in at least two consecutive leads, (iii) increased cardiac markers (CK‐MB) two times the upper limit of the normal, and (iv) presumably new onset bundle‐branch block. Patients with renal disease, thyroid disease, with a body mass index (BMI) more than 35, chronic inflammatory diseases, recent major surgery, malignancy, and infection were excluded from this study. Healthy subjects were selected randomly from subjects attending out patient department of our hospital for minor ailments or routine medical check‐up. All subjects were assessed by clinical examination and some laboratory tests including electrocardiogram (ECG) and routine biochemical tests. Body weight and height were recorded. BMI was calculated as kg/m2. History of smoking and alcohol consumption was noted in details. Patients and healthy subjects were also investigated for conventional risk factors (BMI, lipid profile).
Sample Collection and Preparation
In order to standardize the samples, the salivary samples were collected from patients who did not have any caries in their mouth. People with lesion(s) in their mouths or with muscular trauma were excluded from the study. All participants received detailed information about the collection protocol. Both venous blood and salivary samples were obtained simultaneously from each patient on the first 24 hr and first 48 hr after occurrence of AMI. They had been advised not to eat, to brush teeth, or smoke 1 hr before the sample collection. All participants rinsed their mouths thoroughly with tap water before the collection. Saliva was sampled for 5 min with the mouth closed without stimulation and transferred to plastic test tubes. Salivary samples were collected by established methods 19. Whole saliva was collected into a 10‐ml test tube, and then centrifuged at 10,000 × g for 10 min. Venous blood samples were obtained from the antecubital fossa of the arm following salivary sampling. Blood samples were centrifuged at 3,800 × g for 10 min. Then serum and salivary supernatants were isolated and stored in aliquots at −20°C until the time for analysis.
Biochemical Analysis
We measured serum lipid profile, aspartate aminotransferase (AST), alanine aminotransferase (ALT) activities and urea, creatinine, sodium (Na), and potassium (K) levels. High‐density lipoprotein cholesterol (HDL‐C) levels were determined with direct enzymatic method without precipitation (Randox, UK). Low‐density lipoprotein cholesterol (LDL‐C) levels were calculated with Friedewald formula. Estimation of other parameters was done by routine methods using autoanalyzer (Synchron LX20 system, Beckman Coulter, CA, USA). The serum concentrations of CK‐MB and cTnI were determined by using UniCel DxI 800 analyzer (Beckman Coulter, CA, USA). The cut‐off values for CK‐MB and cTnI were set at 6.3 ng/ml and 0.04 ng/ml, respectively. The measurement of all the cardiac markers using the analyzer in each sample was completed within 50 min.
IMA Analysis
A manual colorimetric assay described by Bar‐Or et al. 20 was used to access the ability of binding exogenous cobalt Co(II) to human albumin in serum. Fifty microliters of water solution of 0.1% cobalt chloride (CoCl26H2O) was added to 200 μl of plasma, gently mixed and after 10 min (for adequate cobalt albumin binding), the 50 μL of dithiothreitiol (DTT) solution (1.5 mg/ml H2O) was added as a colorizing agent and the reaction was quenched 2 min later by adding 1.0 ml of 0.9% NaCl. Color development with DTT was measured spectrophotometrically at 470 nm in comparison with a plasma cobalt blank without DTT. The results were given in absorbance units (ABSU).
Statistical Analysis
Statistical analyses were performed using SPSS (version 16.0 (SPSS Inc., IL). To compare the ratio of categorical variables, we used the Chi‐squared test. The normality of the variables was evaluated using the one‐sample Kolmogorov–Smirnov test. Total cholesterol (TC), LDL‐C, urea, K, age, and weight were distributed parametrically but creatinine, HDL‐C, triglycerides (TG), AST, ALT, Na, height, and BMI were not normally distributed nonparametrically. Independent Samples t‐test and Mann–Whitney U test were used for comparing mean and the median values, respectively. We performed (intergroup comparisons) independent samples t‐test to compare the difference in the levels of serum and salivary IMA between healthy subjects and AMI patients. In addition, intragroup comparisons (first day and second day) were performed by paired‐sample t‐test. The correlations between variables were tested by Pearson's correlation test. All data are expressed as mean ± standard deviations (SDs). Differences were considered significant at a probability level of P < 0.05.
RESULTS
Clinical characteristics and biochemical parameters of the subjects are presented in Table 1. Body weight, BMI, TC, TG, AST, ALT, urea, creatinine, LDL‐C, and TC levels of the AMI patients were significantly higher, whereas HDL‐C levels were lower than those of the healthy subjects. In addition, no significant differences were observed in age, gender, height, serum Na, and K levels in AMI patients and healthy subjects.
Table 1.
Clinical Characteristics and Biochemical Variables of the Patients with AMI and the Control Group
| AMI | Controls | ||
|---|---|---|---|
| Characteristics | (n = 60) | (n = 40) | P |
| Gender (F/M) | 8/52 | 6/34 | 0.518 |
| Age (years) | 56.25 ± 12.1 | 52.45 ± 8.9 | 0.082 |
| Weight (kg) | 78.45 ± 12.2 | 73.47 ± 7.4 | 0.017 |
| Height (cm) | 169.42 ± 9.1 | 170.22 ± 8.6 | 0.703 |
| BMI (kg/m2) | 27.34 ± 4.0 | 25.15 ± 2.9 | 0.003 |
| Current smoking (%) | 80 | – | – |
| Hypertension (%) | 21.66 | – | – |
| Diabetes mellitus (%) | 16.66 | – | – |
| Total‐Cholesterol (mg/dl)a | 191.26 ± 48.5 | 174.38 ± 21.4 | 0.027 |
| Triglycerides (mg/dl)a | 166.96 ± 129.0 | 95.30 ± 44.1 | <0.001 |
| HDL‐C (mg/dl)a | 35.18 ± 11.5 | 44.73 ± 11.5 | <0.001 |
| LDL‐C (mg/dl)a | 122.96 ± 35.0 | 114.13 ± 27.3 | 0.170 |
| AST (U/l)a | 71.75 ± 77.1 | 18.65 ± 3.1 | <0.001 |
| ALT (U/l)a | 29.54 ± 17.6 | 17.30 ± 4.6 | <0.001 |
| Urea (mg/dl)a | 30.86 ± 9.8 | 26.35 ± 8.8 | 0.022 |
| Creatinine (mg/dl)a | 0.97 ± 0.5 | 0.72 ± 0.1 | <0.001 |
| Sodium (mEq/l)a | 137.28 ± 4.9 | 138.90 ± 2.6 | 0.074 |
| Potassium (mEq/l)a | 4.31 ± 0.5 | 4.24 ± 0.5 | 0.567 |
| cTnI (ng/ml)a | 28.41 ± 37.3 | – | – |
| CK‐MB (ng/ml)a | 119.30 ± 114.3 | – | – |
Serum samples were obtained at the time of hospitalization.
BMI, body mass index; HDL‐C, high‐density lipoprotein‐cholesterol; LDL‐C, low‐density lipoprotein‐cholesterol; AST, aspartate aminotransferase; ALT, alanine aminotransferase; cTnI, cardiac troponin I; CK‐MB, creatine kinase MB.
Serum and salivary IMA levels of the groups are presented in Table 2. Serum IMA levels were significantly higher in the first and second day and salivary IMA levels were significantly higher only in the first day of AMI patients than the healthy subjects (Fig. 1). In addition, there were no different between serum and salivary IMA levels in the first day and second day of AMI patients.
Table 2.
Serum and Salivary IMA Levels of the Patients with AMI and the Control Group
| AMI | Controls | ||
|---|---|---|---|
| Parameters | (n = 60) | (n = 40) | • P |
| Serum IMA (ABSU) | |||
| First day | 1.30 ± 0.1 | 0.92 ± 0.1 | <0.001 |
| Second day | 1.28 ± 0.1 | <0.001 | |
| Saliva IMA (ABSU) | |||
| First day | 1.07 ± 0.3 | 0.90 ± 0.4 | 0.027 |
| Second day | 0.99 ± 0.3 | 0.217 | |
| Serum IMA (ABSU) | First day | Second day | •• P |
| 1.30 ± 0.1 | 1.28 ± 0.1 | 0.233 | |
| Saliva IMA (ABSU) | First day | Second day | •• P |
| 1.07 ± 0.3 | 0.99 ± 0.3 | 0.076 | |
IMA, ischemia‐modified albumin; •Independent samples t‐test; ••Paired samples t‐test.
Figure 1.
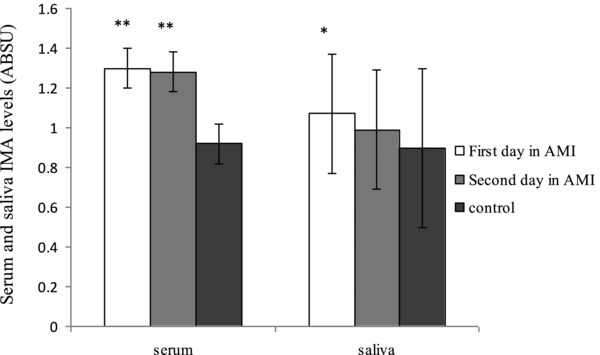
Ischemia‐modified albumin (IMA) levels in serum and saliva in patients with acute myocardial infarction (AMI) and controls. *P < 0.05, **P < 0.001 compared with control group.
Simple correlation analysis was performed to investigate the association between serum and salivary IMA levels. As shown in Table 3, on the first day, AMI patients serum levels of IMA were positively correlated with the salivary levels and on the second day serum levels of IMA were positively correlated with the salivary levels. On the other hand, serum IMA levels were positively correlated with salivary levels in healthy subjects.
Table 3.
Results of Correlation Analysis Between Serum and Salivary Ischemia‐Modified Albumin (IMA) Levels
| AMI patiens | Controls | |
|---|---|---|
| First day | ||
| Serum‐saliva | r = 0.298 | r = 0.454 |
| P = 0.040 | P = 0.003 | |
| Second day | ||
| Serum‐saliva | r = 0.319 | |
| P = 0.024 | ||
Acute myocardial infarction (AMI).
DISCUSSION
Accurate and rapid diagnosis of AMI is essential because it has high mortality rates. Our results have demonstrated that, both on the first and second day, salivary and serum IMA levels were increased in patients with AMI compared to the healthy subjects. Moreover, salivary IMA levels correlated with serum levels in two groups. To the best of our knowledge, the salivary IMA levels of AMI patients have not been investigated yet.
AMI is the main cause of death in the world 1. In the diagnosis of AMI, markers of myocardial necrosis such as cTn and CK‐MB are used as the gold standard 21. There is an increasing interest in discovering new biomarkers that could be more sensitive or require shorter time periods for testing compared to the common biomarkers such as cardiac‐specific troponins. Moreover, it may reduce the time of assessment to rule out ACS in patients in the emergency department, thus reducing length of stay times and potentially hospital costs 22. IMA is one of these markers. Myocardial ischemia that precedes AMI is associated with changes in human serum albumin that results in decreased divalent cobalt ion (Co2+) binding. The principle of this reaction depends on colorimetric Co2+‐albumin binding assay, which is an indirect measure of IMA. It was shown that IMA was a useful diagnostic test for the diagnosis of myocardial ischemia in potential ACS patients 8. This method has been developed to determine IMA in the setting of cardiac ischemia, which could improve the sensitivity of diagnosing ACS. Although some studies supported the use of IMA in patients presenting symptoms of ACS 12, 23, 24, 25, the others did not support the use of IMA alone in the diagnosis of ACS or AMI 26, 27, 28, 29, 30. IMA levels in our study are consistent with some previous reports, that serum IMA levels of patients with AMI were significantly higher than those of healthy subjects.
The composition of saliva is originated from typical extracellular fluids such as plasma, but in the salivary glands, the active transport and secretion mechanisms may change the composition of saliva. It might be an excellent alternative to serum as a biological fluid analyzed for diagnostic aims. Because salivary collection is very easy and is a plausible method to screen large populations, it is a good alternative for patients whose blood is difficult to obtain or when compliance is a problem 14. Moreover, the laboratory procedures are easier than blood samples since it does not clot, thus reducing the number of required manipulations 31. On the other hand, saliva has a dynamic composition that may be affected by many physiologic variables such as diet, pH, health conditions, and salivary flow rate 32. Under standardized conditions, saliva could be a good option. It was reported that saliva has been proposed for monitoring drugs such as digoxin, ethanol, cocaine, marijuana, and opioids. Measurements of salivary tests are increasing rapidly with recent technological advances 33. It was demonstrated in many reports that saliva could be used for drug monitoring and diagnosis of hereditary disorders, autoimmune diseases, infectious diseases, endocrine disorders, and cancers 14, 34, 35. There are a few papers investigating salivary cardiac biomarkers in patients with AMI 16, 17, 36. Floriano et al. 36 reported significant differences of both established and novel cardiac biomarkers in serum and saliva produced from AMI patients and healthy controls. The saliva‐based biomarker panel of C‐reactive protein, myoglobin, and myeloperoxidase exhibited significant diagnostic capability and in conjunction with ECG yielded strong screening capacity for AMI comparable to that of the panel (brain natriuretic peptide, cTnI, CK‐MB, myoglobin). They concluded that complementary to ECG, saliva‐based tests may provide a convenient and rapid screening method for cardiac events in pre‐hospital stages for AMI patients. Recently, it was reported that both CK and CK‐MB levels in the saliva of AMI patients were significantly higher than those of the controls. The authors suggested that the underlying mechanism of this increase is yet unknown. But plasma is obviously the main source of salivary secretions, and any change in the blood levels can lead to a similar, though to a lesser extent, modification in salivary content of this necrotic biomarkers 16, 17. However, in another study, it has been shown that salivary CK‐MB levels do not show a significant difference between AMI patients and controls 36.
CONCLUSION
In the present study, we determined higher serum and salivary IMA levels on the first day of AMI and higher serum IMA levels on the second day of AMI in patients as compared to the healthy subjects. Salivary IMA levels correlated significantly with serum IMA levels in both days. The present study has some limitations. We assayed IMA levels in serum and saliva at the first 24 hr and first 48 hr after occurrence of AMI. We did not measure the salivary IMA levels in the first few hours after the onset of AMI. The patients often admitted to emergency service 4–8 hr after the onset of the chest pain. Moreover, we did not include patients with unstable angina. Therefore, we could not have any data about the IMA levels on the onset of this condition and in the cases with suspected but not diagnosed as AMI. Further studies are needed for the assessment of salivary IMA levels on the time of admission and in larger study groups.
We concluded that salivary IMA levels at the first day of AMI might be used for developing an alternative diagnostic biomarker for myocardial infarction, especially in the first day.
REFERENCES
- 1. Welch TD, Yang EH, Reeder GS, Gersh BJ. Modern management of acute myocardial infarction. Curr Probl Cardiol 2012;37:237–310. [DOI] [PubMed] [Google Scholar]
- 2. Mueller C, Muller B, Perruchoud AP. Biomarkers: Past, present, and future. Swiss Med Wkly 2008;138:225–229. [DOI] [PubMed] [Google Scholar]
- 3. Alpert JS, Thygesen K, Antman E, Bassand JP. Myocardial infarction redefined–A consensus document of The Joint European Society of Cardiology/American College of Cardiology Committee for the redefinition of myocardial infarction. J Am Coll Cardiol 2000;36:959–969. [DOI] [PubMed] [Google Scholar]
- 4. Wu AH, Apple FS, Gibler WB, Jesse RL, Warshaw MM, Valdes R, Jr . National Academy of Clinical Biochemistry Standards of Laboratory Practice: Recommendations for the use of cardiac markers in coronary artery diseases. Clin Chem 1999;45:1104–1121. [PubMed] [Google Scholar]
- 5. Bertrand ME, Simoons ML, Fox KA, et al. Management of acute coronary syndromes in patients presenting without persistent ST‐segment elevation. Eur Heart J 2002;23:1809–1840. [DOI] [PubMed] [Google Scholar]
- 6. Alhadi HA, Fox KA. Do we need additional markers of myocyte necrosis: The potential value of heart fatty‐acid‐binding protein. QJM 2004;97:187–198. [DOI] [PubMed] [Google Scholar]
- 7. Christenson RH, Duh SH, Sanhai WR, et al. Characteristics of an Albumin Cobalt Binding Test for assessment of acute coronary syndrome patients: A multicenter study. Clin Chem 2001;47:464–470. [PubMed] [Google Scholar]
- 8. Bhagavan NV, Lai EM, Rios PA, et al. Evaluation of human serum albumin cobalt binding assay for the assessment of myocardial ischemia and myocardial infarction. Clin Chem 2003;49:581–585. [DOI] [PubMed] [Google Scholar]
- 9. Sinha MK, Roy D, Gaze DC, Collinson PO, Kaski JC. Role of “ischemia modified albumin”, a new biochemical marker of myocardial ischaemia, in the early diagnosis of acute coronary syndromes. Emerg Med J 2004;21:29–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Anwaruddin S, Januzzi JL, Jr. , Baggish AL, Lewandrowski EL, Lewandrowski KB. Ischemia‐modified albumin improves the usefulness of standard cardiac biomarkers for the diagnosis of myocardial ischemia in the emergency department setting. Am J Clin Pathol 2005;123:140–145. [DOI] [PubMed] [Google Scholar]
- 11. Van Belle E, Dallongeville J, Vicaut E, Degrandsart A, Baulac C, Montalescot G. Ischemia‐modified albumin levels predict long‐term outcome in patients with acute myocardial infarction. The French Nationwide OPERA study. Am Heart J 2010;159:570–576. [DOI] [PubMed] [Google Scholar]
- 12. Keating L, Benger JR, Beetham R, et al. The PRIMA study: Presentation ischaemia‐modified albumin in the emergency department. Emerg Med J 2006;23:764–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Charpentier S, Ducasse JL, Cournot M, et al. Clinical assessment of ischemia‐modified albumin and heart fatty acid‐binding protein in the early diagnosis of non‐ST‐elevation acute coronary syndrome in the emergency department. Acad Emerg Med 2010;17:27–35. [DOI] [PubMed] [Google Scholar]
- 14. Kaufman E, Lamster IB. The diagnostic applications of saliva a review. Crit Rev Oral Biol Med 2002;13:197–212. [DOI] [PubMed] [Google Scholar]
- 15. Hofman LF. Human saliva as a diagnostic specimen. J Nutr 2001;131:1621–1625. [DOI] [PubMed] [Google Scholar]
- 16. Mirzaii‐Dizgah I, Jafari‐Sabet M. Unstimulated whole saliva creatine phosphokinase in acute myocardial infarction. Oral Dis 2011;17:597–600. [DOI] [PubMed] [Google Scholar]
- 17. Mirzaii‐Dizgah I, Hejazi SF, Riahi E, Salehi MM. Saliva‐based creatine kinase MB measurement as a potential point‐of‐care testing for detection of myocardial infarction. Clin Oral Investig 2012;16:775–779. [DOI] [PubMed] [Google Scholar]
- 18. Tunstall‐Pedoe H, Kuulasmaa K, Amouyel P, Arveiler D, Rajakangas AM, Pajak A. Myocardial infarction and coronary deaths in the World Health Organization MONICA Project. Registration procedures, event rates, and case‐fatality rates in 38 populations from 21 countries in four continents. Circulation 1994;90:583–612. [DOI] [PubMed] [Google Scholar]
- 19. Janket S, Meurman JH, Baird AE, et al. Salivary immunoglobulins and prevalent coronary artery disease. C 2010;89:389–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bar‐Or D, Lau E, Winkler JV. A novel assay for cobalt‐albumin binding and its potential as a marker for myocardial ischemia‐A preliminary report. J Emerg Med 2000;19:311–315. [DOI] [PubMed] [Google Scholar]
- 21. Thygesen K, Alpert JS, White HD, et al. Universal definition of myocardial infarction. Circulation 2007;116:2634–2653. [DOI] [PubMed] [Google Scholar]
- 22. Lin S, Yokoyama H, Rac VE, Brooks SC. Novel biomarkers in diagnosing cardiac ischemia in the emergency department: A systematic review. Resuscitation 2012;83:684–691. [DOI] [PubMed] [Google Scholar]
- 23. Dekker MS, Mosterd A, van ‘t Hof AW, Hoes AW. Novel biochemical markers in suspected acute coronary syndrome: systematic review and critical appraisal. Heart 2010;96:1001–1010. [DOI] [PubMed] [Google Scholar]
- 24. Collinson PO, Gaze DC, Bainbridge K, et al. Utility of admission cardiac troponin and “Ischemia Modified Albumin” measurements for rapid evaluation and rule out of suspected acute myocardial infarction in the emergency department. Emerg Med J 2006;23:256–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Peacock F, Morris DL, Anwaruddin S, et al. Meta‐analysis of ischemia‐modified albumin to rule out acute coronary syndromes in the emergency department. Am Heart J 2006;152:253–262. [DOI] [PubMed] [Google Scholar]
- 26. Kim JS, Hwang HJ, Ko YG, et al. Ischemia‐modified albumin: Is it a reliable diagnostic and prognostic marker for myocardial ischemia in real clinical practice? Cardiology 2010;116:123–129. [DOI] [PubMed] [Google Scholar]
- 27. Liyan C, Jie Z, Yonghua W, Xiaozhou H. Assay of ischemia‐modified albumin and C‐reactive protein for early diagnosis of acute coronary syndromes. J Clin Lab Anal 2008;22:45–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Talwalkar SS, Bon Homme M, Miller JJ, Elin RJ. Ischemia modified albumin, a marker of acute ischemic events: A pilot study. Ann Clin Lab Sci 2008;38:132–137. [PubMed] [Google Scholar]
- 29. Hjortshoj S, Kristensen SR, Ravkilde J. Diagnostic value of ischemia‐modified albumin in patients with suspected acute coronary syndrome. Am J Emerg Med 2010;28:170–176. [DOI] [PubMed] [Google Scholar]
- 30. Lin RM, Fatovich DM, Grasko JM, Vasikaran SD. Ischaemia modified albumin cannot be used for rapid exclusion of acute coronary syndrome. Emerg Med J 2010;27:668–671. [DOI] [PubMed] [Google Scholar]
- 31. Lee YH, Wong DT. Saliva: An emerging biofluid for early detection of diseases. Am J Dent 2009;22:241–248. [PMC free article] [PubMed] [Google Scholar]
- 32. Crossner CG. Salivary flow rate in children and adolescents. Swed Dent J 1984;8:271–276. [PubMed] [Google Scholar]
- 33. Drobitch RK, Svensson CK. Therapeutic drug monitoring in saliva. An update. Clin Pharmacokinet 1992;23:365–379. [DOI] [PubMed] [Google Scholar]
- 34. Streckfus CF, Bigler LR. Saliva as a diagnostic fluid. Oral Dis 2002;8:69–76. [DOI] [PubMed] [Google Scholar]
- 35. Wong DT. Salivary diagnostics powered by nanotechnologies, proteomics and genomics. J Am Dent Assoc 2006;137:313–321. [DOI] [PubMed] [Google Scholar]
- 36. Floriano PN, Christodoulides N, Miller CS, et al. Use of saliva‐based nano‐biochip tests for acute myocardial infarction at the point of care: a feasibility study. Clin Chem 2009;55:1530–1538. [DOI] [PMC free article] [PubMed] [Google Scholar]


