Abstract
NMO‐IgGagainst aquaporin‐4 (AQP4) is a specific marker for neuromyelitis optica (NMO). We evaluated the performance of different NMO‐IgG detecting methods. In 124 sera (from 54 with NMO spectrum disorders including nine with NMO, ten with multiple sclerosis including two with OSMS, and 60 with other neurological diseases), NMO‐IgG was measured with tissue‐based indirect immunofluorescence (IIF‐tissue) using mouse cerebellum, cell‐based IIF (IIF‐AQP4) using transfected HEK293 cells which express human AQP4, and AQP4 autoantibody detecting enzyme linked immunosorbent assay (ELISA‐AQP4). The sensitivities and specificities of three assays were 44.4–55.6% and 87.0–92.2% for detecting NMO, and 11.1–20.4% and 95.7–97.1% for detecting NMO spectrum disorders. Although there was no significant difference, the patients with NMO or NMO spectrum disorders showed higher rates of seropositivity in the ELISA‐AQP4 vs. IIF assays. Out of the 19 sera with NMO‐IgG, in at least one test, only six (31.6%) were found to be positive by all three assays. Among the three methods, the ranges of co‐negativities, co‐positivities, and agreement were 77.4–97.4%, 42.9–75.0%, and 91.1–95.2% (kappa 0.475–0.641), respectively. In patients who had positive ELISA‐AQP4 results, IIF‐AQP4 positivity was associated with NMO (P = 0.01). In summary, we observed an increased prevalence of NMO‐IgG in patients with NMO and NMO spectrum disorders. ELISA‐AQP4 may be more sensitive and specific when confirmed by IIF‐AQP4. J. Clin. Lab. Anal. 26:184‐189, 2012. © 2012 Wiley Periodicals, Inc.
Keywords: neuromyelitis optica, NMO spectrum disorders, NMO‐IgG, Aquaporin‐4
INTRODUCTION
Neuromyelitis optica (NMO) is a severe inflammatory demyelinating disorder of the central nervous system characterized by optic neuritis (ON) and acute transverse myelitis (TM) 1, 2. Typical characteristics of typical NMO patients include recurrent attacks of myelitis with longitudinally extensive spinal cord lesion extending over three or more vertebral segment as viewed by MRI, severe ON resulting in frequent relapses with little or no remission, and absence of brain involvement based on clinical evidence 3, 4. It is often very difficult to distinguish between cases of NMO and multiple sclerosis (MS) in the early phase of disease. However, NMO has a worse prognosis, and early diagnosis is critical for the prompt initiation of immunosuppressive medication and long‐term treatment 2, 5, 6, 7, 8, 9, 10, 11.
NMO‐IgG has been recognized as a specific serologic marker for NMO 12, and aquaporin 4 (AQP4) has been identified as a target antigen 13. AQP4 is a water‐channel protein and a component of the dystroglycan protein complex that is located on the conglomerated astrocyte foot process of the blood brain barrier 13. To measure NMO‐IgG levels, several different techniques have been proposed. NMO‐IgG was first detected by indirect immunofluorescence assay using mouse brain tissue (IIF‐tissue). This technique has been proposed as the gold standard for the new diagnostic criteria, particularly for IIF in monkey cerebellum 14. Cell‐based IIF (IIF‐AQP4) uses AQP4‐transfected mammalian cells, which express AQP4 on their cell membranes. This has been reported to be a more sensitive and reproducible method than IIF‐tissue 15, 16. Recently, ELISA‐based tests to detect AQP4 antibody have been developed and they provide quantitative results with sensitivity and specificity comparable to those of conventional IIF 17.
Although several commercial kits based on different assays are currently available, the performance of these assays varies between different study groups 4, 14, 17, 18, and the comparative analysis for them is insufficient. Since populations of anti‐AQP4‐positive patients are heterogeneous, determining the sensitivity and specificity of the various assays for different groups of these individuals is necessary. In this study, we tested NMO‐IgG using tissue‐, cell‐based IIF, and ELISA, and compared the results according to the clinical characteristics of neuronal disease.
MATERIALS AND METHODS
Patients
This study was approved by the Institutional Review Board of Seoul St. Mary's Hospital. We included consecutive 124 sera samples that were requested for the detection of NMO‐IgG. All sera were from 54 patients with NMO spectrum disorders including NMO (n = 9), optic neuritis (n = 32), and transverse myelitis (n = 13); 10 patients with MS including two patients with optico‐spinal MS (OSMS), and 60 patients with other neurological diseases. These neurological diseases included Parkinson disease (n = 11), peripheral neuropathy (n = 11), paraneoplastic neurological disorders (n = 6), cerebral infarction (n = 5), encephalitis (n = 5), vasculitis (n = 4), neurosis (n = 3), and miscellaneous conditions (n = 15). All patients in this study were admitted and followed up in our hospital between 2008 and 2011.
The medical records of the 124 patients were reviewed to gather information about the patients’ gender, age, clinical manifestations, clinically defined diagnosis, laboratory findings, and brain and/or spinal cord MRI results. NMO and MS were diagnosed based on the revised Wingerchuk criteria excluding the NMO‐IgG and McDonald's criteria, respectively 14, 19. To detect NMO‐IgG, all sera were initially tested by cell‐based and tissue‐based IIF assays and then stored at −80°C until they were tested by ELISA‐AQP4.
Indirect Immunofluorescence Assay (IIF)
IIF‐tissue and IIF‐AQP4 were performed using Neurology Mosaics kit (EUROIMMUN, AG, Luebeck, Germany). IIF‐tissue uses mouse cerebellum tissue sections to detect NMO‐IgG. IIF‐AQP4 uses HEK293 cells transfected with recombinant full‐length human AQP4 to detect anti‐AQP4 antibodies and wild‐type HEK293 cells as a control substrate. According to the manufacturer's instruction, 25 μL of 1:10 diluted sera was applied to slides on which mouse tissue or HEK cells were attached. After 30 min of incubation at room temperature, the slides were washed and fluorescein‐labeled anti‐human globulin was added. Fluorescence was observed with a microscope after 30 min of incubation by two independent evaluators (Y‐J K, E‐J O). The positive and negative results of IIF‐tissues between two evaluators were concordant. The evaluators were unaware of the clinical diagnosis and scored the IIF results in direct comparison with control sera. NMO‐IgG‐positive positivity was detected when the small brain vessels, pia, subpia, and Virchow Robin space were stained in the IIF‐tissue test or when the cytoplasm of AQP4‐transfected cells showed a flat, smooth, and file granular fluorescence staining pattern in the IIF‐AQP4 test (Fig. 1). Positive fluorescence intensity was graded on a 4‐point scale (+1 to +4).
Figure 1.
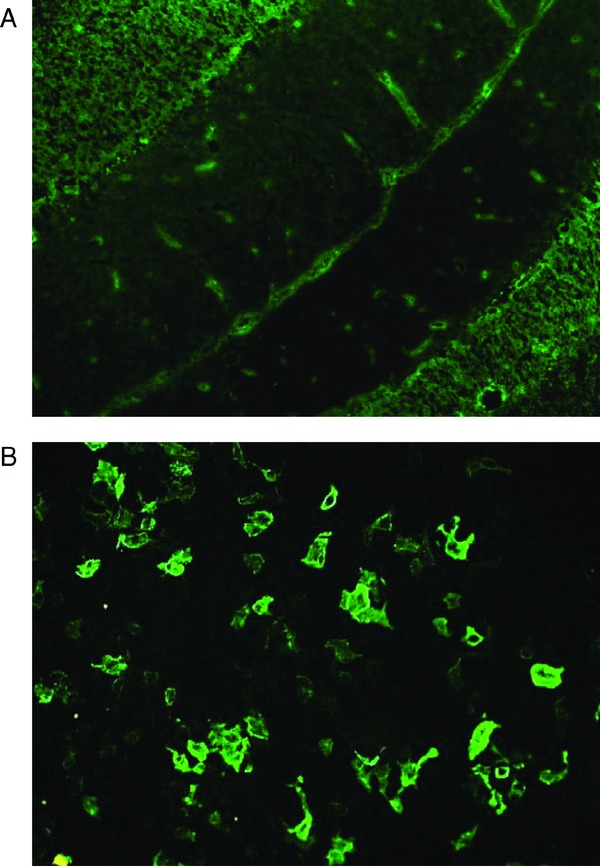
Immunofluorescence patterns of NMO‐IgG in mouse cerebellum tissue (A) showing fluorescence of the small vessels, the pia, the subpia, and Virchow Robins space and in AQP4‐transfected cells (B).
ELISA‐AQP4
Anti‐AQP4 autoantibodies were detected using an Aquaporin‐4 autoantibody ELISA kit (DLD Diagnostika GmbH, Hamburg, Germany) that uses recombinant human AQP4 produced in a baculovirus/insect cell expression system. According to the manufacturer's instructions, 50 μL of serum and reconstituted AQP4‐biotin were added to AQP4 antigen‐coated ELISA plate wells and incubated for 2 hr at room temperature with shaking. After washing, 100 μL of streptavidin peroxidase was added to each well and the plate incubated for 20 min at room temperature. After incubation with 100 μL of TMB substrate in the dark, optical absorbance of each well was measured at 450 nm using an ELISA reader. The concentrations of AQP4 antibody were determined by a calibration curve in which 5 U/mL was considered to be the cut‐off value.
Statistical Analysis
Statistical analyses were performed with SPSS version 12.0 (SPSS, Chicago, IL). The results were compared using a chi‐square test for categorical data and Mann–Whitney U‐test for non‐normally distributed variables. Agreement between the NMO‐IgG results was assessed according to Kappa coefficients (0.001–0.2 indicated slight concurrence, 0.201–0.4 indicated fair agreement, 0.401–0.6 showed moderate agreement, 0.601–0.8 indicated substantial concurrence, and 0.801–0.999 showed excellent agreement). All P‐values were two‐tailed and ones <0.05 were considered to be statistically significant.
RESULTS
Seropositivity rates for NMO‐IgG determined by IIF‐tissue, IIF‐AQP4, and ELISA‐AQP4 are shown according to clinical diagnoses (Table 1). Out of 124 sera, NMO‐IgG antibodies were detected in 19 samples (15.3%) and were specific for NMO spectrum disorders (P = 0.017). NMO‐IgG‐positive results from at least one assay were obtained for 55.6% (5/9) of patients with clinically defined NMO and 24.1% (13/54) of patients with NMO spectrum disorders. Out of the 13 patients with TM, two that were NMO‐IgG positive had TM relapses.
Table 1.
Seropositivity Rates for NMO‐IgG and AQP4 Antibodies of the Three Methods for Patients with Different Groups of Diseases
| Disease groups | N | NMO‐IgG (+)a | IIF‐tissue (+) | IIF‐AQP4 (+) | ELISA‐AQP4 (+) |
|---|---|---|---|---|---|
| Neuromyelitis optica | 9 | 5 (55.6%) | 4 (44.4%) | 5 (55.6%) | 5 (55.6%) |
| Optic neuritis | 32 | 6 (18.8%) | 2 (6.3%) | 0 (0.0%) | 4 (12.5%) |
| Transverse myelitis | 13 | 2 (15.4%) | 1 (7.7%) | 1 (7.7%) | 2 (15.4%) |
| NMO spectrum disorders | 54 | 13 (24.1%) | 7 (13.0%) | 6 (11.1%) | 11 (20.4%) |
| Multiple sclerosis | 10 | 3 (30.0%) | 1 (10.0%) | 1 (10.0%) | 3 (30.0%) |
| (Optico‐spinal multiple sclerosis) | (2) | 2 (100%) | 0 (0%) | 0 (0%) | 2 (100%) |
| Other neurological diseases | 60 | 3 (5.0%) | 1 (1.7%) | 2 (3.3%) | 0 (0.0%) |
| Disease controls | 70 | 6 (8.6%) | 2 (2.9%) | 3 (4.3%) | 3 (4.3%) |
NMO‐IgG‐positive results from at least one assay.
When comparing the seropositive rates determined by the different methods, only six (31.6%) out of 19 sera were identified as positive by all three assays. Out of nine patients with NMO, four (44.4%) were seropositive according to IIF‐tissue vs. five patients (55.6%) who were positive based on IIF‐AQP and ELISA‐AQP4 (P = 1.00). Although not statistically significant (P >0.05), patients with NMO spectrum disorders had a higher rate of seropositivity according to the ELISA‐AQP4 (20.4%, 11/54) than those determined to be positive by IIF‐tissue (13.0%, 7/54) or IIF‐AQP4 (11.1%, 5/54). The specificities for patients with NMO or NMO spectrum disorders were 87.0–97.1% by the three assays, 87.0 and 97.1% by IIF‐tissue, 92.2 and 95.7% by IIF‐AQP4, and 92.2 and 95.7% by ELISA‐AQP4, respectively. However, the sensitivities and specificities of three methods were not significantly different for any groups of patients in this study.
Table 2 shows the co‐negativities, co‐positivities, and agreement of the results among the three assays. IIF‐tissue showed 95.2% (kappa 0.641) and 91.1% (0.475) agreement with IIF‐AQP4 and ELISA‐AQP4, respectively. While IIF‐tissue assay had about 97% co‐negativities with the other two AQP4 antibodies detecting methods, co‐positivities were less than 67%. The three sera that were positive according to IIF‐tissue alone were from two patients with optic neuritis and one patient with Parkinson's disease (Table 3). One serum sample from an NMO patient was positive according to IIF‐AQP4 and ELISA‐AQP4 but was negative based on the IIF‐tissue assay.
Table 2.
Concordance Rates for the Detection of NMO‐IgG in 124 Sera between IIF‐Tissue, IIF‐AQP4, and ELISA‐AQP4
| Methods | Co‐negativity | Co‐positivity | % agreement (kappa value) |
|---|---|---|---|
| IIF‐tissue vs. IIF‐AQP4 | 97.4 | 66.7 | 95.2 (0.641) |
| IIF‐ tissue vs. ELISA‐AQP4 | 97.3 | 42.9 | 91.1 (0.475) |
| IIF‐AQP4 vs. ELISA‐AQP4 | 77.4 | 75.0 | 92.7 (0.571) |
Table 3.
Clinical Diagnosis According to the Results of the Three NMO‐IgG Detection Methods
| IIF‐tissue | IIF‐AQP4 | ELISA‐AQP4 | N | Clinical diagnosis (n) |
|---|---|---|---|---|
| ‐ | ‐ | ‐ | 105 | NMO (4), ON (26), TM (11), MS (7), OND (57) |
| + | ‐ | ‐ | 3 | ON (2), OND (1) |
| ‐ | + | ‐ | 2 | OND (2) |
| ‐ | ‐ | + | 7 | ON (4), TM (1), OSMS (2) |
| ‐ | + | + | 1 | NMO (1) |
| + | + | + | 6 | NMO (4), TM (1), MS (1) |
NMO, neuromyelitis optica; ON, optic neuritis; OSMS, optico‐spinal multiple sclerosis; TM, transverse myelitis; MS, multiple sclerosis; OND, other neurological diseases.
The IIF‐AQP4 and ELISA‐AQP4 results showed 92.7% agreement (kappa 0.571) for the detection of AQP4 autoantibodies; the co‐negativity and co‐positivity rates were 77.4 and 75.0%, respectively. The two IIF‐AQP4(+)/ELISA‐AQP4(‐) sera samples were weakly positive (1+ fluorescence intensity) on the IIF‐AQP4 and were obtained from patients with other neurological diseases. The seven IIF‐AQP4(‐)/ELISA‐AQP4(+) sera samples were from five patients with NMO spectrum disorders (four with optic neuritis and one with transverse myelitis) and two patients with OSMS. Out of 14 ELISA‐AQP4(+) sera, seven were also positive on the IIF‐AQP4 assay. Five (71.4%) out of seven ELISA‐AQP4(+)/IIF‐AQP4 (+) sera were from patients with NMO while none of the seven ELISA‐AQP4(+)/IIF‐AQP4(‐) samples were from NMO patients (P = 0.01).
When comparing the clinical and laboratory features of the ELISA‐AQP4 seronegative and seropositive patients, ELISA‐AQP4 positivity was associated with female individuals, NMO, NMO spectrum disorders, ON, TM, and longitudinal extended spinal cord lesion (Table 4). However, among 14 ELISA‐AQP4(+) patients, there was no difference in the demographic and clinical characteristics (gender, mean onset age, optic neuritis, acute myelitis, CSF oligoclonal band, other autoimmune disorder or autoantibodies) between the seven patients who were seropositive and seven who were seronegative based on the IIF‐AQP4 assay.
Table 4.
Clinical and Laboratory Features of the Patients Seronegative and Seropositive for AQP4 Antibodies Based on the ELISA‐AQP4
| ELISA‐AQP4 (‐) | ELISA‐AQP4 (+) | ||
|---|---|---|---|
| (n = 110) | (n = 14) | P‐value | |
| Female | 54 (49.1%)a | 13 (92.9%) | 0.002 |
| Age (mean ± SD) | 49 ± 17 | 44 ± 14 | ns |
| Neuromyelitis optica (NMO) | 4 (3.6%) | 5 (35.7%) | <0.001 |
| NMO spectrum disorders | 43 (39.1%) | 11 (78.6%) | 0.008 |
| Multiple sclerosis | 7 (6.4%) | 3 (21.4%) | ns |
| Optico‐spinal multiple sclerosis | 0 (0%) | 2 (100%) | 0.012 |
| Optic neuritis | 34 (30.9%) | 11 (78.6%) | 0.001 |
| Transverse myelitis | 20 (18.2%) | 8 (57.1%) | 0.001 |
| Longitudinal extended spinal cord lesion | 6 (5.5%) | 6 (42.9%) | <0.001 |
| Brain MRI lesion compatible with inflammatory demyelination | 11 (0.1%) | 0 (0%) | ns |
| CSF oligoclonal band | 5 (4.5%) | 0 (0%) | ns |
| Patients with other autoantibodies | 19 (17.3%) | 5 (35.7%) | ns |
Number of cases (%)
ns, not significant; CSF, cerebrospinal fluid
DISCUSSION
NMO, also known as Devic's syndrome, is an immune‐mediated neurologic disease involving optic nerves and spinal cord. Detection of NMO‐IgG or anti‐AQP4 antibodies represents a valuable tool for the early diagnosis of NMO and NMO spectrum disorders. In the present study, the presence of NMO‐IgG was specific for NMO spectrum disorders, confirming the results of a previous report 20. In NMO patients, the seropositive rates of NMO‐IgG according to the three methods we evaluated were 44.4 to 55.6%; these were similar to the previously reported data 12, 16, 20, 21. Although these rates were significantly higher than those for MS patients (30%), seroprevalence was lower compared to that of previous reports 20, 22. This difference may be due to inadequate performance of the assays used or possible bias associated with clinical evaluation.
We compared different assays to evaluate their ability to detect NMO‐IgG or anti‐AQP4 antibodies. Although differences in sensitivity and specificity among three methods were not statistically significant for any groups of patients in this study, out of 19 NMO‐IgG‐positive sera, only 31.6% were identified as positive by all three assays.
The results from the IIF‐tissue assay showed more than 90% agreement with the IIF‐AQP4 or ELISA‐AQP4 results. While the IIF‐tissue assay shared a 97% co‐negativity rate with other two methods, co‐positivities were as low as 66.7 and 42.9% as compared to the IIF‐AQP4 and ELISA‐AQP4, respectively. These results may be due to differences in the antigens used and concur with previous reports showing the lower seropositive rates for IIF‐tissue than IIF‐AQP4 12, 18. When comparing the IIF‐AQP4 to the ELISA‐AQP4, a previous report concluded that differences in the results from these two assays were caused by differences in rat and human amino acid sequences 17. However, both IIF‐AQP4 and ELISA‐AQP4 assays in this study used human AQP4 as the target antigen, and agreement between these two assays was 92.7% for detecting AQP4 antibodies.
When analyzing sera that produced discrepant results between the IIF‐AQP4 and ELISA‐AQP4, two IIF‐AQP4(+)/ELISA‐AQP4(‐) sera were from patients with other neurological diseases, and seven IIF‐AQP4(‐)/ELISA‐AQP4(+) samples were from patients with NMO spectrum disorders or OSMS. These findings suggest that the ELISA‐AQP4 may be more sensitive for identifying patients with NMO spectrum disorders than the IIF‐AQP4. In addition, we confirmed previous data showing that the clinical and laboratory features of AQP4 antibody‐positive patients were similar to those of NMO patients 2, 23, 24.
In patients with ELISA‐AQP4(+) sera, IIF‐AQP4 positivity was associated with cases of NMO (P = 0.01). None of the seven ELISA‐AQP4(+)/IIF‐AQP4(‐) sera was from NMO patients, suggesting that non‐specific binding of human serum IgG may produce false positive ELISA‐AQP4 results. Since anti‐AQP4 antibodies may play a role in the pathogenesis of NMO and removal of anti‐AQP4 by plasma exchange has been suggested as treatment for NMO 25, confirmation by IIF‐AQP4 in ELISA‐AQP4(+) sera would be beneficial and more specific to detect anti‐AQP4 antobodies.
Despite our encouraging results, this study had a few limitations. We included a relatively small number of NMO patients and did not examine the prognostic value of AQP4 antibodies for predicting the patient's clinical course or response to treatment. However, the comparative analysis data from this study might be helpful for interpreting the results of NMO‐IgG and AQP4 antibodies detecting assays.
In conclusion, we confirmed the potential clinical usefulness of NMO‐IgG detection for differentiating patients with NMO and NMO spectrum disorders from individuals with MS or other neurological diseases. In particular, identification of anti‐AQP4 autoantibodies by ELISA‐AQP4 would be more sensitive and specific when confirmed by IIF‐AQP4. However, prospective studies with a larger number of cases and standardization of different methods are necessary.
ACKNOWLEDGMENTS
This research was supported by a grant of the Korea Healthcare technology R&D Project, Ministry for Health, Welfare & Family Affairs, Republic of Korea (A092258).
Authors’ Disclosures of Potential Conflicts of Interest:
None of the authors have any potential conflicts of interest relevant to this article.
REFERENCES
- 1. Wingerchuk DM, Lennon VA, Lucchinetti CF, Pittock SJ, Weinshenker BG. The spectrum of neuromyelitis optica. Lancet Neurol 2007;6:805–815. [DOI] [PubMed] [Google Scholar]
- 2. Wingerchuk DM, Hogancamp WF, O'Brien PC, Weinshenker BG. The clinical course of neuromyelitis optica (Devic's syndrome). Neurology 1999;53:1107–1114. [DOI] [PubMed] [Google Scholar]
- 3. Matiello M, Jacob A, Wingerchuk DM, Weinshenker BG. Neuromyelitis optica. Curr Opin Neurol 2007;20:255–260. [DOI] [PubMed] [Google Scholar]
- 4. Fazio R, Radaelli M, Furlan R. Neuromyelitis optica: Concepts in evolution. J Neuroimmunol 2011;231:100–104. [DOI] [PubMed] [Google Scholar]
- 5. Ghezzi A, Bergamaschi R, Martinelli V, et al. Clinical characteristics, course and prognosis of relapsing Devic's Neuromyelitis Optica. J Neurol 2004;251:47–52. [DOI] [PubMed] [Google Scholar]
- 6. Wingerchuk DM, Weinshenker BG. Neuromyelitis optica. Curr Treat Options Neurol 2005;7:173–182. [DOI] [PubMed] [Google Scholar]
- 7. Compston A, Coles A. Multiple sclerosis. Lancet 2008;372:1502–1517. [DOI] [PubMed] [Google Scholar]
- 8. Papeix C, Vidal JS, de Seze J, et al. Immunosuppressive therapy is more effective than interferon in neuromyelitis optica. Mult Scler 2007;13:256–259. [DOI] [PubMed] [Google Scholar]
- 9. Cree BA, Lamb S, Morgan K, Chen A, Waubant E, Genain C. An open label study of the effects of rituximab in neuromyelitis optica. Neurology 2005;64:1270–1272. [DOI] [PubMed] [Google Scholar]
- 10. Jacob A, Weinshenker BG, Violich I, et al. Treatment of neuromyelitis optica with rituximab: Retrospective analysis of 25 patients. Arch Neurol 2008;65:1443–1448. [DOI] [PubMed] [Google Scholar]
- 11. Mandler RN, Ahmed W, Dencoff JE. Devic's neuromyelitis optica: A prospective study of seven patients treated with prednisone and azathioprine. Neurology 1998;51:1219–1220. [DOI] [PubMed] [Google Scholar]
- 12. Lennon VA, Wingerchuk DM, Kryzer TJ, et al. A serum autoantibody marker of neuromyelitis optica: Distinction from multiple sclerosis. Lancet 2004;364:2106–2112. [DOI] [PubMed] [Google Scholar]
- 13. Lennon VA, Kryzer TJ, Pittock SJ, Verkman AS, Hinson SR. IgG marker of optic‐spinal multiple sclerosis binds to the aquaporin‐4 water channel. J Exp Med 2005;202:473–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wingerchuk DM, Lennon VA, Pittock SJ, Lucchinetti CF, Weinshenker BG. Revised diagnostic criteria for neuromyelitis optica. Neurology 2006;66:1485–1489. [DOI] [PubMed] [Google Scholar]
- 15. Jarius S, Probst C, Borowski K, et al. Standardized method for the detection of antibodies to aquaporin‐4 based on a highly sensitive immunofluorescence assay employing recombinant target antigen. J Neurol Sci 2010;291:52–56. [DOI] [PubMed] [Google Scholar]
- 16. Fazio R, Malosio ML, Lampasona V, et al. Antiacquaporin 4 antibodies detection by different techniques in neuromyelitis optica patients. Mult Scler 2009;15:1153–1163. [DOI] [PubMed] [Google Scholar]
- 17. Hayakawa S, Mori M, Okuta A, et al. Neuromyelitis optica and anti‐aquaporin‐4 antibodies measured by an enzyme‐linked immunosorbent assay. J Neuroimmunol 2008;196:181–187. [DOI] [PubMed] [Google Scholar]
- 18. Chan KH, Kwan JS, Ho PW, Ho JW, Chu AC, Ramsden DB. Aquaporin‐4 autoantibodies in neuromyelitis optica spectrum disorders: Comparison between tissue‐based and cell‐based indirect immunofluorescence assays. J Neuroinflammation 2010;7:50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Polman CH, Reingold SC, Edan G, et al. Diagnostic criteria for multiple sclerosis: 2005 revisions to the “McDonald Criteria”. Ann Neurol 2005;58:840–846. [DOI] [PubMed] [Google Scholar]
- 20. Waters P, Vincent A. Detection of anti‐aquaporin‐4 antibodies in neuromyelitis optica: Current status of the assays. Int MS J 2008;15:99–105. [PubMed] [Google Scholar]
- 21. Marignier R, De Sèze J, Vukusic S, et al. NMO‐IgG and Devic's neuromyelitis optica: A French experience. Mult Scler 2008;14:440–445. [DOI] [PubMed] [Google Scholar]
- 22. Saiz A, Zuliani L, Blanco Y, Tavolato B, Giometto B, Graus F. Revised diagnostic criteria for neuromyelitis optica (NMO). Application in a series of suspected patients. J Neurol 2007;254:1233–1237. [DOI] [PubMed] [Google Scholar]
- 23. Nakashima I, Fujihara K, Miyazawa I, et al. Clinical and MRI features of Japanese patients with multiple sclerosis positive for NMO‐IgG . J Neurol Neurosurg Psychiatry 2006;77:1073–1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kira J. Multiple sclerosis in the Japanese population. Lancet Neurol 2003;2:117–127. [DOI] [PubMed] [Google Scholar]
- 25. Watanabe S, Nakashima I, Misu T, et al. Therapeutic efficacy of plasma exchange in NMO‐IgG‐positive patients with neuromyelitis optica. Mult Scler 2007;13:128–132. [DOI] [PubMed] [Google Scholar]


